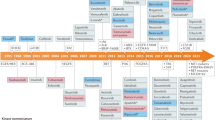
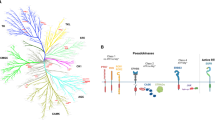
Abstract
Protein kinases regulate nearly all aspects of cell life, and alterations in their expression, or mutations in their genes, cause cancer and other diseases. Here, we review the remarkable progress made over the past 20 years in improving the potency and specificity of small-molecule inhibitors of protein and lipid kinases, resulting in the approval of more than 70 new drugs since imatinib was approved in 2001. These compounds have had a significant impact on the way in which we now treat cancers and non-cancerous conditions. We discuss how the challenge of drug resistance to kinase inhibitors is being met and the future of kinase drug discovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cohen, P. Protein kinases — the major drug _targets of the twenty-first century? Nat. Rev. Drug Discov. 1, 309–315 (2002). A review of the history of the development of protein kinase inhibitors up to the time that imatinib was approved for clinical use, and which provides the background to this article.
Witte, O. N., Dasgupta, A. & Baltimore, D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature 283, 826–831 (1980).
Veale, D., Ashcroft, T., Marsh, C., Gibson, G. J. & Harris, A. L. Epidermal growth factor receptors in non-small cell lung cancer. Br. J. Cancer 55, 513–516 (1987).
Haeder, M. et al. Epidermal growth factor receptor expression in human lung cancer cell lines. Cancer Res. 48, 1132–1136 (1988).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004).
Shigematsu, H. et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl Cancer Inst. 97, 339–346 (2005).
Berger, A. H. et al. High-throughput phenotyping of lung cancer somatic mutations. Cancer Cell 30, 214–228 (2016).
Campbell, J. D. et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 48, 607–616 (2016).
Breccia, M. & Alimena, G. Nilotinib: a second-generation tyrosine kinase inhibitor for chronic myeloid leukemia. Leuk. Res. 34, 129–134 (2010).
Kantarjian, H., Jabbour, E., Grimley, J. & Kirkpatrick, P. Dasatinib. Nat. Rev. Drug Discov. 5, 717–718 (2006).
Mealing, S. et al. The relative efficacy of imatinib, dasatinib and nilotinib for newly diagnosed chronic myeloid leukemia: a systematic review and network meta-analysis. Exp. Hematol. Oncol. 2, 5 (2013).
Cortes, J. E. et al. Matching-adjusted indirect comparison of bosutinib, dasatinib and nilotinib effect on survival and major cytogenetic response in treatment of second-line chronic phase chronic myeloid leukemia. Curr. Med. Res. Opin. 35, 1615–1622 (2019).
Redaelli, S. et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J. Clin. Oncol. 27, 469–471 (2009).
Cortes, J. E. et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J. Clin. Oncol. 36, 231–237 (2018).
Noronha, G. et al. Inhibitors of ABL and the ABL-T315I mutation. Curr. Top. Med. Chem. 8, 905–921 (2008).
O’Hare, T., Zabriskie, M. S., Eiring, A. M. & Deininger, M. W. Pushing the limits of _targeted therapy in chronic myeloid leukaemia. Nat. Rev. Cancer 12, 513–526 (2012).
O’Hare, T. et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 16, 401–412 (2009).
Moy, B., Kirkpatrick, P., Kar, S. & Goss, P. Lapatinib. Nat. Rev. Drug Discov. 6, 431–432 (2007).
Christensen, J. G. et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 6, 3314–3322 (2007). An interesting preclinical example of the polypharmacology of a kinase inhibitor.
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
Solomon, B. J. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177 (2014).
Mok, T. et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 31, 1056–1064 (2020).
Peters, S. et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377, 829–838 (2017).
Camidge, D. R. et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 379, 2027–2039 (2018).
Shaw, A. T. et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N. Engl. J. Med. 383, 2018–2029 (2020).
Rajabi, M. & Mousa, S. A. The role of angiogenesis in cancer treatment. Biomedicines 5, 34 (2017).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Faivre, S., Demetri, G., Sargent, W. & Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 6, 734–745 (2007).
Bukowski, R. M., Yasothan, U. & Kirkpatrick, P. Pazopanib. Nat. Rev. Drug Discov. 9, 17–18 (2010).
Keating, G. M. Axitinib: a review in advanced renal cell carcinoma. Drugs 75, 1903–1913 (2015).
Rathi, N., Maughan, B. L., Agarwal, N. & Swami, U. Mini-review: cabozantinib in the treatment of advanced renal cell carcinoma and hepatocellular carcinoma. Cancer Manag. Res. 12, 3741–3749 (2020).
Davies, S. P., Reddy, H., Caivano, M. & Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 (2000).
Bain, J. et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 (2007). This paper and Davies et al. (2000) introduce and popularize the use of kinase profiling panels to assess the specificities of kinase inhibitors.
Yang, G. et al. HCK is a survival determinant transactivated by mutated MYD88, and a direct _target of ibrutinib. Blood 127, 3237–3252 (2016).
Shaw, A. T. et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 371, 1963–1971 (2014).
Rodig, S. J. & Shapiro, G. I. Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr. Opin. Investig. Drugs 11, 1477–1490 (2010).
Suh, K. J. et al. Analysis of adverse events associated with dasatinib and nilotinib treatments in chronic-phase chronic myeloid leukemia patients outside clinical trials. Int. J. Hematol. 106, 229–239 (2017).
Klaeger, S. et al. The _target landscape of clinical kinase drugs. Science 358, eaan4368 (2017).
Elkins, J. M. et al. Comprehensive characterization of the published kinase inhibitor set. Nat. Biotechnol. 34, 95–103 (2016).
Ciceri, P. et al. Dual kinase-bromodomain inhibitors for rationally designed polypharmacology. Nat. Chem. Biol. 10, 305–312 (2014). The paper demonstrating that protein kinases inhibitors frequently bind to BRD-containing proteins, and that dual kinase–BRD inhibitors can be designed rationally.
Malik, N. et al. Suppression of interferon β gene transcription by inhibitors of bromodomain and extra-terminal (BET) family members. Biochem. J. 468, 363–372 (2015).
Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020).
Zhang, Q. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020).
Kolch, W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351, 289–305 (2000).
Roy, F., Laberge, G., Douziech, M., Ferland-McCollough, D. & Therrien, M. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 16, 427–438 (2002).
Terrell, E. M. & Morrison, D. K. Ras-mediated activation of the Raf family kinases. Cold Spring Harb. Perspect. Med. 9, a033746 (2019).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002). This study exploits the sequence of the human genome to identify protein kinases mutated in human cancers. The analysis reveals the prevalence of the BRAF V600E mutation in malignant melanoma and other cancers.
Peng, S. B. et al. Inhibition of RAF isoforms and active dimers by LY3009120 leads to anti-tumor activities in RAS or BRAF mutant cancers. Cancer Cell 28, 384–398 (2015).
Robert, C. et al. Five-year outcomes with dabrafenib plus trametinib in metastaticmelanoma. N. Engl. J. Med. 381, 626–636 (2019). Review of the clinical studies that revealed the remarkable improvement in the treatment of metastatic melanoma produced by combining inhibitors of BRAF and MEK, compared with either kinase inhibitor used alone.
Long, G. V. et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 377, 1813–1823 (2017).
Dummer, R. et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N. Engl. J. Med. 383, 1139–1148 (2020).
Planchard, D. et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 17, 984–993 (2016).
Planchard, D. et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 18, 1307–1316 (2017).
Hall-Jackson, C. A. et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chem. Biol. 6, 559–568 (1999).
Poulikakos, P. I., Zhang, C., Bollag, G., Shokat, K. M. & Rosen, N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464, 427–430 (2010).
Karoulia, Z., Gavathiotis, E. & Poulikakos, P. I. New perspectives for _targeting RAF kinase in human cancer. Nat. Rev. Cancer 17, 676–691 (2017).
Su, F. et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 366, 207–215 (2012).
Khan, Z. M. et al. Structural basis for the action of the drug trametinib at KSR-bound MEK. Nature 588, 509–514 (2020).
Poh, A. Dual RAF-MEK inhibitor assessed. Cancer Discov. 11, 5–6 (2020).
van Geel, R. et al. Phase 1 study of the pan-HER inhibitor dacomitinib plus the MEK1/2 inhibitor PD-0325901 in patients with KRAS-mutation-positive colorectal, non-small-cell lung and pancreatic cancer. Br. J. Cancer 122, 1166–1174 (2020).
de Miguel, M. & Calvo, E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell 38, 326–333 (2020).
Giraldo, N. A. et al. The clinical role of the TME in solid cancer. Br. J. Cancer 120, 45–53 (2019).
Baldewijns, M. M. et al. VHL and HIF signalling in renal cell carcinogenesis. J. Pathol. 221, 125–138 (2010).
Liu, P. et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat. Commun. 10, 1486 (2019). The first evidence that a protein TKI is capable of inducing immunogenic cell death.
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Dummer, R. et al. Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma: safety run-in and biomarker cohorts of COMBI-i. Nat. Med. 26, 1557–1563 (2020).
P. Nathan, R. D. et al. LBA43 - Spartalizumab plus dabrafenib and trametinib (Sparta-DabTram) in patients (pts) with previously untreated BRAF V600–mutant unresectable or metastatic melanoma: Results from the randomized part 3 of the phase III COMBI-i trial. Ann. Oncol. 31, S1142–S1215 (2020).
Yang, J. C. et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M-positive NSCLC following previous EGFR TKI therapy: CAURAL Brief Report. J. Thorac. Oncol. 14, 933–939 (2019).
Spigel, D. R. et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation - positive advanced non-small cell lung cancer (CheckMate 370). J. Thorac. Oncol. 13, 682–688 (2018).
Glodde, N. et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity 47, 789–802.e789 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04139317 (2021).
Hemmings, B. A. & Restuccia, D. F. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 4, a011189 (2012).
Flinn, I. W. et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood 123, 3406–3413 (2014).
Sapon-Cousineau, V., Sapon-Cousineau, S. & Assouline, S. PI3K inhibitors and their role as novel agents for _targeted therapy in lymphoma. Curr. Treat. Options Oncol. 21, 51 (2020).
Andre, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Smyth, L. M. et al. Capivasertib, an AKT kinase inhibitor, as monotherapy or in combination with fulvestrant in patients with AKT1 E17K-mutant, ER-positive metastatic breast cancer. Clin. Cancer Res. 26, 3947–3957 (2020).
Rudolph, M. et al. AKT1 (E17K) mutation profiling in breast cancer: prevalence, concurrent oncogenic alterations, and blood-based detection. BMC Cancer 16, 622 (2016).
Jones, R. H. et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 21, 345–357 (2020).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010).
Oizumi, S. et al. Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 Trial. Oncologist 17, 863–870 (2012).
Hosomi, Y. et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J. Clin. Oncol. 38, 115–123 (2020).
Noronha, V. et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J. Clin. Oncol. 38, 124–136 (2020).
Reungwetwattana, T. et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J. Clin. Oncol. 36, 3290–3297 (2018).
Camidge, D. R. et al. Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J. Clin. Oncol. 36, 2693–2701 (2018).
Solomon, B. J. et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 19, 1654–1667 (2018).
Subbiah, V. et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol. 29, 1869–1876 (2018).
Lin, J. J. et al. Efficacy of alectinib in patients with ALK-positive NSCLC and symptomatic or large CNS metastases. J. Thorac. Oncol. 14, 683–690 (2019).
Yang, J. C. H. et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J. Clin. Oncol. 38, 538–547 (2020).
Ou, S. H., Sommers, K. R., Azada, M. C. & Garon, E. B. Alectinib induces a durable (>15 months) complete response in an ALK-positive non-small cell lung cancer patient who progressed on crizotinib with diffuse leptomeningeal carcinomatosis. Oncologist 20, 224–226 (2015).
Goodwin, S., McPherson, J. D. & McCombie, W. R. Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet. 17, 333–351 (2016).
Cescon, D. W., Bratman, S. V., Chan, S. M. & Siu, L. L. Circulating tumor DNA and liquid biopsy in oncology. Nat. Cancer 1, 276–290 (2020). This review discusses the current landscape of ctDNA liquid-biopsy applications across cancer and highlights opportunities for clinical investigation.
Li, J. W., Cao, S. H., Xu, J. L. & Zhong, H. De novo MET amplification promotes intrinsic resistance to first-generation EGFR tyrosine kinase inhibitors. Cancer Biol. Ther. 20, 1183–1186 (2019).
Yu, H. A. et al. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann. Oncol. 25, 423–428 (2014).
Wander, S. A. et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 10, 1174–1193 (2020).
Guo, R. et al. MET inhibitor resistance in patients with MET exon 14-altered lung cancers. J. Clin. Oncol. 37, 9006–9006 (2019).
Lovly, C. M. & Shaw, A. T. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clin. Cancer Res. 20, 2249–2256 (2014).
Robey, R. W. et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 18, 452–464 (2018).
Eyers, P. A., Craxton, M., Morrice, N., Cohen, P. & Goedert, M. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem. Biol. 5, 321–328 (1998).
Clark, K. et al. Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proc. Natl Acad. Sci. USA 109, 16986–16991 (2012).
Gorre, M. E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293, 876–880 (2001). One of the first examples of clinical drug resistance to a kinase inhibitor mediated by a gatekeeper mutation.
Nguyen, K. S., Kobayashi, S. & Costa, D. B. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin. Lung Cancer 10, 281–289 (2009).
Yun, C. H. et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl Acad. Sci. USA 105, 2070–2075 (2008).
Drilon, A. et al. Repotrectinib (TPX-0005) Is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov. 8, 1227–1236 (2018).
Solomon, B. J. et al. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J. Thorac. Oncol. 15, 541–549 (2020).
Cocco, E. et al. TRK xDFG mutations trigger a sensitivity switch from type I to II kinase inhibitors. Cancer Discov. 11, 126–141 (2021).
Volm, M. & Efferth, T. Prediction of cancer drug resistance and implications for personalized medicine. Front. Oncol. 5, 282 (2015).
Husain, H. et al. Strategies to overcome bypass mechanisms mediating clinical resistance to EGFR tyrosine kinase inhibition in lung cancer. Mol. Cancer Ther. 16, 265–272 (2017).
Lin, J. J. et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann. Oncol. 31, 1725–1733 (2020).
Lavaud, P. & Andre, F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med. 12, 132 (2014).
D’Amato, V. et al. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat. Rev. 41, 877–883 (2015).
Yang, C. et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36, 2255–2264 (2017).
Herrera-Abreu, M. T. et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 76, 2301–2313 (2016).
Min, A. et al. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 430, 123–132 (2018).
Pandey, K. et al. Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers 12, 3566 (2020).
Bordi, P. et al. Detection of ALK and KRAS mutations in circulating tumor DNA of patients with advanced ALK-positive NSCLC with disease progression during crizotinib treatment. Clin. Lung Cancer 18, 692–697 (2017).
Suzawa, K. et al. Activation of KRAS mediates resistance to _targeted therapy in MET exon 14-mutant non-small cell lung cancer. Clin. Cancer Res. 25, 1248–1260 (2019).
Ohashi, K. et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc. Natl Acad. Sci. USA 109, E2127–E2133 (2012).
de Bruin, E. C. et al. Reduced NF1 expression confers resistance to EGFR inhibition in lung cancer. Cancer Discov. 4, 606–619 (2014).
Jamme, P. et al. Alterations in the PI3K pathway drive resistance to MET inhibitors in NSCLC harboring MET exon 14 skipping mutations. J. Thorac. Oncol. 15, 741–751 (2020).
Costa, C. et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov. 10, 72–85 (2020).
Juric, D. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518, 240–244 (2015).
Sos, M. L. et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 69, 3256–3261 (2009).
Solca, F. et al. _target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J. Pharmacol. Exp. Ther. 343, 342–350 (2012).
Engelman, J. A. et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 67, 11924–11932 (2007).
Cross, D. A. E. et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR Inhibitors in lung cancer. Cancer Discov. 4, 1046–1061 (2014).
Kim, E. S. Olmutinib: first global approval. Drugs 76, 1153–1157 (2016).
Mok, T. S. et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung Ccancer. N. Engl. J. Med. 376, 629–640 (2017).
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50 (2019).
Leonetti, A. et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 121, 725–737 (2019).
Thress, K. S. et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat. Med. 21, 560–562 (2015).
Katayama, R. et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci. Transl Med. 4, 120ra117 (2012).
Crinò, L. et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J. Clin. Oncol. 34, 2866–2873 (2016).
Shaw, A. T. et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 17, 234–242 (2016).
Gettinger, S. N. et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 17, 1683–1696 (2016).
Gainor, J. F. et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 6, 1118–1133 (2016).
Yoda, S. et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 8, 714–729 (2018).
Recondo, G. et al. Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin. Cancer Res. 26, 242–255 (2020).
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002). The comprehensive classification and annotation of the 500 plus protein kinases (the human kinome) and its subdivision into subfamilies, which has greatly facilitated the understanding of kinome relationships.
Morgan Jones, M. et al. The Structural Genomics Consortium: A Knowledge Platform for Drug Discovery (RAND Corporation, 2014).
Murray, C. W. & Blundell, T. L. Structural biology in fragment-based drug design. Curr. Opin. Struct. Biol. 20, 497–507 (2010).
Thomas, S. E. et al. Structure-guided fragment-based drug discovery at the synchrotron: screening binding sites and correlations with hotspot mapping. Phil. Trans. R. Soc. A 377, 20180422 (2019).
Abdeldayem, A., Raouf, Y. S., Constantinescu, S. N., Moriggl, R. & Gunning, P. T. Advances in covalent kinase inhibitors. Chem. Soc. Rev. 49, 2617–2687 (2020).
Liu, Q. et al. Developing irreversible inhibitors of the protein kinase cysteinome. Chem. Biol. 20, 146–159 (2013). This article and Abdeldayem et al. (2020) review advances in developing kinase inhibitors that bind covalently to conserved cysteine residues located near the catalytic site, and contributed to the increasing numbers of these inhibitors that are being developed and approved for clinical use.
Arighi, E., Borrello, M. G. & Sariola, H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor. Rev. 16, 441–467 (2005).
Gautschi, O. et al. _targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J. Clin. Oncol. 35, 1403–1410 (2017).
Drilon, A. et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N. Engl. J. Med. 383, 813–824 (2020).
Subbiah, V. et al. Precision _targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 8, 836–849 (2018).
Kollareddy, M. et al. Aurora kinase inhibitors: progress towards the clinic. Invest. New Drugs 30, 2411–2432 (2012).
Komlodi-Pasztor, E., Sackett, D. L. & Fojo, A. T. Inhibitors _targeting mitosis: tales of how great drugs against a promising _target were brought down by a flawed rationale. Clin. Cancer Res. 18, 51–63 (2012).
Sherr, C. J. & Bartek, J. Cell cycle–_targeted cancer therapies. Annu. Rev. Cancer Biol. 1, 41–57 (2017).
Ashton, S. et al. Aurora kinase inhibitor nanoparticles _target tumors with favorable therapeutic index in vivo. Sci. Transl Med. 8, 325ra317 (2016).
Heffron, T. P. Challenges of developing small-molecule kinase inhibitors for brain tumors and the need for emphasis on free drug levels. Neuro-Oncol. 20, 307–312 (2017).
Skerratt, S. E. & Storer, R. I. in Kinase Drug Discovery: Modern Approaches (eds Ward, R. A. & Goldberg, F. W.) 128–180 (Royal Society of Chemistry, 2019). This article and Heffron (2017) discuss the unmet need for kinase inhibitors to achieve free brain penetration, and the chemistry design approaches and challenges to achieve inhibitors with appropriate properties.
Johnson, T. W. et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J. Med. Chem. 57, 4720–4744 (2014).
Zeng, Q. et al. Discovery and evaluation of clinical candidate AZD3759, a potent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor. J. Med. Chem. 58, 8200–8215 (2015).
Shibuya, M. & Suzuki, Y. Treatment of cerebral vasospasm by a protein kinase inhibitor AT 877 [Japanese]. No Shinkei 45, 819–824 (1993).
Doggrell, S. A. Rho-kinase inhibitors show promise in pulmonary hypertension. Expert Opin. Investig. Drugs 14, 1157–1159 (2005).
Nagumo, H. et al. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am. J. Physiol. Cell Physiol. 278, C57–65 (2000).
Heitman, J., Movva, N. R. & Hall, M. N. _targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253, 905–909 (1991).
Cohen, P. _targeting protein kinases for the development of anti-inflammatory drugs. Curr. Opin. Cell Biol. 21, 317–324 (2009).
Zarrin, A. A., Bao, K., Lupardus, P. & Vucic, D. Kinase inhibition in autoimmunity and inflammation. Nat. Rev. Drug Discov. 20, 39–63 (2021).
Shuai, K. & Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3, 900–911 (2003).
Fragoulis, G. E., McInnes, I. B. & Siebert, S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology 58, i43–i54 (2019). Recent review of the increasing number of JAK inhibitors being developed for the treatment of rheumatoid arthritis and other immune diseases.
A, T. V., Haikarainen, T., Raivola, J. & Silvennoinen, O. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs 33, 15–32 (2019).
Baxter, E. J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
Kralovics, R. et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352, 1779–1790 (2005).
Kuykendall, A. T. et al. Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann. Hematol. 97, 435–441 (2018).
Talpaz, M., Erickson-Viitanen, S., Hou, K., Hamburg, S. & Baer, M. R. Evaluation of an alternative ruxolitinib dosing regimen in patients with myelofibrosis: an open-label phase 2 study. J. Hematol. Oncol. 11, 101 (2018).
Kim, T. W. et al. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J. Exp. Med. 204, 1025–1036 (2007).
Nanda, S. K. et al. Distinct signals and immune cells drive liver pathology and glomerulonephritis in ABIN1[D485N] mice. Life Sci. Alliance 2, e201900533 (2019).
Seganish, W. M. Inhibitors of interleukin-1 receptor-associated kinase 4 (IRAK4): a patent review (2012–2015). Expert. Opin. Ther. Pat. 26, 917–932 (2016).
Picard, C., Casanova, J. L. & Puel, A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IκBα deficiency. Clin. Microbiol. Rev. 24, 490–497 (2011).
Picard, C. et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine 89, 403–425 (2010). Review of the remarkable long-term clinical study of IRAK4-deficient patients, which revealed why IRAK4 inhibition is unlikely to cause life-threatening microbial infections in adults, and de-risked this drug _target.
Danto, S. I. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of PF-06650833, a selective interleukin-1 receptor-associated kinase 4 (IRAK4) inhibitor, in single and multiple ascending dose randomized phase 1 studies in healthy subjects. Arthritis Res. Ther. 21, 269 (2019).
Wiese, M. D., Manning-Bennett, A. T. & Abuhelwa, A. Y. Investigational IRAK-4 inhibitors for the treatment of rheumatoid arthritis. Expert Opin. Investig. Drugs 29, 475–482 (2020).
Darling, N. J., Toth, R., Arthur, J. S. & Clark, K. Inhibition of SIK2 and SIK3 during differentiation enhances the anti-inflammatory phenotype of macrophages. Biochem. J. 474, 521–537 (2017).
Galapagos. Galapagos’ R&D Roundtable showcases Toledo program. GlobeNewswire https://www.globenewswire.com/news-release/2020/10/27/2115341/0/en/Galapagos-R-D-Roundtable-showcases-Toledo-program.html (2020).
Mujahid, N. et al. A UV-independent topical small-molecule approach for melanin production in human skin. Cell Rep. 19, 2177–2184 (2017).
Wein, M. N. et al. SIKs control osteocyte responses to parathyroid hormone. Nat. Commun. 7, 13176 (2016).
Kim, M. K. et al. Salt-inducible kinase 1 regulates bone anabolism via the CRTC1-CREB-Id1 axis. Cell Death Dis. 10, 826 (2019).
Lee, J. C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994).
Cuenda, A. et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364, 229–233 (1995).
Eyers, P. A., van den, I. P., Quinlan, R. A., Goedert, M. & Cohen, P. Use of a drug-resistant mutant of stress-activated protein kinase 2a/p38 to validate the in vivo specificity of SB 203580. FEBS Lett. 451, 191–196 (1999).
Hammaker, D. & Firestein, G. S. “Go upstream, young man”: lessons learned from the p38 saga. Ann. Rheum. Dis. 69, i77–i82 (2010).
Alam, J. J. Selective brain-_targeted antagonism of p38 MAPKα reduces hippocampal IL-1β levels and improves Morris water maze performance in aged rats. J. Alzheimers Dis. 48, 219–227 (2015).
Alam, J., Blackburn, K. & Patrick, D. Neflamapimod: clinical phase 2b-ready oral small molecule inhibitor of p38α to reverse synaptic dysfunction in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 4, 273–278 (2017).
Fagiani, F., Lanni, C., Racchi, M. & Govoni, S. _targeting dementias through cancer kinases inhibition. Alzheimers Dement. 6, e12044 (2020).
Cohen, P. & Goedert, M. GSK3 inhibitors: development and therapeutic potential. Nat. Rev. Drug Discov. 3, 479–487 (2004).
Martinez, A., Alonso, M., Castro, A., Pérez, C. & Moreno, F. J. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J. Med. Chem. 45, 1292–1299 (2002).
Serenó, L. et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis. 35, 359–367 (2009).
Paisán-Ruiz, C., Lewis, P. A. & Singleton, A. B. LRRK2: cause, risk, and mechanism. J. Parkinsons Dis. 3, 85–103 (2013).
Alessi, D. R. & Sammler, E. LRRK2 kinase in Parkinson’s disease. Science 360, 36–37 (2018).
Di Maio, R. et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl Med. 10, eaar5429 (2018).
Wong, M. M. K. et al. Neurodegeneration in SCA14 is associated with increased PKCγ kinase activity, mislocalization and aggregation. Acta Neuropathol. Commun. 6, 99 (2018).
Mabillard, H. & Sayer, J. A. The molecular genetics of Gordon syndrome. Genes 10, 986 (2019).
Di Maira, G. et al. The protein kinase CK2 contributes to the malignant phenotype of cholangiocarcinoma cells. Oncogenesis 8, 61 (2019).
D’Amore, C., Borgo, C., Sarno, S. & Salvi, M. Role of CK2 inhibitor CX-4945 in anti-cancer combination therapy - potential clinical relevance. Cell. Oncol. 43, 1003–1016 (2020).
Boumahdi, S. & de Sauvage, F. J. The great escape: tumour cell plasticity in resistance to _targeted therapy. Nat. Rev. Drug Discov. 19, 39–56 (2020). Interesting review of the emerging concept of drug-tolerant cells (also referred to as minimal residual disease) that can drive a drug-refractory phenotypic state that no longer depends on the drug-_targeted pathway.
Oikkonen, J. et al. Prospective longitudinal ctDNA workflow reveals clinically actionable alterations in ovarian cancer. JCO Precis. Oncol. 3, 1–12 (2019).
Jiang, J. et al. Plasma-based longitudinal mutation monitoring as a potential predictor of disease progression in subjects with adenocarcinoma in advanced non-small cell lung cancer. BMC Cancer 20, 885 (2020).
Msaouel, P., Genovese, G., Gao, J., Sen, S. & Tannir, N. M. TAM kinase inhibition and immune checkpoint blockade- a winning combination in cancer treatment? Expert Opin. Ther. _targets 25, 141–151 (2021).
Xun, Q., Wang, Z., Hu, X., Ding, K. & Lu, X. Small-molecule CSF1R inhibitors as anticancer agents. Curr. Med. Chem. 27, 3944–3966 (2020).
Moen, M. D., McKeage, K., Plosker, G. L. & Siddiqui, M. A. A. Imatinib. Drugs 67, 299–320 (2007).
Joensuu, H. et al. Adjuvant imatinib for high-risk GI stromal tumor: analysis of a randomized trial. J. Clin. Oncol. 34, 244–250 (2016).
Wu, Y. L. et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 383, 1711–1723 (2020). First approval of a kinase inhibitor for reducing risk of recurrence in early-stage resectable NSCLC.
Abbosh, C., Birkbak, N. J. & Swanton, C. Early stage NSCLC - challenges to implementing ctDNA-based screening and MRD detection. Nat. Rev. Clin. Oncol. 15, 577–586 (2018). Overview of the technical challenges and feasibility of low-frequency mutation detection using NGS-based ctDNA profiling to deliver the potential paradigm shifting approach needed for early disease and minimal residual disease detection.
Whyte, J. L., Smith, A. A. & Helms, J. A. Wnt signaling and injury repair. Cold Spring Harb. Perspect. Biol. 4, a008078 (2012).
Lu, X., Yang, J., Zhao, S. & Liu, S. Advances of Wnt signalling pathway in dental development and potential clinical application. Organogenesis 15, 101–110 (2019).
Neves, V. C., Babb, R., Chandrasekaran, D. & Sharpe, P. T. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci. Rep. 7, 39654 (2017). An interesting and unexpected example of the repurposing of a protein kinase inhibitor for a completely different clinical application.
Cross, D. A., Alessi, D. R., Cohen, P., Andjelkovich, M. & Hemmings, B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995).
Hudis, C. A. Trastuzumab–mechanism of action and use in clinical practice. N. Engl. J. Med. 357, 39–51 (2007).
Garcia, J. et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 86, 102017 (2020).
Yun, J. et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR–MET bispecific antibody, in diverse models of EGFR exon 20 insertion–driven NSCLC. Cancer Discov. 10, 1194–1209 (2020).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Hashimoto, Y. et al. A novel HER3-_targeting antibody-drug conjugate, U3-1402, exhibits potent therapeutic efficacy through the delivery of cytotoxic payload by efficient internalization. Clin. Cancer Res. 25, 7151–7161 (2019).
Zeng, S. et al. Proteolysis _targeting chimera (PROTAC) in drug discovery paradigm: recent progress and future challenges. Eur. J. Med. Chem. 210, 112981 (2020).
Henning, R. K. et al. Degradation of Akt using protein-catalyzed capture agents. J. Pept. Sci. 22, 196–200 (2016).
Tovell, H. et al. Design and characterization of SGK3-PROTAC1, an isoform specific SGK3 kinase PROTAC degrader. ACS Chem. Biol. 14, 2024–2034 (2019).
Burslem, G. M. et al. _targeting BCR-ABL1 in chronic myeloid leukemia by PROTAC-mediated _targeted protein degradation. Cancer Res. 79, 4744–4753 (2019).
Lu, X., Smaill, J. B. & Ding, K. New promise and opportunities for allosteric kinase inhibitors. Angew. Chem. Int. Ed. 59, 13764–13776 (2020).
Wylie, A. A. et al. The allosteric inhibitor ABL001 enables dual _targeting of BCR-ABL1. Nature 543, 733–737 (2017).
Hughes, T. P. et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N. Engl. J. Med. 381, 2315–2326 (2019). The first clinical data demonstrating efficacy of an allosteric ABL inhibitor in patients with CML who had failed prior ATP-competitive ABL inhibitors.
To, C. et al. Single and dual _targeting of mutant EGFR with an allosteric inhibitor. Cancer Discov. 9, 926–943 (2019).
Eide, C. A. et al. Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell 36, 431–443.e435 (2019).
Acknowledgements
The authors thank R. Ward at AstraZeneca for valuable input, including help in generating Figs. 4 and 5, and R. Marais for valuable discussions.
Author information
Authors and Affiliations
Contributions
P.C., D.C. and P.J. researched, wrote and edited this Review.
Corresponding authors
Ethics declarations
Competing interests
P.C. has shares in Alliance Pharma, AstraZeneca and GlaxoSmithKline and is a member of the Scientific Advisory Boards of Mission Therapeutics, Ubiquigent and Biocatalyst International. D.C. is an employee and shareholder of AstraZeneca. P.A.J. has received consulting fees from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche/Genentech, Takeda Oncology, ACEA Biosciences, Eli Lilly and Company, Araxes Pharma, Ignyta, Mirati Therapeutics, Novartis, LOXO Oncology, Daiichi Sankyo, Sanofi Oncology, Voronoi, SFJ Pharmaceuticals, Biocartis, Novartis Oncology, Nuvalent, Esai, Bayer, Transcenta and Silicon Therapeutics; receives post-marketing royalties from DFCI-owned intellectual property on EGFR mutations licensed to Lab Corp; has sponsored research agreements with AstraZeneca, Daichi-Sankyo, PUMA, Boehringer-Ingelheim, Eli Lilly and Company, Revolution Medicines, and Astellas Pharmaceuticals; and has stock ownership in Gatekeeper Pharmaceuticals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Protein kinases
-
Enzymes that catalyse transfer of the γ-phosphate of ATP to amino acid side chains in substrate proteins, such as serine, threonine and tyrosine residues.
- Polypharmacology
-
A term to describe a feature of kinase inhibitors that can inhibit multiple kinase _targets at clinically achievable concentrations. This feature can lead to a broader range of clinical activity and/or increased toxicity.
- Next-generation inhibitor
-
A kinase inhibitor that is improved sufficiently compared with the original first-generation drug that it is effective in preventing disease progression in patients who are no longer responsive to the original drug.
- Bromodomain
-
(BRD). A particular type of protein domain comprising approximately 110 amino acids that recognizes acetylated lysine residues in other proteins, frequently leading to the remodelling of chromatin and changes in gene transcription. Many drugs initially developed as protein kinase inhibitors also bind to and inhibit the functions of BRDs.
- Pseudokinase
-
Pseudokinases are proteins that possess a domain that closely resembles the catalytic domains of protein kinases, but that lack one or more amino acid residues essential for catalysis by other kinases, and are therefore presumed to be catalytically inactive. Some pseudokinases have subsequently been found to display kinase catalytic activity for interesting reasons or to have acquired entirely novel catalytic functions.
- Oncogenic addiction
-
A process in which cancers with genetic, epigenetic or chromosomal irregularities become dependent on one or a few genes for maintenance and survival. Such cancers can be exquisitely sensitive to killing by particular kinase inhibitors if one of the genes required for cancer survival encodes a particular protein kinase or a regulator of kinase activation.
- Blood–brain barrier
-
(BBB). A highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the extracellular fluid of the central nervous system.
- Acquired drug resistance
-
Clinical drug resistance, characterized by tumour growth while on treatment, that develops in patients following an initial clinical benefit (a clinical response or prolonged stable disease).
- Gatekeeper mutation
-
The gatekeeper is a conserved amino acid residue near the ATP-binding site that, when occupied by an amino acid with a small side chain (typically threonine), creates a small hydrophobic pocket that is _targeted by many protein kinase inhibitors. The mutation of the gatekeeper to an amino acid with a bulkier side chain fills the hydrophobic pocket, making it inaccessible to the kinase inhibitor, but not ATP. Such mutations are a frequent cause of drug resistance.
- Solvent-front mutations
-
The solvent front is a region of the ATP pocket in the catalytic domain that has a relatively high solvent exposure, and where multiple kinase inhibitors typically make contact. Mutation of residues in this region frequently cause resistance to kinase inhibitors.
- Kinase profiling
-
A technology for assessing which of the 500 plus protein kinases encoded by the human genome is inhibited by a particular kinase inhibitor. Some technologies used for kinase profiling can identify _targets of such drugs that are not protein kinases.
- Covalent inhibitors
-
Kinase inhibitors that bind irreversibly to their _targets, most commonly by making a covalent chemical bond with a cysteine residue of the kinase.
Rights and permissions
About this article
Cite this article
Cohen, P., Cross, D. & Jänne, P.A. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discov 20, 551–569 (2021). https://doi.org/10.1038/s41573-021-00195-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-021-00195-4
This article is cited by
-
Application of nanotechnology in the treatment of glomerulonephritis: current status and future perspectives
Journal of Nanobiotechnology (2024)
-
DBPP-Predictor: a novel strategy for prediction of chemical drug-likeness based on property profiles
Journal of Cheminformatics (2024)
-
Potentials and future perspectives of multi-_target drugs in cancer treatment: the next generation anti-cancer agents
Cell Communication and Signaling (2024)
-
CircHAS2 activates CCNE2 to promote cell proliferation and sensitizes the response of colorectal cancer to anlotinib
Molecular Cancer (2024)
-
A first-in-class selective inhibitor of EGFR and PI3K offers a single-molecule approach to _targeting adaptive resistance
Nature Cancer (2024)