Abstract
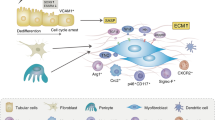
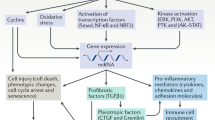
Renal fibrosis is the common end point of virtually all progressive kidney diseases. Renal fibrosis should not be viewed as a simple and uniform 'scar', but rather as a dynamic system that involves extracellular matrix components and many, if not all, renal and infiltrating cell types. The involved cells exhibit enormous plasticity or phenotypic variability—a fact that we are only beginning to appreciate. Only a detailed understanding of the underlying mechanisms of renal fibrosis can facilitate the development of effective treatments. In this Review, we discuss the most recent advances in renal, or more specifically, tubulointerstitial fibrosis. Novel mechanisms as well as potential treatment _targets based on different cell types are described. Problems that continue to plague the field are also discussed, including specific therapeutic _targeting of the kidney, the development of improved diagnostic methods to assess renal fibrosis and the shortcomings of available animal models.
Key Points
-
Renal fibrosis is the common end point for all progressive renal diseases
-
This entity involves virtually all intrinsic and infiltrating cells and is characterized by alterations in their phenotype
-
Whether these alterations are part of a regenerative program or are largely pathological is still not clear
-
Many potential treatment _targets for renal fibrosis have been identified in animal models
-
The lack of noninvasive diagnostic tools for renal fibrosis hinders efficient translation of these _targets into clinical practice
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
23 September 2010
In the version of this article initially published online, there was a mistake in Figure 4. The errors have been corrected in all electronic versions of the text.
References
Zeisberg, M. & Neilson, E. G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119, 1429–1437 (2009).
Schlondorff, D. O. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 74, 860–866 (2008).
Chevalier, R. L. Obstructive nephropathy: towards biomarker discovery and gene therapy. Nat. Clin. Pract. Nephrol. 2, 157–168 (2006).
Kaissling, B. & Le Hir, M. The renal cortical interstitium: morphological and functional aspects. Histochem. Cell Biol. 130, 247–262 (2008).
Ricardo, S. D., van Goor, H. & Eddy, A. A. Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 (2008).
Floege, J., Eitner, F. & Alpers, C. E. A new look at platelet-derived growth factor in renal disease. J. Am. Soc. Nephrol. 19, 12–23 (2008).
Docherty, N. G., O'Sullivan, O. E., Healy, D. A., Fitzpatrick, J. M. & Watson, R. W. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am. J. Physiol. Renal Physiol. 290, F4–F13 (2006).
Kurts, C., Heymann, F., Lukacs-Kornek, V., Boor, P. & Floege, J. Role of T cells and dendritic cells in glomerular immunopathology. Semin. Immunopathol. 29, 317–335 (2007).
Sung, S. S. & Bolton, W. K. T cells and dendritic cells in glomerular disease: the new glomerulotubular feedback loop. Kidney Int. 77, 393–399 (2010).
Holdsworth, S. R. & Summers, S. A. Role of mast cells in progressive renal diseases. J. Am. Soc. Nephrol. 19, 2254–2261 (2008).
Liu, Y. New insights into epithelial–mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 21, 212–222 (2010).
Boor, P., Sebeková, K., Ostendorf, T. & Floege, J. Treatment _targets in renal fibrosis. Nephrol. Dial. Transplant. 22, 3391–3407 (2007).
Chang, H. Y. et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl Acad. Sci. USA 99, 12877–12882 (2002).
Sorrell, J. M. & Caplan, A. I. Fibroblasts—a diverse population at the center of it all. Int. Rev. Cell Mol. Biol. 276, 161–214 (2009).
Hinz, B. The myofibroblast: paradigm for a mechanically active cell. J. Biomech. 43, 146–155 (2010).
Eyden, B. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct. Pathol. 25, 39–50 (2001).
Ru, Y., Eyden, B., Curry, A., McWilliam, L. J. & Coyne, J. D. Actin filaments in human renal tubulo-interstitial fibrosis: significance for the concept of epithelial–myofibroblast transformation. J. Submicrosc. Cytol. Pathol. 35, 221–233 (2003).
Muchaneta-Kubara, E. C. & el Nahas, A. M. Myofibroblast phenotypes expression in experimental renal scarring. Nephrol. Dial. Transplant. 12, 904–915 (1997).
Lin, S. L., Kisseleva, T., Brenner, D. A. & Duffield, J. S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Picard, N., Baum, O., Vogetseder, A., Kaissling, B. & Le Hir, M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem. Cell Biol. 130, 141–155 (2008).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Sommer, M. et al. Abnormal growth and clonal proliferation of fibroblasts in an animal model of unilateral ureteral obstruction. Nephron 82, 39–50 (1999).
Kilarski, W. W., Samolov, B., Petersson, L., Kvanta, A. & Gerwins, P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat. Med. 15, 657–664 (2009).
Rohatgi, R. & Flores, D. Intratubular hydrodynamic forces influence tubulointerstitial fibrosis in the kidney. Curr. Opin. Nephrol. Hypertens. 19, 65–71 (2010).
Li, L. et al. Aberrant planar cell polarity induced by urinary tract obstruction. Am. J. Physiol. Renal Physiol. 297, F1526–F1533 (2009).
Fujigaki, Y. et al. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Arch. 446, 164–176 (2005).
Takeji, M. et al. Smooth muscle alpha-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J. Biol. Chem. 281, 40193–40200 (2006).
Taneda, S. et al. Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. J. Am. Soc. Nephrol. 14, 2544–2555 (2003).
Boor, P. et al. PDGF-D inhibition by CR002 ameliorates tubulointerstitial fibrosis following experimental glomerulonephritis. Nephrol. Dial. Transplant. 22, 1323–1331 (2007).
Ostendorf, T. et al. Antagonism of PDGF-D by human antibody CR002 prevents renal scarring in experimental glomerulonephritis. J. Am. Soc. Nephrol. 17, 1054–1062 (2006).
Kliem, V. et al. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 49, 666–678 (1996).
Alpers, C. E., Seifert, R. A., Hudkins, K. L., Johnson, R. J. & Bowen-Pope, D. F. PDGF-receptor localizes to mesangial, parietal epithelial, and interstitial cells in human and primate kidneys. Kidney Int. 43, 286–294 (1993).
Hawthorne, T. et al. A phase I study of CR002, a fully-human monoclonal antibody against platelet-derived growth factor-D. Int. J. Clin. Pharmacol. Ther. 46, 236–244 (2008).
Floege, J. et al. Localization of PDGF alpha-receptor in the developing and mature human kidney. Kidney Int. 51, 1140–1150 (1997).
Floege, J., Hudkins, K. L., Davis, C. L., Schwartz, S. M. & Alpers, C. E. Expression of PDGF alpha-receptor in renal arteriosclerosis and rejecting renal transplants. J. Am. Soc. Nephrol. 9, 211–223 (1998).
Eitner, F. et al. PDGF-C expression in the developing and normal adult human kidney and in glomerular diseases. J. Am. Soc. Nephrol. 14, 1145–1153 (2003).
Eitner, F. et al. Expression of a novel PDGF isoform, PDGF-C, in normal and diseased rat kidney. J. Am. Soc. Nephrol. 13, 910–917 (2002).
Eitner, F. et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J. Am. Soc. Nephrol. 19, 281–289 (2008).
Ding, H. et al. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat. Genet. 36, 1111–1116 (2004).
Huang, X. R., Chung, A. C., Wang, X. J., Lai, K. N. & Lan, H. Y. Mice overexpressing latent TGF-beta1 are protected against renal fibrosis in obstructive kidney disease. Am. J. Physiol. Renal Physiol. 295, F118–F127 (2008).
Huang, X. R., Chung, A. C., Zhou, L., Wang, X. J. & Lan, H. Y. Latent TGF-beta1 protects against crescentic glomerulonephritis. J. Am. Soc. Nephrol. 19, 233–242 (2008).
Zeisberg, M. Bone morphogenic protein-7 and the kidney: current concepts and open questions. Nephrol. Dial. Transplant. 21, 568–573 (2006).
Zeisberg, M. et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am. J. Physiol. Renal Physiol. 285, F1060–F1067 (2003).
Zeisberg, M. et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968 (2003).
Zeisberg, M., Shah, A. A. & Kalluri, R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J. Biol. Chem. 280, 8094–8100 (2005).
Yanagita, M. Modulator of bone morphogenetic protein activity in the progression of kidney diseases. Kidney Int. 70, 989–993 (2006).
Tanaka, M. et al. Loss of the BMP antagonist USAG-1 ameliorates disease in a mouse model of the progressive hereditary kidney disease Alport syndrome. J. Clin. Invest. 120, 768–777 (2010).
Grgic, I. et al. Renal fibrosis is attenuated by _targeted disruption of KCa3.1 potassium channels. Proc. Natl Acad. Sci. USA 106, 14518–14523 (2009).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
Krupa, A. et al. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J. Am. Soc. Nephrol. 21, 438–447 (2010).
Kato, M. et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc. Natl Acad. Sci. USA 104, 3432–3437 (2007).
Wang, Q. et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 22, 4126–4135 (2008).
Rastaldi, M. P. et al. Epithelial–mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 62, 137–146 (2002).
Hertig, A. et al. Early epithelial phenotypic changes predict graft fibrosis. J. Am. Soc. Nephrol. 19, 1584–1591 (2008).
Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004).
Li, L., Zepeda-Orozco, D., Black, R. & Lin, F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am. J. Pathol. 176, 1767–1778 (2010).
Docherty, N. G. et al. Increased E-cadherin expression in the ligated kidney following unilateral ureteric obstruction. Kidney Int. 75, 205–213 (2009).
Iwano, M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 (2002).
Humphreys, B. D. et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291 (2008).
Faulkner, J. L., Szcykalski, L. M., Springer, F. & Barnes, J. L. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am. J. Pathol. 167, 1193–1205 (2005).
Sheerin, N. S. & Sacks, S. H. Leaked protein and interstitial damage in the kidney: is complement the missing link? Clin. Exp. Immunol. 130, 1–3 (2002).
Rangan, G. K., Pippin, J. W., Coombes, J. D. & Couser, W. G. C5b-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney Int. 67, 492–503 (2005).
Rangan, G. K., Pippin, J. W. & Couser, W. G. C5b-9 regulates peritubular myofibroblast accumulation in experimental focal segmental glomerulosclerosis. Kidney Int. 66, 1838–1848 (2004).
Boor, P. et al. Complement C5 mediates experimental tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 18, 1508–1515 (2007).
Pan, H. et al. Anaphylatoxin C5a contributes to the pathogenesis of cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal Physiol. 296, F496–F504 (2009).
Hartleben, B. et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 120, 1084–1096 (2010).
Kume, S. et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 120, 1043–1055 (2010).
Cybulsky, A. V. Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int. 77, 187–193 (2010).
Inagi, R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp. Nephrol. 112, e1–e9 (2009).
Periyasamy-Thandavan, S., Jiang, M., Schoenlein, P. & Dong, Z. Autophagy: molecular machinery, regulation, and implications for renal pathophysiology. Am. J. Physiol. Renal Physiol. 297, F244–F256 (2009).
Periyasamy-Thandavan, S. et al. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 74, 631–640 (2008).
Pallet, N. et al. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy 4, 783–791 (2008).
Gozuacik, D. et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 15, 1875–1886 (2008).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Menke, J. et al. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J. Clin. Invest. 119, 2330–2342 (2009).
Ruan, X. Z., Varghese, Z. & Moorhead, J. F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 5, 713–721 (2009).
Cho, K. H., Kim, H. J., Kamanna, V. S. & Vaziri, N. D. Niacin improves renal lipid metabolism and slows progression in chronic kidney disease. Biochim. Biophys. Acta 1800, 6–15 (2010).
Cho, K. H., Kim, H. J., Rodriguez-Iturbe, B. & Vaziri, N. D. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am. J. Physiol. Renal Physiol. 297, F106–F113 (2009).
Kim, H. J., Moradi, H., Yuan, J., Norris, K. & Vaziri, N. D. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am. J. Physiol. Renal Physiol. 296, F1297–F1306 (2009).
Wu, J. et al. Peroxisome proliferator-activated receptors and renal diseases. Front. Biosci. 14, 995–1009 (2009).
Toblli, J. E. et al. Antifibrotic effects of pioglitazone on the kidney in a rat model of type 2 diabetes mellitus. Nephrol. Dial. Transplant. 24, 2384–2391 (2009).
Kawai, T. et al. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab. Invest. 89, 47–58 (2009).
Higgins, D. F., Kimura, K., Iwano, M. & Haase, V. H. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle 7, 1128–1132 (2008).
Kimura, K. et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am. J. Physiol. Renal Physiol. 295, F1023–F1029 (2008).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M. & Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19, 2282–2287 (2008).
Li, J., Qu, X. & Bertram, J. F. Endothelial–myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am. J. Pathol. 175, 1380–1388 (2009).
Zhou, X. J. et al. The aging kidney. Kidney Int. 74, 710–720 (2008).
Wilkinson, L. et al. Loss of renal microvascular integrity in postnatal Crim1 hypomorphic transgenic mice. Kidney Int. 76, 1161–1171 (2009).
Hakroush, S. et al. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am. J. Pathol. 175, 1883–1895 (2009).
Sakai, N. et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc. Natl Acad. Sci. USA 103, 14098–14103 (2006).
Niedermeier, M. et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc. Natl Acad. Sci. USA 106, 17892–17897 (2009).
Sakai, N. et al. Fibrocytes are involved in the pathogenesis of human chronic kidney disease. Hum. Pathol. 41, 672–678 (2010).
Pilling, D., Fan, T., Huang, D., Kaul, B. & Gomer, R. H. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 4, e7475 (2009).
Wynn, T. A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 4, 583–594 (2004).
Shao, D. D., Suresh, R., Vakil, V., Gomer, R. H. & Pilling, D. Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J. Leukoc. Biol. 83, 1323–1333 (2008).
Lin, S. L., Castaño, A. P., Nowlin, B. T., Lupher, M. L. Jr & Duffield, J. S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 183, 6733–6743 (2009).
Roufosse, C. et al. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J. Am. Soc. Nephrol. 17, 775–782 (2006).
Broekema, M. et al. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J. Am. Soc. Nephrol. 18, 165–175 (2007).
Henderson, N. C. et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 172, 288–298 (2008).
Henderson, N. C. & Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 230, 160–171 (2009).
Ma, F. Y., Liu, J., Kitching, A. R., Manthey, C. L. & Nikolic-Paterson, D. J. _targeting renal macrophage accumulation via c-fms kinase reduces tubular apoptosis but fails to modify progressive fibrosis in the obstructed rat kidney. Am. J. Physiol. Renal Physiol. 296, F177–F185 (2009).
Lin, S. L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl Acad. Sci. USA 107, 4194–4199 (2010).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Nishida, M. & Hamaoka, K. Macrophage phenotype and renal fibrosis in obstructive nephropathy. Nephron Exp. Nephrol. 110, e31–e36 (2008).
Wang, Y. et al. By homing to the kidney, activated macrophages potently exacerbate renal injury. Am. J. Pathol. 172, 1491–1499 (2008).
Nishida, M. et al. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem. Biophys. Res. Commun. 332, 11–16 (2005).
Krüger, T. et al. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J. Am. Soc. Nephrol. 15, 613–621 (2004).
Macconi, D. et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J. Am. Soc. Nephrol. 20, 123–130 (2009).
Heymann, F. et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J. Clin. Invest. 119, 1286–1297 (2009).
Sakamoto, I. et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int. 75, 828–838 (2009).
Matsui, K. et al. Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J. Am. Soc. Nephrol. 14, 1981–1989 (2003).
Kriz, W. & LeHir, M. Pathways to nephron loss starting from glomerular diseases—insights from animal models. Kidney Int. 67, 404–419 (2005).
Zhang, T. et al. Disturbance of lymph circulation develops renal fibrosis in rats with or without contralateral nephrectomy. Nephrology (Carlton) 13, 128–138 (2008).
Zhang, T. et al. Functional, histological and biochemical consequences of renal lymph disorder in mononephrectomized rats. J. Nephrol. 22, 109–116 (2009).
Kerjaschki, D. et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 15, 603–612 (2004).
Stuht, S. et al. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. Am. J. Transplant. 7, 377–384 (2007).
El-Koraie, A. F., Baddour, N. M., Adam, A. G., El Kashef, E. H. & El Nahas, A. M. Role of stem cell factor and mast cells in the progression of chronic glomerulonephritides. Kidney Int. 60, 167–172 (2001).
Roberts, I. S. & Brenchley, P. E. Mast cells: the forgotten cells of renal fibrosis. J. Clin. Pathol. 53, 858–862 (2000).
Kondo, S. et al. Role of mast cell tryptase in renal interstitial fibrosis. J. Am. Soc. Nephrol. 12, 1668–1676 (2001).
Timoshanko, J. R., Kitching, R., Semple, T. J., Tipping, P. G. & Holdsworth, S. R. A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J. Am. Soc. Nephrol. 17, 150–159 (2006).
Kanamaru, Y. et al. Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. J. Immunol. 176, 5607–5615 (2006).
Miyazawa, S. et al. Role of mast cells in the development of renal fibrosis: use of mast cell-deficient rats. Kidney Int. 65, 2228–2237 (2004).
Hochegger, K. et al. Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur. J. Immunol. 35, 3074–3082 (2005).
Kim, D. H. et al. Mast cells decrease renal fibrosis in unilateral ureteral obstruction. Kidney Int. 75, 1031–1038 (2009).
Silver, R. B. et al. Mast cells: a unique source of renin. Proc. Natl Acad. Sci. USA 101, 13607–13612 (2004).
Fan, Y. Y. et al. Contribution of chymase-dependent angiotensin II formation to the progression of tubulointerstitial fibrosis in obstructed kidneys in hamsters. J. Pharmacol. Sci. 111, 82–90 (2009).
Shweke, N. et al. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am. J. Pathol. 173, 631–642 (2008).
Huang, L. et al. Transglutaminase inhibition ameliorates experimental diabetic nephropathy. Kidney Int. 76, 383–394 (2009).
Abrass, C. K., Hansen, K. M. & Patton, B. L. Laminin alpha4-null mutant mice develop chronic kidney disease with persistent overexpression of platelet-derived growth factor. Am. J. Pathol. 176, 839–849 (2010).
Xie, P. et al. C/EBP-beta modulates transcription of tubulointerstitial nephritis antigen in obstructive uropathy. J. Am. Soc. Nephrol. 20, 807–819 (2009).
Ramachandra Rao, S. P. et al. Pirfenidone is renoprotective in diabetic kidney disease. J. Am. Soc. Nephrol. 20, 1765–1775 (2009).
Takakuta, K. et al. Renoprotective properties of pirfenidone in subtotally nephrectomized rats. Eur. J. Pharmacol. 629, 118–124 (2010).
Cho, M. E. & Kopp, J. B. Pirfenidone: an anti-fibrotic therapy for progressive kidney disease. Expert Opin. Investig. Drugs 19, 275–283 (2010).
Cho, M. E., Smith, D. C., Branton, M. H., Penzak, S. R. & Kopp, J. B. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2, 906–913 (2007).
Cook, H. T. The origin of renal fibroblasts and progression of kidney disease. Am. J. Pathol. 176, 22–24 (2010).
Little, M. H. & Bertram, J. F. Is there such a thing as a renal stem cell? J. Am. Soc. Nephrol. 20, 2112–2117 (2009).
Hopkins, C., Li, J., Rae, F. & Little, M. H. Stem cell options for kidney disease. J. Pathol. 217, 265–281 (2009).
Psihogios, N. G. et al. Evaluation of tubulointerstitial lesions' severity in patients with glomerulonephritides: an NMR-based metabonomic study. J. Proteome Res. 6, 3760–3770 (2007).
Nickolas, T. L., Barasch, J. & Devarajan, P. Biomarkers in acute and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 17, 127–132 (2008).
Tanaka, T. et al. Urinary L-type fatty acid-binding protein can reflect renal tubulointerstitial injury. Am. J. Pathol. 174, 1203–1211 (2009).
Yamamoto, T. et al. Renal L-type fatty acid—binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 18, 2894–2902 (2007).
Negishi, K. et al. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int. 73, 1374–1384 (2008).
Miranda, K. C. et al. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 78, 191–199 (2010).
Sato, Y. et al. Urine podocyte mRNAs mark progression of renal disease. J. Am. Soc. Nephrol. 20, 1041–1052 (2009).
Hartono, C., Muthukumar, T. & Suthanthiran, M. Noninvasive diagnosis of acute rejection of renal allografts. Curr. Opin. Organ Transplant. 15, 35–41 (2010).
Ghoul, B. E. et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin. J. Am. Soc. Nephrol. 5, 205–210 (2010).
Ju, W. et al. Renal gene and protein expression signatures for prediction of kidney disease progression. Am. J. Pathol. 174, 2073–2085 (2009).
Sato, Y. et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 26, 431–442 (2008).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Jones, L. K. et al. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol. Dial. Transplant. 24, 3024–3032 (2009).
Li, Y. et al. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 1907–1918 (2009).
Zhang, G. et al. A novel signaling pathway: fibroblast nicotinic receptor alpha1 binds urokinase and promotes renal fibrosis. J. Biol. Chem. 284, 29050–29064 (2009).
Pang, M. et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Renal Physiol. 297, F996–F1005 (2009).
Noh, H. et al. Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am. J. Physiol. Renal Physiol. 297, F729–F739 (2009).
Grande, M. T. et al. _targeted genomic disruption of H-ras and N-ras has no effect on early renal changes after unilateral ureteral ligation. World J. Urol. doi:10.1007/s00345-009-0399-8.
Grande, M. T. et al. Deletion of H-Ras decreases renal fibrosis and myofibroblast activation following ureteral obstruction in mice. Kidney Int. 77, 509–518 (2010).
Liao, T. D. et al. N-acetyl-seryl-aspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass: council for high blood pressure research. Hypertension 55, 459–467 (2010).
Kassiri, Z. et al. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J. Am. Soc. Nephrol. 20, 1223–1235 (2009).
Jung, G. S. et al. The orphan nuclear receptor SHP attenuates renal fibrosis. J. Am. Soc. Nephrol. 20, 2162–2170 (2009).
Hewitson, T. D. et al. Endogenous relaxin is a naturally occurring modulator of experimental renal tubulointerstitial fibrosis. Endocrinology 148, 660–669 (2007).
Teerlink, J. R. et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 373, 1429–1439 (2009).
Yuen, D. A. et al. Culture-modified bone marrow cells attenuate cardiac and renal injury in a chronic kidney disease rat model via a novel antifibrotic mechanism. PLoS ONE 5, e9543 (2010).
Benigni, A., Morigi, M. & Remuzzi, G. Kidney regeneration. Lancet 375, 1310–1317 (2010).
Sakairi, T. et al. Nestin expression in the kidney with an obstructed ureter. Kidney Int. 72, 307–318 (2007).
Nguyen, T. Q. et al. CTGF inhibits BMP-7 signaling in diabetic nephropathy. J. Am. Soc. Nephrol. 19, 2098–2107 (2008).
Acknowledgements
We apologize to all authors whose important work could not be cited owing to space limitations. We wish to thank Dr B. Hintz and members of J. Floege's laboratory for valuable discussions. Our work described in this Review was supported by grants from the Deutsche Forschungsgemeinschaft (SFB/TRR 57), projects P14, P17 and P19 (to T. Ostendorf, J. Floege).
Author information
Authors and Affiliations
Contributions
P. Boor wrote the manuscript; P. Boor, T. Ostendorf and J. Floege contributed equally to discussing content, and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. Floege has received speaker honoraria and grant support from Amgen. P. Boor and T. Ostendorf declare no competing interests.
Rights and permissions
About this article
Cite this article
Boor, P., Ostendorf, T. & Floege, J. Renal fibrosis: novel insights into mechanisms and therapeutic _targets. Nat Rev Nephrol 6, 643–656 (2010). https://doi.org/10.1038/nrneph.2010.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.120