Abstract
Bariatric surgery is known to reduce leptin and increase adiponectin levels, but the influence of sleeve gastrectomy on the leptin: adiponectin ratio (LAR), a measure of insulin sensitivity and cardiovascular risk, has not previously been described. We sought to determine the influence of sleeve gastrectomy on LAR in adults with severe obesity.In a single centre prospective cohort study of adults undergoing laparoscopic sleeve gastrectomy over a four-month period in our unit, we measured LAR preoperatively and 12 months after surgery. Of 22 patients undergoing sleeve gastrectomy, 17 (12 females, 12 with type 2 diabetes) had follow-up LAR measured at 12.1 ± 1 months. Mean body weight decreased from 130.6 ± 30.8 kg to 97.6 ± 21.6 kg, body mass index (BMI) from 46.9 ± 7.8 to 35.3 ± 7.2 kg m−2 and excess body weight from 87.5 ± 31.3 to 41.3 ± 28.8% (all p < 0.001). The reduction in leptin from 40.7 ± 24.9 to 30.9 ± 30.5 ng/ml was not significant (p = 0.11), but adiponectin increased from 4.49 ± 1.6 to 8.93 ± 6.36 µg/ml (p = 0.005) and LAR decreased from 8.89 ± 4.8 to 5.26 ± 6.52 ng/µg (p = 0.001), equivalent to a 70.9% increase in insulin sensitivity. The correlation with the amount of weight lost was stronger for LAR than it was for leptin or adiponectin alone. In this single-centre, interventional prospective cohort, patients undergoing laparoscopic sleeve gastrectomy had a substantial reduction in their LAR after 12 months which was proportional to the amount of weight lost. This may indicate an improvement in insulin sensitivity and a reduction in cardiovascular risk.
Similar content being viewed by others
Introduction
Obesity is a major determinant of type 2 diabetes risk, primarily through its adverse effects on insulin sensitivity1. Bariatric surgery to treat severe obesity leads to significant improvements in diabetes control2, cardiovascular risk3,4, microalbuminuria5 and systemic inflammation6. However, the influence of bariatric surgery on insulin sensitivity is not well defined7,8, partly because quantifying insulin sensitivity can be challenging. For example, the hyperinsulinaemic-euglycaemic clamp allows precise measurement of insulin sensitivity in muscle and liver9 but is a technically elaborate and expensive technique that is only available in specialized research units. The leptin: adiponectin ratio (LAR) is a measure of whole body insulin sensitivity that has been validated in large population-based studies10,11. Both of these hormones are adipokines, produced by adipocytes and they influence metabolic homeostasis. Leptin works through receptors in the hypothalamus to regulate dietary intake as well as energy expenditure12 and has been shown to be elevated in individuals with obesity13. Conversely, adiponectin reduces circulating free fatty acids by increasing tissue fat oxidation: Adiponectin levels tend to be lower in individuals with obesity14. Several studies have described reductions in leptin and increases in adiponectin in adults with severe obesity undergoing bariatric surgery3,6,7,8,15. Recently, investigators described changes in the adiponectin: leptin ratio (ALR) in a cohort of 25 Spanish adults with type 2 diabetes who underwent Roux-en-Y gastric bypass16. To date, the influence of sleeve gastrectomy on the ratio of these two hormones has not been described. We sought to quantify the effects of sleeve gastrectomy on leptin, adiponectin and LAR in adults with severe obesity, and to assess whether the magnitude of weight loss after surgery influenced the change in LAR.
Methods
We conducted a single-centre, interventional prospective cohort study of all patients undergoing laparoscopic sleeve gastrectomy at our hospital over four months between September and December 2016. We performed follow-up measures 12.1 ± 1 months after surgery. All study participants provided written informed consent and the study was approved by the Galway University Hospital’s Research Ethics Committee (reference C.A. 2058). The study was conducted adhering to the STROBE (Strengthening the Reporting of OBservational studies in Epidemiology) guidelines17.
Inclusion criteria for bariatric surgery at our institution are consistent with those internationally. We define severe obesity as a body mass index (BMI) ≥ 40 kgm−2 (or ≥ 35 kgm−2 with co-morbidities such as type 2 diabetes and obstructive sleep apnea syndrome). Male and female patients aged 18 years or older, put forward for consideration for sleeve gastrectomy by the bariatric multidisciplinary team at our institution (nurse, dietitian, physician, psychologist, surgeon) were all eligible for inclusion. Our clinical practice is to refer these patients for surgical consideration after completion of a ten-week structured lifestyle modification programme that we have described in detail previously18. Patients must have undertaken a formal psychological assessment of the suitability of sleeve gastrectomy for their treatment. Those with a recent myocardial infarction (within six months), untreated arrhythmia, untreated left ventricular failure, recent cholelithiasis (within the past year), type 1 diabetes, untreated major psychiatric disorders, eating disorders, undergoing cancer treatment, or a BMI < 35 kg m−2 or those deemed unlikely to attend for post-operative follow-up (e.g. frequent clinic non-attendance) were excluded from undergoing sleeve gastrectomy. The study population was a convenience sample and its size was determined by the number of sleeve gastrectomies done during the study period. All metabolic and anthropometric baseline and follow-up measures were conducted at our bariatric out-patient clinic.
Weight was measured on a Tanita® scale and height with a Seca® wall-mounted stadiometer. Blood pressure was measured with an automated oscillometric device (Omron®) using a large cuff on the right arm, after participants had been seated quietly for five minutes. Three measures were recorded at one-minute intervals and the average of the three was recorded. Blood samples were drawn from patients in the fasted state on the morning of their sleeve gastrectomy. Glycosylated haemoglobin (HbA1c) was measured using High Performance Liquid Chromatography (HPLC) on the Menarini® HA8160 analyser. Leptin and adiponectin were measured using separate two-site micro titre plate-based DELFIA assays manufactured by R&D Systems Europe, Abingdon UK. The adiponectin assay measured “total” adiponectin and our “in-house” analyses have found a between batch imprecision of 5.4% at 3.6 µg/ml, 5.2% at 9.2 µg/ml and 5.8% at 15.5 µg/ml, as previously described19. For leptin, the between batch imprecision was 7.1% at 2.7 ng/ml, 3.9% at 14.9 ng/ml and 5.7% at 54.9 ng/ml.
All statistical analyses were conducted with SPSS® version 24. Changes in anthropometric and metabolic variables between baseline and follow-up were assessed using the student’s paired t-test, assuming equal variances. The relationship between the degree of weight loss over 12 months and changes in LAR was determined using Pearson correlation and linear regression modelling.
Human and animal rights
This study was conducted according to the principles established in the Declaration of Helsinki and described by the International Conference for the Harmonisation of Good Clinical Practice.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Results
Of 148 patients with severe obesity who were on the waiting list for sleeve gastrectomy at the time the study started, 22 were put forward for surgery (based on clinical prioritisation and length of time waiting, under a short-term, government-funded “waiting list initiative”). All of these patients were invited to the study and all agreed to participate. However, one patient subsequently died six months after sleeve gastrectomy, from an aggressive oesophageal carcinoma with liver metastases which first presented three months after his sleeve gastrectomy. This was despite the patient having a normal barium swallow and ultrasound before his bariatric surgery. Four patients attended for bariatric follow-up at other institutions and were unable to attend within our specified window of 10–14 months post-surgery for follow-up measures. Thus, we report on 17 patients for whom baseline and 12-month follow-up measures were available. 12 patients were female and 12 had type 2 diabetes at baseline. The mean duration of diabetes was 9.9 (range 3–27) years. Mean age was 52.2 ± 8.3 (range 39–71) years, with a follow- up interval of 12.1 ± 1 (range 10–13) months. Baseline and follow-up anthropometric and metabolic characteristics are presented in Table 1. There were substantial reductions in weight, BMI and excess body weight over 12.1 months, as shown, with a mean weight loss of 33.0 ± 21.6 kg and an absolute reduction in excess body weight percentage (EBW%) of 47.2 ± 28.8% (all p < 0.001), equivalent to a percentage total weight loss of 24.3 ± 12%.
There was a non-significant trend to reduced HbA1c overall after 12 months, which was more pronounced (though remained non-significant) in the subgroup of patients with diabetes. Two patients were taking insulin preoperatively and both remained on insulin 12 months later. Of two patients taking sodium glucose linked transported 2 (SGLT2) inhibitor therapy preoperatively, one remained on it and a second had stopped it. A third patient had started the drug by the time of their follow-up. Of 12 patients taking metformin at baseline, three had stopped this by 12 months. Of five patients taking glucagon like peptide 1 (GLP1) agonist therapy at baseline, one remained on this at follow-up. All three patients on gliptin therapy for diabetes had this stopped at the time of their surgery, while no patients were taking sulphonylureas or glitazones at baseline or follow-up. Ten patients were taking statin therapy at baseline and this was continued in all patients routinely post-operatively. There was a reduction in the prevalence of patients taking antihypertensive therapy from 71 to 29% over 12 months.
Overall, there was a (non-significant) trend to reduced leptin levels, while there was a more pronounced and statistically significant increase in adiponectin. This equated to an overall reduction in the LAR which was consistent with a 70.9% increase in insulin sensitivity in the cohort 12 months after sleeve gastrectomy. When examining changes in the subgroup of patients with diabetes, results were similar to the group overall, as shown.
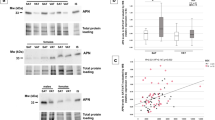
Next, we sought to determine the correlation between the percentage weight lost and changes in leptin, adiponectin and the LAR. As outlined in Fig. 1a–c, there was a modest correlation only between the percentage weight lost with leptin and with adiponectin individually, such that the percentage weight loss accounted for approximately 39.5% of the reduction in leptin (r2 = 0.3948, p = 0.007) and 49.6% of the increase in adiponectin (r2 = 0.4961, p = 0.002), respectively. However, there was a much stronger correlation seen for the LAR, such that approximately 82.2% of the reduction in LAR was accounted for by the percentage weight lost (r2 = 0.8222, p < 0.001).
Discussion
We have shown that in a predominantly white cohort of adults with severe obesity who underwent laparoscopic sleeve gastrectomy, there was a substantial reduction in the LAR after 12 months, which may indicate an increase in insulin sensitivity. While several studies have measured leptin or adiponectin separately in patients after bariatric surgery3,6,7,8,15, we are aware of only one other study by Unamuno et al. that has considered them together as a ratio and as an indicator of adipocyte dysfunction16. Ours is the first study to consider these adipokines as a ratio in patients undergoing laparoscopic sleeve gastrectomy. We found that the reductions in LAR were driven primarily by an increase in adiponectin rather than a reduction in leptin, and these changes were proportional to the reduction in weight after surgery, consistent with the Unamuno study. The changes we observed in leptin and adiponectin concentrations were less than those described after bypass bariatric surgery3,6,7 and are more consistent with those previously described after banding8.
We did not see a statistically significant reduction in leptin, but we think this is due to inadequate power, given that a recent randomised controlled trial confirmed that leptin decreases after sleeve gastrectomy20. As the primary effect of leptin is to supress appetite and defend against weight gain via hypothalamic signalling, it might seem counterintuitive to find a reduction rather than an increase after sleeve gastrectomy. However this phenomenon is well described and may be due to ‘leptin resistance’, whereby individuals with obesity demonstrate paradoxically high levels of circulating leptin but diminished leptin sensitivity21. Previous studies have suggested that decreased leptin levels after bariatric surgery do not attenuate weight loss, because of compensatory increased leptin sensitivity in the hypothalamus22. The mechanisms by which leptin sensitivity might be restored remain to be determined, but other adipokines such as fibroblast growth factor-21 (FGF-21) may mediate this effect via direct signalling in the central nervous system or through augmenting the secretion of adiponectin23. Another consideration is the interaction between leptin and adiponectin. For example, in normal-weight individuals, leptin enhances adiponectin secretion, but this effect is lost in patients with obesity through the action of caveolin-1, which attenuates leptin-dependant increases in adiponectin24.
Increased circulating levels of adiponectin are not always indicative of improvements in health or reductions in cardiovascular risk: the so-called “adiponectin paradox” refers to the observation that while adiponectin mediates a variety of essentially beneficial effects and levels of adiponectin correlate positively with better long term cardiovascular outcomes in young healthy populations they also correlate with increased risk of premature death in high-risk populations, especially older patients or those with ischemic heart disease, heart failure or renal failure25.
Of note, the mean HbA1c was low at baseline in our patients with diabetes. This is likely due to relatively short-term improvements in glycaemic control that occur with our pre-operative “high protein diet” that we use in order to reduce the size of the liver. Data regarding HbA1c in the period preceding surgery are not to hand. However, we don’t think that this would affect our findings as it is likely that the pre-operative diet would have led to increased insulin sensitivity, reflected in a reduced LAR at baseline, thus if anything attenuating our ability to detect a change in LAR after surgery. Future studies could incorporate measurement of HbA1c, leptin, adiponectin and LAR just before the pre-operative high protein diet commences, in order to determine the extent to which these variables change before surgery.
A key limitation of our study is its modest size (reflected in the borderline statistical significance of the reduction in LAR, for example), but the effects of bariatric surgery on metabolic outcomes and cardiovascular disease risk tend to be so profound that even in randomised controlled trials, surgical interventions require population sizes2 that are orders of magnitude smaller than those in drug26 or lifestyle intervention trials27. Our cohort size is similar to other surgical studies3,6,7,8,15 and our findings around the correlation between weight loss and reduced LAR are statistically robust. Rather than using LAR as the only index of insulin sensitivity, future studies could also examine indices related to fasting glucose and insulin (such as the homeostasis model assessment, HOMA28), in order to strengthen causal inference on the impact of bariatric interventions on insulin sensitivity.
There were changes in diabetes medication usage within the cohort that are unavoidable in an observational study such as this, which might have influenced our results. For example, metformin is known to have a weak positive effect on insulin sensitivity29 and potentially a lowering effect on circulating levels of leptin30, so it is possible that the cessation of metformin diminished the reduction in the LAR observed in our patients. Also, GLP agonist therapy has previously been reported to attenuate the reduction in circulating levels of leptin after weight loss31, so the cessation of GLP agonist therapy in four of our patients may have diminished the reduction in leptin that we observed. Similar considerations apply to the three patients who stopped gliptin medications, as these drugs decrease leptin and increase adiponectin32. SGLT2 inhibitors decrease leptin and increase adiponectin33, so we do not think that initiation of this treatment in one patient and its cessation in another is likely to have led to the observed difference in LAR after the programme. Angiotensin converting enzyme (ACE) inhibitors increase adiponectin34, so we think their cessation would have attenuated rather than enhanced the observed reduction in LAR. Thus, we would expect that the overall reductions in the usage of these drugs would have led to attenuation, rather than exaggeration of the measured differences in adipokines and LAR that we observed.
Ultimately, this work could inform definitive interventions and aetiological trials in larger populations of patients to overcome these limitations and determine the efficacy of sleeve gastrectomy and other interventions to reduce insulin resistance. Whether LAR is a good way to identify “responders” to surgery remains to be determined in large, prospective studies.
Conclusions
This single-centre, interventional prospective cohort study of adults with severe and complicated obesity undergoing laparoscopic sleeve gastrectomy found that after twelve months there was a substantial reduction in the leptin: adiponectin ratio and that this reduction was proportional to the amount of weight lost after surgery. Given the heterogenous nature and small size of the study population, the findings must be regarded as preliminary. Nonetheless they suggest a change in adipocyte function consistent with improved insulin sensitivity. Further studies in larger, more specifically defined patient subgroups would help to further elucidate the relevance of adipokine measurement in these patients and the mechanistic basis for metabolic improvements after bariatric surgery.
References
Laaksonen, M. A. et al. The relative importance of modifiable potential risk factors of type 2 diabetes: a meta-analysis of two cohorts. Eur. J. Epidemiol. 25, 115–124. https://doi.org/10.1007/s10654-009-9405-0 (2010).
Schauer, P. R. et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N. Engl. J. Med. 376, 641–651. https://doi.org/10.1056/NEJMoa1600869 (2017).
Lambert, G. et al. Early regression of carotid intima-media thickness after bariatric surgery and its relation to serum leptin reduction. Obes. Surg. 28, 226–233. https://doi.org/10.1007/s11695-017-2839-7 (2018).
Vest, A. R., Heneghan, H. M., Schauer, P. R. & Young, J. B. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 127, 945–959. https://doi.org/10.1161/circulationaha.112.103275 (2013).
Navaneethan, S. D. et al. Urinary albumin excretion, HMW adiponectin, and insulin sensitivity in type 2 diabetic patients undergoing bariatric surgery. Obes. Surg. 20, 308–315. https://doi.org/10.1007/s11695-009-0026-1 (2010).
Freitas, W. R. Jr. et al. Systemic inflammation in severe obese patients undergoing surgery for obesity and weight-related diseases. Obes. Surg. 28, 1931–1942. https://doi.org/10.1007/s11695-017-3104-9 (2018).
Yadav, R. et al. Effect of Roux-en-Y bariatric surgery on lipoproteins, insulin resistance, and systemic and vascular inflammation in obesity and diabetes. Front. Immunol. 8, 1512. https://doi.org/10.3389/fimmu.2017.01512 (2017).
Urbanavicius, V. et al. A prospective 4-year study of insulin resistance and adipokines in morbidly obese diabetic and non-diabetic patients after gastric banding. Wideochir Inne Tech. Maloinwazyjne 12, 147–153. https://doi.org/10.5114/wiitm.2017.67207 (2017).
DeFronzo, R. A., Tobin, J. D. & Andres, R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiol. 237, E214-223. https://doi.org/10.1152/ajpendo.1979.237.3.E214 (1979).
Lopez-Jaramillo, P. et al. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol. Biol. Clin. Investig. 18, 37–45. https://doi.org/10.1515/hmbci-2013-0053 (2014).
Finucane, F. M. et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia 52, 2345–2349. https://doi.org/10.1007/s00125-009-1508-3 (2009).
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770. https://doi.org/10.1038/27376 (1998).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295. https://doi.org/10.1056/NEJM199602013340503 (1996).
Turer, A. T. & Scherer, P. E. Adiponectin: mechanistic insights and clinical implications. Diabetologia 55, 2319–2326. https://doi.org/10.1007/s00125-012-2598-x (2012).
Woelnerhanssen, B. et al. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Surg. Obes. Relat. Dis. 7, 561–568. https://doi.org/10.1016/j.soard.2011.01.044 (2011).
Unamuno, X. et al. Increase of the adiponectin/leptin ratio in patients with obesity and type 2 diabetes after Roux-en-Y gastric bypass. Nutrients https://doi.org/10.3390/nu11092069 (2019).
Vandenbroucke, J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int. J. Surg. 12, 1500–1524. https://doi.org/10.1016/j.ijsu.2014.07.014 (2014).
Crowe, C. et al. Effects of an eight-week supervised, structured lifestyle modification programme on anthropometric, metabolic and cardiovascular risk factors in severely obese adults. BMC Endocr. Disord. 15, 37. https://doi.org/10.1186/s12902-015-0038-x (2015).
Semple, R. K. et al. Elevated plasma adiponectin in humans with genetically defective insulin receptors. J. Clin. Endocrinol. Metab. 91, 3219–3223. https://doi.org/10.1210/jc.2006-0166 (2006).
Kalinowski, P. et al. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass-results of a randomized clinical trial. Surg. Obes. Relat. Dis. 13, 181–188. https://doi.org/10.1016/j.soard.2016.08.025 (2017).
Gruzdeva, O., Borodkina, D., Uchasova, E., Dyleva, Y. & Barbarash, O. Leptin resistance: underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. 12, 191–198. https://doi.org/10.2147/DMSO.S182406 (2019).
Mazahreh, T. S. et al. The effects of laparoscopic sleeve gastrectomy on the parameters of leptin resistance in obesity. Biomolecules https://doi.org/10.3390/biom9100533 (2019).
BonDurant, L. D. & Potthoff, M. J. Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annu. Rev. Nutr. 38, 173–196. https://doi.org/10.1146/annurev-nutr-071816-064800 (2018).
Singh, P. et al. Differential effects of leptin on adiponectin expression with weight gain versus obesity. Int. J. Obes. (Lond) 40, 266–274. https://doi.org/10.1038/ijo.2015.181 (2016).
Baker, J. F. et al. The adiponectin paradox in the elderly: associations with body composition, physical functioning, and mortality. J. Gerontol. A Biol. Sci. Med. Sci. 74, 247–253. https://doi.org/10.1093/gerona/gly017 (2019).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128. https://doi.org/10.1056/NEJMoa1504720 (2015).
Wing, R. R. et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 369, 145–154. https://doi.org/10.1056/NEJMoa1212914 (2013).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. https://doi.org/10.1007/BF00280883 (1985).
Pernicova, I. & Korbonits, M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 10, 143–156. https://doi.org/10.1038/nrendo.2013.256 (2014).
Fruhbeck, G., Catalan, V., Rodriguez, A. & Gomez-Ambrosi, J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 7, 57–62. https://doi.org/10.1080/21623945.2017.1402151 (2018).
Iepsen, E. W. et al. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int. J. Obes. (London) 39, 834–841. https://doi.org/10.1038/ijo.2014.177 (2015).
Ametov, A. S. & Gusenbekova, D. G. Effect of dipeptidyl peptidase-4 inhibitors on lipid metabolism in patients with type 2 diabetes mellitus. Ter Arkh 86, 85–89 (2014).
Wu, P. et al. Systematic review and meta-analysis of randomized controlled trials on the effect of SGLT2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes. Horm. Metab. Res. 51, 487–494. https://doi.org/10.1055/a-0958-2441 (2019).
Phillips, S. A. & Kung, J. T. Mechanisms of adiponectin regulation and use as a pharmacological _target. Curr. Opin. Pharmacol. 10, 676–683. https://doi.org/10.1016/j.coph.2010.08.002 (2010).
Author information
Authors and Affiliations
Contributions
M.F. Rafey and C.E.H. Fang contributed to the study design, conducted data analysis and prepared the manuscript. I. Ioana, H. Griffin, T. O’Brien, P.O’Shea and M. Hynes contributed to the study design and revised the manuscript. O. McAnena and C. Collins performed the surgical procedures, contributed to study design and revised the manuscript. C. Davenport contributed to data analysis and revision of the manuscript. F.M. Finucane supervised the drafting of the manuscript and the data analysis. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
M.F. Rafey, C.E.H. Fang, I. Ioana, H. Griffin, M. Hynes, O. McAnena, P. O’Shea and C. Collins declare that they have no competing interests. T. O’Brien has served on advisory boards for MSD and Novo Nordisk. C. Davenport has received honoraria and served on advisory boards for Novo Nordisk. F.M. Finucane have received honoraria, travel grants and have served on advisory boards for Novo Nordisk, Eli Lilly, Ethicon, Pfizer Inc., Sanofi-Aventis, Astra Zeneca, Merck-Serono, Boehringer Ingelheim, Janssen and Novartis up until 2017 but none since then.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rafey, M.F., Fang, C.E.H., Ioana, I. et al. The leptin to adiponectin ratio (LAR) is reduced by sleeve gastrectomy in adults with severe obesity: a prospective cohort study. Sci Rep 10, 16270 (2020). https://doi.org/10.1038/s41598-020-73520-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73520-3
This article is cited by
-
Explorative research on glucolipid metabolism and levels of adipokines in pseudohypoparathyroidism type 1 patients
Orphanet Journal of Rare Diseases (2023)