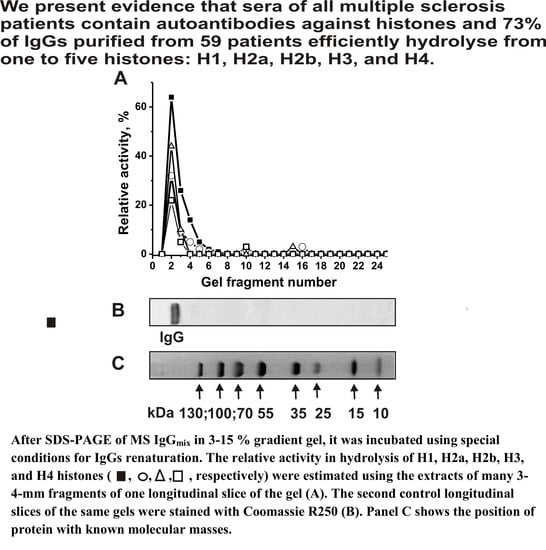
Antibodies from the Sera of Multiple Sclerosis Patients Efficiently Hydrolyze Five Histones
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals, Donors, and Patients
2.2. Antibody Purification
2.3. ELISA of Anti-Histones Autoantibodies
2.4. Ab proteolytic Activity Assay
2.5. SDS-PAGE Assay of Ab Proteolytic Activity
2.6. Determination of the Kinetic Parameters
2.7. Statistical Analysis
3. Results
3.1. IgG Purification and Characterization
3.2. Titers of IgGs to Different Histones
3.3. Catalytic Activity and Application of the Strict Criteria
3.4. Estimation of the Relative Proteolytic Activity
3.5. Type of Proteolytic Activity
3.6. The Effect of External Metal Ions on the Activity of IgGs
3.7. pH Dependencies of Histones Hydrolysis
3.8. Affinity of IgGs for Histones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ab | antibody |
| Abz | abzyme |
| AI | autoimmune |
| CC | coefficient of correlation |
| EDTA | ethylenediaminetetraacetic acid |
| FPLC | fast protein liquid chromatography |
| IgA, IgG, or IgM | immunoglobulins of A, G and M types, respectively |
| MS | multiple sclerosis |
| MBP, | myelin basic protein |
| PMSF | phenylmethanesulfonylfluoride |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
| SLE | systemic lupus erythematosus |
| TBS | neutral buffer |
| RA | relative activity |
References
- Lerner, R.A.; Tramontano, A. Antibodies as enzymes. Trends Bioch. Sci. 1987, 12, 427–438. [Google Scholar] [CrossRef]
- Schultz, P.G.; Lerner, R.A. From molecular diversity to catalysis: Lessons from the immune system. Science 1995, 269, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- David, B.S. Catalytic Antibodies; Keinan, E., Ed.; Wiley-VCH Verlag GmbH and Co. KgaA: Weinheim, Germany, 2005; pp. 1–586. [Google Scholar]
- Nevinsky, G.A.; Buneva, V.N. Natural catalytic antibodies–abzymes. In Catalytic Antibodies; Keinan, E., Ed.; VCH-Wiley Press: Weinheim, Germany, 2005; pp. 505–569. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in autoimmune diseases. In Autoimmune Diseases: Symptoms, Diagnosis and Treatment; Brenner, K.J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 1–107. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in HIV-infected patients. In Understanding HIV/AIDS Management and Care—Pandemic Approaches the 21st Century; Kasenga, F.H., Ed.; InTech: Rijeka, Croatia, 2011; pp. 151–192. [Google Scholar]
- Nevinsky, G.A. Autoimmune processes in multiple sclerosis: Production of harmful catalytic antibodies associated with significant changes in the hematopoietic stem cell differentiation and proliferation. In Multiple Sclerosis; Conzalez-Quevedo, A., Ed.; InTech: Rijeka, Croatia, 2016; pp. 100–147. [Google Scholar]
- Nevinsky, G.A. Catalytic antibodies in norm and systemic lupus erythematosus. In Lupus; Khan, W.A., Ed.; InTech: Rijeka, Croatia, 2017; pp. 41–101. [Google Scholar]
- Izadyar, L.; Friboulet, A.; Remy, M.H.; Roseto, A.; Thomas, D. Monoclonal anti-idiotypic antibodies as functional internal images of enzyme active sites: Production of a catalytic antibody with a cholinesterase activity. Proc. Natl. Acad. Sci. USA 1993, 90, 8876–8880. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, A.V.; Kozyr, A.V.; Alexandrova, E.S.; Koralewski, F.; Demin, A.; Titov, M.I.; Avalle, B.; Tramontane, A.; Paul, S.; Thomas, D.; et al. Enzyme mimicry by the antiidiotypic antibody approach. Proc. Natl. Acad. Sci. USA 2000, 97, 13526–13531. [Google Scholar] [CrossRef]
- Shuster, A.M.; Gololobov, G.V.; Kvashuk, O.A.; Bogomolova, A.E.; Smirnov, I.V.; Gabibov, A.G. DNA hydrolyzing autoantibodies. Science 1992, 256, 665–667. [Google Scholar] [CrossRef]
- Nevinsky, G.A.; Buneva, V.N. Human catalytic RNA- and DNA-hydrolyzing antibodies. J. Immunol. Methods 2002, 269, 235–249. [Google Scholar] [CrossRef]
- Savel’ev, A.N.; Eneyskaya, E.V.; Shabalin, K.A.; Filatov, M.V.; Neustroev, K.N. Antibodies with amylolytic activity. Protein Peptide Lett. 1999, 6, 179–181. [Google Scholar]
- Paul, S.; Li, L.; Kalaga, R.; O’Dell, J.R.; Dannenbring, R.E.; Swindells, S.; Hinrichs, S.; Caturegli, P.; Rose, N.R. Characterization of thyroglobulin-directed and polyreactive catalytic antibodies in autoimmune disease. J. Immunol. 1997, 159, 1530–1536. [Google Scholar]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J. Cell Mol. Med. 2004, 8, 359–368. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Nevinsky, G.A.; Favorova, O.O. Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol. Lett. 2006, 103, 75–81. [Google Scholar] [CrossRef]
- Paul, S.; Planque, S.A.; Nishiyama, Y.; Hanson, C.V.; Massey, R.J. Nature and nurture of catalytic antibodies. Adv. Exp. Med. Biol. 2012, 750, 56–75. [Google Scholar] [PubMed]
- Planque, S.A.; Nishiyama, Y.; Hara, M.; Sonoda, S.; Murphy, S.K.; Watanabe, K.; O’Nuallain, B. Physiological IgM class catalytic antibodies selective for transthyretin amyloid. J. Biol. Chem. 2014, 289, 13243–13258. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.C.; Bar-Or, A.; Hafler, D.A. The neuroimmunology of multiple sclerosis: Possible roles of T and B lymphocytes in immunopathogenesis. J. Clin. Immunol. 2001, 21, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Archelos, J.J.; Storch, M.K.; Hartung, H.P. The role of B cells and autoantibodies in multiple sclerosis. Ann. Neurol. 2000, 47, 694–706. [Google Scholar] [CrossRef]
- Hemmer, B.; Archelos, J.J.; Hartung, H.P. New concepts in the immunopathogenesis of multiple sclerosis. Nat. Rev. Neurosci. 2002, 3, 291–301. [Google Scholar] [CrossRef]
- Williamson, R.A.; Burgoon, M.P.; Owens, G.P.; Ghausi, O.; Leclerc, E.; Firme, L.; Carlson, S.; Corboy, J.; Parren, P.W.; Sanna, P.P.; et al. Anti-DNA antibodies are a major component of the intrathecal B cell response in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2001, 98, 1793–1798. [Google Scholar] [CrossRef]
- Baranovskii, A.G.; Kanyshkova, T.G.; Mogelnitskii, A.S.; Naumov, V.A.; Buneva, V.N.; Gusev, E.I.; Boiko, A.N.; Zargarova, T.A.; Favorova, O.O.; Nevinsky, G.A. Polyclonal antibodies from blood and cerebrospinal fluid of patients with multiple sclerosis effectively hydrolyze DNA and RNA. Biochemistry 1998, 63, 1239–1248. [Google Scholar]
- Baranovskii, A.G.; Ershova, N.A.; Buneva, V.N.; Kanyshkova, T.G.; Mogelnitskii, A.S.; Doronin, B.M.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Catalytic heterogeneity of polyclonal DNA-hydrolyzing antibodies from the sera of patients with multiple sclerosis. Immunol. Lett. 2001, 76, 163–167. [Google Scholar] [CrossRef]
- Legostaeva, G.A.; Polosukhina, D.I.; Bezuglova, A.M.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with multiple sclerosis. J. Cell Mol. Med. 2010, 14, 699–709. [Google Scholar] [CrossRef]
- Bezuglova, A.V.; Konenkova, L.P.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity and metal-dependence of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with systemic lupus erythematosus. J. Mol. Recognit. 2011, 24, 960–974. [Google Scholar] [CrossRef]
- Savel’ev, A.N.; Kulminskaya, A.A.; Ivanen, D.R.; Nevinsky, G.A.; Neustroev, K.N. Human antibodies with amylolytic activity. Trends Glycosci. Glycotechnol. 2004, 16, 17–31. [Google Scholar]
- Parkhomenko, T.A.; Doronin, V.B.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of DNA-hydrolyzing antibodies from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e93001. [Google Scholar] [CrossRef] [PubMed]
- Doronin, V.B.; Parkhomenko, T.A.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of antibodies hydrolyzing myelin basic protein from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e107807. [Google Scholar] [CrossRef] [PubMed]
- Doronin, V.B.; Parkhomenko, T.A.; Castellazzi, M.; Cesnik, E.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of antibodies with amylase activity from cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2016, 11, e0154688. [Google Scholar] [CrossRef] [PubMed]
- Nevinsky, G.A.; Buneva, V.N. Catalytic antibodies in healthy humans and patients with autoimmune and viral pathologies. J. Cell Mol. Med. 2003, 7, 265–276. [Google Scholar] [CrossRef]
- Kozyr, A.V.; Kolesnikov, A.V.; Aleksandrova, E.S.; Sashchenko, L.P.; Gnuchev, N.V.; Favorov, P.V.; Alekberova, Z.S. Novel functional activities of anti-DNA autoantibodies from sera of patients with lymphoproliferative and autoimmune diseases. Appl. Biochem. Biotechnol. 1998, 75, 45–61. [Google Scholar] [CrossRef]
- Founel, S.; Muller, S. Antinucleosome antibodies and T-cell response in systemic lupus erythematosus. Ann. Med. Interne. 2002, 153, 513–519. [Google Scholar]
- Chen, R.; Kang, R.; Fan, X.-G.; Tang, D. Release and activity of histone in diseases. Cell Death. Dis. 2014, 5, e1370. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies from the sera of HIV-infected patients efficiently hydrolyze all human histones. J. Mol. Recognit. 2016, 29, 346–362. [Google Scholar] [CrossRef]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sedykh, S.E.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in cell differentiation and proliferation lead to production of abzymes in EAE mice treated with DNA-Histone complexes. J. Cell Mol. Med. 2018, 22, 5816–5832. [Google Scholar] [CrossRef]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurological impairment in multiple sclerosis: An expanded disability scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Fersht, A. Enzyme Structure and Mechanism, 2nd ed.; W.H. Freeman Co.: NewYork, NY, USA, 1985. [Google Scholar]
- Shoenfeld, Y.; Ben-Yehuda, O.; Messinger, Y.; Bentwitch, Z.; Rauch, J.; Isenberg, D.I.; Gadoth, N.G. Autoimmune diseases other than lupus share common anti-DNA idiotypes. Immunol. Lett. 1988, 17, 285–291. [Google Scholar] [CrossRef]
- Blanco, F.; Kalsi, J.; Isenberg, D.A. Analysis of antibodies to RNA in patients with systemic lupus erythematosus and other autoimmune rheumatic diseases. Clin. Exp. Immunol. 1991, 86, 66–70. [Google Scholar] [CrossRef]
- Berneman, A.; Cuilbert, B.; Enschrich, S.; Avrames, S. IgG auto- and polyreactivities of normal human sera. Mol. Immunol. 1993, 30, 1499–1510. [Google Scholar] [CrossRef]
- Paul, S.; Volle, D.J.; Beach, C.M.; Johnson, D.R.; Powell, M.J.; Massey, R.J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science 1989, 244, 1158–1162. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing antibodies from sera of HIV-infected patients. Biochimie 2009, 91, 1081–1086. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing IgM antibodies from sera of HIV-infected patients. Int. Immunol. 2010, 22, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Ikhmyangan, E.N.; Vasilenko, N.L.; Buneva, V.N.; Nevinsky, G.A. Metal ions-dependent peroxidase and oxidoreductase activities of polyclonal IgGs from the sera of Wistar rats. J. Mol. Recognit. 2006, 19, 91–105. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Zaksas, N.P.; Buneva, V.N.; Vasilenko, N.L.; Nevinsky, G.A. Oxidoreductase activities of polyclonal IgGs from the sera of Wistar rats are better activated by combinations of different metal ions. J. Mol. Recognit. 2009, 22, 26–37. [Google Scholar] [CrossRef]
- Doronin, V.B.; Parkhomenko, T.A.; Korablev, A.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaja, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; et al. Changes in different parameters, lymphocyte proliferation and hematopoietic progenitor colony formation in EAE mice treated with myelin oligodendrocyte glycoprotein. J. Cell Mol. Med. 2016, 20, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in haematopoietic progenitor colony differentiation and proliferation and the production of different abzymes in EAE mice treated with DNA. J. Cell Mol. Med. 2017, 21, 3795–3809. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Buneva, V.N.; Nevinsky, G.A. Systemic lupus erythematosus: Molecular cloning and analysis of 22 individual recombinant monoclonal kappa light chains specifically hydrolyzing human myelin basic protein. J. Mol. Recognit. 2015, 28, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Systemic lupus erythematosus: Molecular cloning and analysis of recombinant monoclonal kappa light chain NGTA2-Me-pro-ChTr possessing two different activities-trypsin-like and metalloprotease. Int. Immunol. 2015, 27, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timofeeva, A.M.; Buneva, V.N.; Nevinsky, G.A. Systemic lupus erythematosus: Molecular cloning and analysis of recombinant monoclonal kappa light chain NGTA1-Me-pro with two metalloprotease active centers. Mol. Biosyst. 2016, 12, 3556–3566. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Buneva, V.N.; Nevinsky, G.A. SLE: Unusual recombinant monoclonal light chain NGTA3-Pro-DNase possessing three different activities trypsin-like, metalloprotease and DNase. Lupus Open Access 2017, 2, 127. [Google Scholar] [CrossRef]





| Number | Denotation of Preparation | Relative Activity % * | ||||
|---|---|---|---|---|---|---|
| H1 | H2a | H2b | H3 | H4 | ||
| Debut of Multiple Sclerosis (DMS) ** | ||||||
| 1 | DMS1 | 22.0 ± 2.0 | 11.0 ± 1.5 | 21.0 ± 2.0 | 26.0 ± 3.5 | 48.0 ± 7.0 |
| 2 | DMS2 | 11.0 ± 1.5 | 21.0 ± 2.0 | 5.0 ± 0.7 | 29.0 ± 3.3 | 36.0 ± 4.5 |
| 3 | DMS3 | 14.5 ± 2.0 | 11.0 ± 1.4 | 15.0 ± 1.9 | 44.0 ± 4.9 | 46.0 ± 5.3 |
| 4 | DMS4 | 5.0 ± 0.7 | 15.0 ± 1.8 | 13.0 ± 1.5 | 30.0 ± 4.0 | 35.0 ± 4.6 |
| 5 | DMS5 | 0.0 | 19.0 ± 2.1 | 0.0 | 0.0 | 18.0 ± 2.5 |
| 6 | DMS6 | 66.0 ± 6.0 | 57.0 ± 6.1 | 59.0 ± 6.2 | 51.0 ± 6.0 | 55.0 ± 7.0 |
| 7 | DMS7 | 57.0 ± 7.0 | 68.0 ± 7.0 | 67.0 ± 7.1 | 64.0 ± 6.9 | 63.0 ± 7.3 |
| 8 | DMS8 | 55.0 ± 6.0 | 62.0 ± 7.1 | 66.0 ± 7.0 | 65.0 ± 7.2 | 65.0 ± 6.9 |
| Average Value | 30.9 ± 27.7 | 33.0 ± 24.7 | 30.8 ± 28.3 | 38.6 ± 21.8 | 45.8 ± 15.8 | |
| Average Value for H1–H4 | 35.9 ± 23.4% | |||||
| Median (IQR) | 18.25 (48.0) | 20.0 (46.5) | 18.0 (53.5) | 37.0 (30.0) | 47.0 (23.5) | |
| Remitting Multiple Sclerosis (RMS) | ||||||
| 9 | RMS1 | 0.0 | 0.0 | 0.0 | 39.0 ± 4.0 | 18.0 ± 2.5 |
| 10 | RMS2 | 0.0 | 8.0 ± 1.0 | 0.0 | 16.0 ± 2.0 | 16.0 ± 2.2 |
| 11 | RMS3 | 34.0 ± 4.0 | 37.0 ± 4.1 | 38.0 ± 4.5 | 26.0 ± 3.5 | 47.0 ± 5.2 |
| 12 | RMS4 | 31.0 ± 4.2 | 40.0 ± 5.0 | 40.0 ± 5.4 | 36.0 ± 4.2 | 58.0 ± 6.9 |
| 13 | RMS5 | 25.0 ± 3.0 | 20.0 ± 3.5 | 28.0 ± 2.0 | 29.0 ± 3.4 | 44.0 ± 5.1 |
| 14 | RMS6 | 25.0 ± 3.2 | 28.0 ± 3.7 | 25.0 ± 3.4 | 44.0 ± 5.1 | 32.0 ± 4.0 |
| 15 | RMS7 | 20.0 ± 2.9 | 27.0 ± 3.3 | 12.0 ± 1.9 | 17.0 ± 2.2 | 24.0 ± 3.1 |
| 16 | RMS8 | 20.0 ± 2.0 | 20.0 ± 2.9 | 18.0 ± 2.8 | 33.0 ± 3.9 | 33.0 ± 4.0 |
| 17 | RMS9 | 20.0 ± 3.4 | 45.0 ± 5.0 | 20.0 ± 3.1 | 43.0 ± 5.1 | 27.0 ± 3.2 |
| 18 | RMS10 | 10.0 ± 2.0 | 0.0 | 40.0 ± 4.6 | 0.0 | 0.0 |
| 19 | RMS11 | 40.0 ± 4.9 | 70.0 ± 8.0 | 80.0 ± 9.1 | 85.0 ± 9.3 | 0.0 |
| 20 | RMS12 | 0.0 | 0.0 | 0.0 | 35.0 ± 4.3 | 0.0 |
| 21 | RMS13 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 22 | RMS14 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 23 | RMS15 | 12.0 ± 1.7 | 0.0 | 0.0 | 10.0 ± 2.0 | 0.0 |
| 24 | RMS16 | 9.0 ± 1.8 | 40.0 ± 4.8 | 40.0 ± 5.0 | 50.0 ± 6.2 | 10.0 ± 2.1 |
| 25 | RMS17 | 5.0 ± 1.5 | 0.0 | 0.0 | 10.0 ± 1.7 | 10.0 ± 1.4 |
| 26 | RMS18 | 10.0 ± 1.6 | 10.0 ± 1.5 | 0.0 | 10.0 ± 2.0 | 0.0 |
| 27 | RMS19 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 28 | RMS20 | 11.0 ± 2.1 | 0.0 | 0.0 | 5.0 ± 1.0 | 0.0 |
| 29 | RMS21 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 30 | RMS22 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 31 | RMS23 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 32 | RMS24 | 0.0 | 0.0 | 0.0 | 10.0 ± 2.0 | 0.0 |
| 33 | RMS25 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 34 | RMS26 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 35 | RMS27 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 36 | RMS28 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 37 | RMS29 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 38 | RMS30 | 26.0 ± 2.9 | 80.0 ± 9.1 | 90.0 ± 10.5 | 0.0 | 0.0 |
| 39 | RMS31 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 40 | RMS32 | 90.0 ± 10.0 | 90.0 ± 12.0 | 87.0 ± 9.9 | 90.0 ± 11.0 | 78.0 ± 9.8 |
| 41 | RMS33 | 48.0 ± 6.0 | 76.0 ± 8.2 | 50.0 ± 6.3 | 90.0 ± 12.1 | 90.0 ± 11.0 |
| 42 | RMS34 | 24.0 ± 3.1 | 20.0 ± 3.0 | 14.0 ± 2.4 | 0.0 | 90.0 ± 10.0 |
| 43 | RMS35 | 55.0 ± 7.1 | 0.0 | 15.0 ± 2.1 | 40.0 ± 4.9 | 0.0 |
| 44 | RMS36 | 47.0 ± 5.9 | 0.0 | 0.0 | 10.0 ± 1.7 | 0.0 |
| 45 | RMS37 | 17.0 ± 2.2 | 18.0 ± 2.5 | 19.0 ± 2.3 | 20.0 ± 3.0 | 14.0 ± 2.0 |
| Average Value | 16.0 ± 20.5 | 16.8 ± 26.0 | 16.8 ± 25.6 | 20.2 ± 26.0 | 15.6 ± 26.3 | |
| Average Value for H1–H4 | 17.1 ± 24.9 | |||||
| Median (IQR) | 10.0 (25.0) | 0.0 (27.0) | 0.0 (25.0) | 10.0 (35.0) | 0.0 (24.0) | |
| Secondary Progressive Multiple Sclerosis (SPMS) | ||||||
| 46 | SPMS1 | 40.0 ± 5.4 | 48.0 ± 5.9 | 47.0 ± 5.6 | 35.0 ± 4.2 | 53.0 ± 6.4 |
| 47 | SPMS2 | 32.0 ± 4.1 | 38.0 ± 4.4 | 35.0 ± 4.7 | 32.0 ± 4.9 | 51.0 ± 6.9 |
| 48 | SPMS3 | 30.0 ± 3.9 | 33.0 ± 4.0 | 32.0 ± 4.3 | 30.0 ± 4.2 | 58.0 ± 7.0 |
| 49 | SPMS4 | 33.0 ± 4.4 | 39.0 ± 4.5 | 43.0 ± 5.8 | 13.0 ± 1.9 | 50.0 ± 6.8 |
| 50 | SPMS5 | 26.0 ± 3.2 | 25.0 ± 3.9 | 24.0 ± 3.5 | 18.0 ± 2.1 | 28.0 ± 3.4 |
| 51 | SPMS6 | 24.0 ± 3.7 | 30.0 ± 4.1 | 19.0 ± 2.6 | 25.0 ± 2.9 | 32.0 ± 4.1 |
| 52 | SPMS7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 53 | SPMS8 | 0.0 | 0.0 | 10.0 ± 2.0 | 0.0 | 0.0 |
| 54 | SPMS9 | 0.0 | 5.0 ± 1.5 | 11.0 ± 2.1 | 11.0 ± 2.2 | 10.0 ± 1.9 |
| 55 | SPMS10 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 56 | SPMS11 | 45.0 ± 5.3 | 0.0 | 0.0 | 10.0 ± 2.3 | 90.0 ± 12.0 |
| Average value | 20.9 ± 17.5 | 19.8 ± 18.9 | 20.1 ± 17.4 | 15.8 ± 13.2 | 33.8 ± 29.4 | |
| Average value for H1–H4 | 22.3 ± 19.3 | |||||
| Median (IQR) | 26.0 (33.0) | 25.0 (38.0) | 19.0 (35.0) | 13.0 (30.0) | 32.0 (53.0) | |
| Remittently Progressive Multiple Sclerosis (RPMS) | ||||||
| 57 | RPMS1 | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 ± 1.0 |
| 58 | RPMS2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 59 | RPMS3 | 44.0 ± 5.9 | 50.0 ± 7.2 | 55.0 ± 6.8 | 74.0 ± 8.9 | 10.0 ± 2.1 |
| Average Value | 14.7 ± 25.4 | 16.7 ± 28.9 | 18.3 ± 31.8 | 24.7 ± 42.7 | 5.0 ± 5.0 | |
| Average Value for H1–H4 | 15.9 ± 26.7 | |||||
| Median (IQR) | 0.0 (44.0) | 0.0 (50.0) | 0.0 (55.0) | 0.0 (74.0) | 5.0 (10.0) | |
| Average Value for 59 patients | 18.6 ± 20.9 | 19.5 ± 24.8 | 19.4 ± 24.8 | 22.1 ± 24.8 | 22.5 ± 27.5 | |
| Average Value for H1–H4 | 20.4 ± 24.5 | |||||
| Median (IQR) for 59 Patients | 11.0 (31.0) | 10.0 (37.0) | 11.0 (35.0) | 13.0 (35.0) | 10.0 (46.0) | |
| Denotation of IgG Preparation | Histones | Percent of Inhibition of Different Histones Hydrolysis ** | ||||
|---|---|---|---|---|---|---|
| EDTA 0.1 M | AEBSF | Iodoacetamide | Pepstatin A | Sum of the Effects | ||
| DMS6 | H1 | 27.0 ± 2.0 | 96.0 ± 10.0 | 0.0 | 0.0 | 123.0 |
| H2a | 20.0 ± 1.9 | 23.0 ± 2.6 | 10.0 ± 1.4 | 43.0 ± 4.7 | 96.0 | |
| H2b | 48.0 ± 5.2 | 97.0 ± 11.0 | 8.0 ± 1.2 | 54 ± 6.0 | 212.0 | |
| H4 | 70.0 ± 8.1 | 58.0 ± 6.2 | 23.0 ± 2.9 | 61 ± 7.0 | 212.0 | |
| SPMS1 | H1 | 0.0 | 52.0 ± 6.1 | 20.0 ± 2.0 | 15.0 ± 2.0 | 87.0 |
| H2a | 0.0 | 36.0 ± 4.4 | 22.0 ± 2.8 | 54.0.0 ± 6.1 | 112.0 | |
| H2b | 0.0 | 62.0 ± 6.9 | 5.0 ± 1.0 | 59.0 ± 6.4 | 60.0 | |
| H4 | 0.0 | 19.0 ± 2.5 | 41.0 ± 5.1 | 0.0 | 60.0 | |
| DMS7 | H1 | 43.0 ± 5.0 | 26.0 ± 3.2 | 12.0 ± 1.9 | 0.0 | 81.0 |
| H2a | 54.0 ± 6.0 | 0.0 | 0.0 | 0.0 | 54.0 | |
| H2b | 65.0 ± 7.2 | 43.0 ± 5.9 | 7.0 ± 1.2 | 9.0 ± 1.3 | 124.0 | |
| H4 | 65.0 ± 7.3 | 54.0 ± 6.6 | 36.0 ± 4.2 | 52.0 ± 6.6 | 207.0 | |
| DMS8 | H1 | 32.0 ± 4.4 | 100.0 ± 5.2 | 54.0 ± 6.7 | 0.0 | 186.0 |
| H2a | 78.0 ± 9.2 | 22.0 ± 3.3 | 0.0 | 40.0 ± 5.2 | 140.0 | |
| H2b | 66.0 ± 7.7 | 89.0 ± 10.2 | 0.0 | 54.0 ± 6.7 | 209.0 | |
| H4 | 69.0 ± 7.2 | 98.0 ± 11.0 | 16.0 ± 1.9 | 58.0 ± 7.4 | 241.0 | |
| Average Values | 29.8 ± 28.8 | 54.7 ± 33.0 | 15.9 ± 16 | 31.2 ± 26.1 | 137.8 ± 64.1 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, S.V.; Mikheeva, E.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies from the Sera of Multiple Sclerosis Patients Efficiently Hydrolyze Five Histones. Biomolecules 2019, 9, 741. https://doi.org/10.3390/biom9110741
Baranova SV, Mikheeva EV, Buneva VN, Nevinsky GA. Antibodies from the Sera of Multiple Sclerosis Patients Efficiently Hydrolyze Five Histones. Biomolecules. 2019; 9(11):741. https://doi.org/10.3390/biom9110741
Chicago/Turabian StyleBaranova, Svetlana V., Elena V. Mikheeva, Valentina N. Buneva, and Georgy A. Nevinsky. 2019. "Antibodies from the Sera of Multiple Sclerosis Patients Efficiently Hydrolyze Five Histones" Biomolecules 9, no. 11: 741. https://doi.org/10.3390/biom9110741
APA StyleBaranova, S. V., Mikheeva, E. V., Buneva, V. N., & Nevinsky, G. A. (2019). Antibodies from the Sera of Multiple Sclerosis Patients Efficiently Hydrolyze Five Histones. Biomolecules, 9(11), 741. https://doi.org/10.3390/biom9110741