Abstract
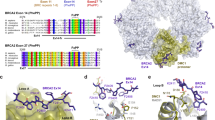
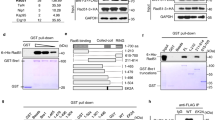
The human breast cancer susceptibility gene BRCA2 is required for the regulation of RAD51-mediated homologous recombinational repair. BRCA2 interacts with RAD51 monomers, as well as nucleoprotein filaments, primarily though the conserved BRC motifs. The unrelated C-terminal region of BRCA2 also interacts with RAD51. Here we show that the BRCA2 C terminus interacts directly with RAD51 filaments, but not monomers, by binding an interface created by two adjacent RAD51 protomers. These interactions stabilize filaments so that they cannot be dissociated by association with BRC repeats. Interaction of the BRCA2 C terminus with the RAD51 filament causes a large movement of the flexible RAD51 N-terminal domain that is important in regulating filament dynamics. We suggest that interactions of the BRCA2 C-terminal region with RAD51 may facilitate efficient nucleation of RAD51 multimers on DNA and thereby stimulate recombination-mediated repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lancaster, J.M. et al. BRCA2 mutations in primary breast and ovarian cancers. Nat. Genet. 13, 238–240 (1996).
Connor, F. et al. Tumorigenesis and a DNA-repair defect in mice with a truncating BRCA2 mutation. Nat. Genet. 17, 423–430 (1997).
West, S.C. Molecular views of recombination proteins and their control. Nat. Rev. Mol. Cell Biol. 4, 435–445 (2003).
Sharan, S.K. et al. Embryonic lethality and radiation hypersensitivity mediated by RAD51 in mice lacking BRCA2. Nature 386, 804–810 (1997).
Wong, A.K.C., Pero, R., Ormonde, P.A., Tavtigian, S.V. & Bartel, P.L. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene BRCA2. J. Biol. Chem. 272, 31941–31944 (1997).
Yang, H. et al. BRCA2 function in DNA binding and recombination from a BRCA2–DSS1-ssDNA structure. Science 297, 1837–1848 (2002).
Godthelp, B.C., Artwert, F., Joenje, H. & Zdzienicka, M.Z. Impaired DNA damage-induced nuclear RAD51 foci formation uniquely characterizes Fanconi anemia group D1. Oncogene 21, 5002–5005 (2002).
Yuan, S.-S.F. et al. BRCA2 is required for ionizing radiation-induced assembly of RAD51 complex in vivo. Cancer Res. 59, 3547–3551 (1999).
Tarsounas, M., Davies, D. & West, S.C. BRCA2-dependent and independent formation of RAD51 nuclear foci. Oncogene 22, 1115–1123 (2003).
Pellegrini, L. et al. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature 420, 287–293 (2002).
Galkin, V.E. et al. The RAD51/RadA N-terminal domain activates nucleoprotein filament ATPase activity. Structure 14, 983–992 (2006).
Davies, A.A. et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol. Cell 7, 273–282 (2001).
Chen, C.F., Chen, P.L., Zhong, Q., Sharp, Z.D. & Lee, W.H. Expression of BRC repeats in breast cancer cells disrupts the BRCA2–RAD51 complex and leads to radiation hypersensitivity and loss of G(2)/M checkpoint control. J. Biol. Chem. 274, 32931–32935 (1999).
Galkin, V.E. et al. BRCA2 BRC motifs bind RAD51-DNA filaments. Proc. Natl. Acad. Sci. USA 102, 8537–8542 (2005).
Shivji, M.K. et al. A region of human BRCA2 containing multiple BRC repeats promotes RAD51-mediated strand exchange. Nucleic Acids Res. 34, 4000–4011 (2006).
Moynahan, M.E., Pierce, A.J. & Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7, 263–272 (2001).
Tutt, A. et al. Mutation in BRCA2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J. 20, 4704–4716 (2001).
Howlett, N.G. et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297, 606–609 (2002).
Wang, X., Andreassen, P.R. & D'Andrea, A.D. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol. Cell. Biol. 24, 5850–5862 (2004).
Esashi, F. et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 434, 598–604 (2005).
Lo, T., Pellegrini, L., Venkitaraman, A.R. & Blundell, T.L. Sequence fingerprints in BRCA2 and RAD51: implications for DNA repair and cancer. DNA Repair (Amst.) 2, 1015–1028 (2003).
Yu, D.S. et al. Dynamic control of RAD51 recombinase by self-association and interaction with BRCA2. Mol. Cell 12, 1029–1041 (2003).
Shin, D.S. et al. Full-length archaeal RAD51 structure and mutants: mechanisms for RAD51 assembly and control by BRCA2. EMBO J. 22, 4566–4576 (2003).
Conway, A.B. et al. Crystal structure of a RAD51 filament. Nat. Struct. Mol. Biol. 11, 791–796 (2004).
Kinebuchi, T. et al. Structural basis for octameric ring formation and DNA interaction of the human homologous-pairing protein DMC1. Mol. Cell 14, 363–374 (2004).
Hakansson, S. et al. Moderate frequency of BRCA1 and BRCA2 germ-line mutations in Scandinavian familial breast cancer. Am. J. Hum. Genet. 60, 1068–1078 (1997).
Mazoyer, S. et al. A polymorphic stop codon in BRCA2. Nat. Genet. 14, 253–254 (1996).
Baumann, P., Benson, F.E., Hajibagheri, N. & West, S.C. Purification of human RAD51 protein by selective spermidine precipitation. Mutat. Res. 384, 65–72 (1997).
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996).
Egelman, E.H. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy 85, 225–234 (2000).
Acknowledgements
We thank R. Court and D. Wigley for help with the DLS analyses, and our lab members for their comments. This work was supported by Cancer Research UK, the Breast Cancer Campaign, the EU DNA Repair Consortium, the Jeantet Foundation (S.C.W.) and US National Institutes of Health grant GM035269 (E.H.E.). F.E. is a recipient of a postdoctoral fellowship from the Human Frontiers Science Program and the Japanese Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
F.E. designed and carried out the biochemical studies. V.E.G. and X.Y. carried out the EM studies. E.H.E. did the image reconstructions. F.E. and S.C.W. were responsible for manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Esashi, F., Galkin, V., Yu, X. et al. Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat Struct Mol Biol 14, 468–474 (2007). https://doi.org/10.1038/nsmb1245
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1245