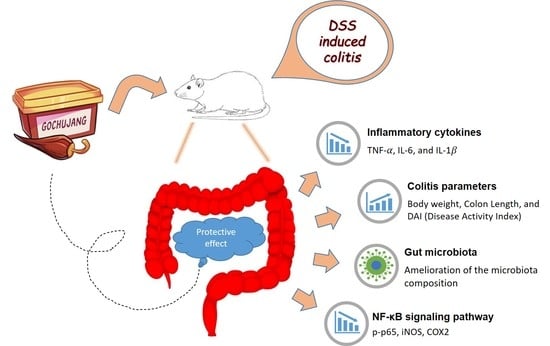
Protective Effect of Gochujang on Inflammation in a DSS-Induced Colitis Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Disease Activity Index (DAI) and Sample Collection
2.3. Histological Analysis
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.6. Western Blot Analysis
2.7. Gut Microbiota Analysis
2.8. Statistics and Analysis
3. Results
3.1. Effect of Gochujang on UC Symptoms
3.2. Effect of Gochujang on Colon Damage
3.3. Effect of Gochujang on Serum Levels of Pro-Inflammatory Cytokine
3.4. Effects of Gochujang on Colonic Inflammation-Related Gene and Protein Expression
3.5. Effect of Gochujang on Gut Microbiota Dysbiosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, N.N.; Jess, T. Has the risk of colorectal cancer in inflammatory bowel disease decreased? World J. Gastroenterol. 2013, 19, 7561–7568. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Abreu, M.T.; Cohen, R.; Tremaine, W.; American Gastroenterological Associatoin. American gastroenterological association institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006, 130, 940–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting therapeutic _targets in inflammatory bowel disease (stride): Determining therapeutic goals for treat-to-_target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T.; Stepankova, R.; Kozakova, H.; Hudcovic, T.; Tlaskalova-Hogenova, H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: Studies in germ-free mice. BMC Immunol. 2008, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Bell, V.; Ferrao, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One health, fermented foods, and gut microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [Green Version]
- Song, J.L.; Choi, J.H.; Seo, J.H.; Lim, Y.I.; Park, K.Y. Anti-colitic effects of kanjangs (fermented soy sauce and sesame sauce) in dextran sulfate sodium-induced colitis in mice. J. Med. Food 2014, 17, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.A.; Jang, S.E.; Jeong, J.J.; Yu, D.H.; Han, M.J.; Kim, D.H. Doenjang, a korean soybean paste, ameliorates TNBS-induced colitis in mice by suppressing gut microbial lipopolysaccharide production and NF-κB activation. J. Funct. Foods 2014, 11, 417–427. [Google Scholar] [CrossRef]
- Monteleone, I.; Marafini, I.; Dinallo, V.; Di Fusco, D.; Troncone, E.; Zorzi, F.; Laudisi, F.; Monteleone, G. Sodium chloride-enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J. Crohns. Colitis 2017, 11, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee, H.J.; Shin, H.C.; Lee, J.M.; Park, K.H.; Moon, J.H. Behavior of flavonoid glycosides contained in korean red pepper paste (gochujang) during fermentation: Participation of a β-glucosidase inhibitor. Food Sci. Biotechnol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Park, K.Y.; Kong, K.R.; Jung, K.O.; Rhee, S.H. Inhibitory effects of kochujang extracts on the tumor formation and lung metastasis in mice. Prev. Nutr. Food Sci. 2001, 6, 187–191. [Google Scholar]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, Y.S.; Shin, D.W.; Park, S. Kochujang, a korean fermented red pepper plus soybean paste, improves glucose homeostasis in 90% pancreatectomized diabetic rats. Nutrition 2009, 25, 790–799. [Google Scholar] [CrossRef]
- Ahn, I.S.; Do, M.S.; Kim, S.O.; Jung, H.S.; Kim, Y.I.; Kim, H.J.; Park, K.Y. Antiobesity effect of kochujang (korean fermented red pepper paste) extract in 3T3-L1 adipocytes. J. Med. Food 2006, 9, 15–21. [Google Scholar] [CrossRef]
- Lee, D.E.; Shin, G.R.; Lee, S.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Metabolomics reveal that amino acids are the main contributors to antioxidant activity in wheat and rice gochujangs (korean fermented red pepper paste). Food Res. Int. 2016, 87, 10–17. [Google Scholar] [CrossRef]
- Ryu, J.A.; Kim, E.; Kim, M.J.; Lee, S.; Yoon, S.R.; Ryu, J.G.; Kim, H.Y. Physicochemical characteristics and microbial communities in gochujang, a traditional korean fermented hot pepper paste. Front. Microbiol. 2020, 11, 620478. [Google Scholar] [CrossRef]
- Jang, Y.K.; Shin, G.R.; Jung, E.S.; Lee, S.; Lee, S.; Singh, D.; Jang, E.S.; Shin, D.J.; Kim, H.J.; Shin, H.W.; et al. Process specific differential metabolomes for industrial gochujang types (pepper paste) manufactured using white rice, brown rice, and wheat. Food Chem. 2017, 234, 416–424. [Google Scholar] [CrossRef]
- Chen, Y.; Le, T.H.; Du, Q.; Zhao, Z.; Liu, Y.; Zou, J.; Hua, W.; Liu, C.; Zhu, Y. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int. Immunopharmacol. 2019, 71, 144–154. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, J.; Zhao, J.; Chen, Y. Genistein improves inflammatory response and colonic function through NF-κB signal in DSS-induced colonic injury. Onco_target 2017, 8, 61385–61392. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Li, N.; Zhang, X. Daidzein ameliorates dextran sulfate sodium-induced experimental colitis in mice by regulating NF-κB signaling. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 29–39. [Google Scholar] [CrossRef]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. mBio 2017, 8, e00470-17. [Google Scholar] [CrossRef] [Green Version]
- Bessler, H.; Djaldetti, M. Capsaicin modulates the immune cross talk between human mononuclears and cells from two colon carcinoma lines. Nutr. Cancer 2017, 69, 14–20. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Cai, S.; Yu, H.; Wang, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri ameliorates intestinal inflammation and modulates gut microbiota and metabolic disorders in dextran sulfate sodium-induced colitis in mice. Nutrients 2020, 12, 2298. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Hong, G.; Huang, C.; Qian, W.; Bai, T.; Song, J.; Song, Y.; Hou, X. Probiotic mixtures with aerobic constituent promoted the recovery of multi-barriers in DSS-induced chronic colitis. Life Sci. 2020, 240, 117089. [Google Scholar] [CrossRef]
- Korkmaz, D. Precipitation titration: “Determination of chloride by the mohr method”. Methods 2001, 2, 1–6. [Google Scholar]
- Tubbs, A.L.; Liu, B.; Rogers, T.D.; Sartor, R.B.; Miao, E.A. Dietary salt exacerbates experimental colitis. J. Immunol. 2017, 199, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Kong, R.; Luo, H.; Wang, N.; Li, J.; Xu, S.; Chen, K.; Feng, J.; Wu, L.; Li, S.; Liu, T.; et al. Portulaca extract attenuates development of dextran sulfate sodium induced colitis in mice through activation of PPARγ. PPAR Res. 2018, 2018, 6079101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.Y.; Chung, K.R.; Yang, H.J.; Jang, D.J. Gochujang (korean red pepper paste): A korean ethnic sauce, its role and history. J. Ethn. Foods 2015, 2, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, M.S.; Lee, M.S.; Park, Y.S.; Lee, H.J.; Kang, S.-A.; Lee, H.S.; Lee, K.-E.; Yang, H.J.; Kim, M.J.; et al. Korean diet: Characteristics and historical background. J. Ethn. Foods 2016, 3, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Kim, Y.S.; Heo, N.J.; Lim, J.H.; Yang, S.Y.; Chung, G.E.; Kim, J.S. High salt intake is associated with atrophic gastritis with intestinal metaplasia. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Bojic, D.; Radojicic, Z.; Nedeljkovic-Protic, M.; Al-Ali, M.; Jewell, D.P.; Travis, S.P. Long-term outcome after admission for acute severe ulcerative colitis in oxford: The 1992–1993 cohort. Inflamm. Bowel Dis. 2009, 15, 823–828. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; You, M.; Tian, W.; Leu, R.K.L.; Topping, D.L.; Conlon, M.A.; Wu, L.; Hu, F. Dietary propolis ameliorates dextran sulfate sodium-induced colitis and modulates the gut microbiota in rats fed a western diet. Nutrients 2017, 9, 875. [Google Scholar] [CrossRef]
- Vetuschi, A.; Latella, G.; Sferra, R.; Caprilli, R.; Gaudio, E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig. Dis Sci. 2002, 47, 1447–1457. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic Th17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Qin, L.; Yao, Z.; Chang, Q.; Zhao, Y.; Liu, N.; Zhu, X.; Liu, Q.; Wang, L.; Yang, A.; Gao, C.; et al. Swimming attenuates inflammation, oxidative stress, and apoptosis in a rat model of dextran sulfate sodium-induced chronic colitis. Onco_target 2017, 8, 7391–7404. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.K.; Chang, H.K.; Park, K.Y. Doenjang prepared with mixed starter cultures attenuates azoxymethane and dextran sulfate sodium-induced colitis-associated colon carcinogenesis in mice. J. Carcinog. 2014, 13, 9. [Google Scholar]
- Morimoto, M.; Watanabe, T.; Yamori, M.; Takebe, M.; Wakatsuki, Y. Isoflavones regulate innate immunity and inhibit experimental colitis. J. Gastroenterol. Hepatol. 2009, 24, 1123–1129. [Google Scholar] [CrossRef]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibel, J.; Molzberger, A.F.; Hertrampf, T.; Leschowski, U.L.; Diel, P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur. J. Nutr. 2009, 48, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Bushmanc, F.D.; Lewis, J.D. Diet, the human gut microbiota, and IBD. Anaerobe 2013, 24, 117–120. [Google Scholar] [CrossRef]
- Vemuri, R.C.; Gundamaraju, R.; Shinde, T.; Eri, R. Therapeutic interventions for gut dysbiosis and related disorders in the elderly: Antibiotics, probiotics or faecal microbiota transplantation? Benef. Microbes 2017, 8, 179–192. [Google Scholar] [CrossRef]
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 2009, 77, 1708–1718. [Google Scholar] [CrossRef] [Green Version]
- Lucke, K.; Miehlke, S.; Jacobs, E.; Schuppler, M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J. Med. Microbiol. 2006, 55, 617–624. [Google Scholar] [CrossRef]
- Balish, E.; Warner, T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am. J. Pathol. 2002, 160, 2253–2257. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chen, H.; He, H.; Du, Y.; Hu, J.; Li, Y.; Li, Y.; Zhou, Y.; Wang, H.; Chen, Y.; et al. Increased Enterococcus faecalis infection is associated with clinically active Crohn disease. Medicine 2016, 95, e5019. [Google Scholar] [CrossRef]
- Dinakaran, V.; Mandape, S.N.; Shuba, K.; Pratap, S.; Sakhare, S.S.; Tabatabai, M.A.; Smoot, D.T.; Farmer-Dixon, C.M.; Kesavalu, L.N.; Adunyah, S.E.; et al. Identification of specific oral and gut pathogens in full thickness colon of colitis patients: Implications for colon motility. Front. Microbiol. 2018, 9, 3220. [Google Scholar] [CrossRef]
- Wu, H.; Rao, Q.; Ma, G.C.; Yu, X.H.; Zhang, C.E.; Ma, Z.J. Effect of triptolide on dextran sodium sulfate-induced ulcerative colitis and gut microbiota in mice. Front. Pharmacol. 2019, 10, 1652. [Google Scholar] [CrossRef]
- Sun, M.C.; Zhang, F.C.; Yin, X.; Cheng, B.J.; Zhao, C.H.; Wang, Y.L.; Zhang, Z.Z.; Hao, H.W.; Zhang, T.H.; Ye, H.Q. Lactobacillus reuteri F-9-35 prevents DSS-induced colitis by inhibiting proinflammatory gene expression and restoring the gut microbiota in mice. J. Food Sci. 2018, 83, 2645–2652. [Google Scholar] [CrossRef]
- Paul, B.; Royston, K.J.; Li, Y.; Stoll, M.L.; Skibola, C.F.; Wilson, L.S.; Barnes, S.; Morrow, C.D.; Tollefsbol, T.O. Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition. PLoS ONE 2017, 12, e0189756. [Google Scholar] [CrossRef] [Green Version]
- Ou, W.; Hu, H.; Yang, P.; Dai, J.; Ai, Q.; Zhang, W.; Zhang, Y.; Mai, K. Dietary daidzein improved intestinal health of juvenile turbot in terms of intestinal mucosal barrier function and intestinal microbiota. Fish Shellfish Immunol. 2019, 94, 132–141. [Google Scholar] [CrossRef]




| Gene Name | Primers | Sequence 5′ to 3′ |
|---|---|---|
| β-actin | Forward | CCCGCGAGTACAACCTTCT |
| Reverse | CGTCATCCATGGCGAACT | |
| TNF-α 1 | Forward | CCCTGGTACTAACTCCCAGAAA |
| Reverse | TGTATGAGAGGGACGGAACC | |
| IL-1β | Forward | CACCTCTCAAGCAGAGCACAG |
| Reverse | GGGTTCCATGGTGAAGTCAAC | |
| IL-6 | Forward | TCCTACCCCAACTTCCAATGCTC |
| Reverse | TTGGATGGTCTTGGTCCTTAGCC |
| TNF-α (pg/mL) | IL-6 (pg/mL) | IL-1β (pg/mL) | |
|---|---|---|---|
| NOR | 49.97 ± 0.21 a | 238.52 ± 3.42 c | 43.76 ± 1.02 b |
| DSS | 50.47 ± 0.22 a | 251.52 ± 1.83 a | 55.66 ± 9.76 a |
| SAL | 50.42 ± 0.24 a | 251.69 ± 1.56 a | 55.93 ± 11.68 a |
| MES | 47.12 ± 1.93 b | 248.87 ± 1.14 b | 45.63 ± 1.16 b |
| GCJ | 49.89 ± 0.11 a | 247.73 ± 1.39 b | 44.81 ± 1.62 b |
| OTU | ACE | CHAO | SHANNON | SIMPSON | Phylogenetic Diversity | |
|---|---|---|---|---|---|---|
| NOR | 292.14 ± 30.93 a | 316.85 ± 32.55 a | 303.01 ± 31.73 a | 2.06 ± 0.16 bc | 0.22 ± 0.04 ab | 285.29 ± 20.97 a |
| DSS | 304.57 ± 41.04 a | 321.36 ± 42.78 a | 310.80 ± 41.59 a | 2.35 ± 0.26 a | 0.18 ± 0.06 b | 254.57 ± 41.56 ab |
| SAL | 200.00 ± 85.24 b | 225.02 ± 80.78 b | 209.94 ± 83.12 b | 1.91 ± 0.15 c | 0.26 ± 0.04 a | 222.43 ± 69.05 b |
| MES | 268.29 ± 46.13 a | 290.75 ± 42.59 a | 277.57 ± 43.95 a | 2.01 ± 0.18 c | 0.27 ± 0.07 a | 282.43 ± 18.88 a |
| GCJ | 313.57 ± 53.54 a | 334.62 ± 53.11 a | 321.00 ± 52.90 a | 2.24 ± 0.19 ab | 0.20 ± 0.06 b | 284.86 ± 42.51 a |
| Species | Composition (%) | ||||
|---|---|---|---|---|---|
| NOR | DSS | SAL | MES | GCJ | |
| Enterococcus faecalis | 1.57 ± 0.53 bc | 8.37 ± 5.91 a | 4.79 ± 2.31 b | 2.66 ± 2.13 bc | 1.15 ± 0.59 c |
| Staphylococcus sciuri group | 1.61 ± 1.38 b | 15.34 ± 11.69 a | 5.69 ± 4.25 b | 0.82 ± 0.80 b | 3.02 ± 3.03 b |
| Akkermansia muciniphila | 0.04 ± 0.04 b | 0.04 ± 0.04 b | 0.08 ± 0.08 b | 0.17 ± 0.10 ab | 0.25 ± 0.26 a |
| Enterococcus faecium group | 25.75 ± 6.88 bc | 21.51 ± 6.88 c | 42.59 ± 9.36 a | 38.30 ± 19.89 ab | 32.61 ± 13.56 abc |
| Escherichia coli group | 26.58 ± 11.94 | 18.54 ± 12.16 | 17.61 ± 12.66 | 22.65 ± 15.46 | 15.16 ± 7.62 |
| Lactobacillus reuteri group | 0.06 ± 0.06 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.14 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahoro, P.; Moon, H.-J.; Yang, H.-J.; Kim, K.-A.; Cha, Y.-S. Protective Effect of Gochujang on Inflammation in a DSS-Induced Colitis Rat Model. Foods 2021, 10, 1072. https://doi.org/10.3390/foods10051072
Mahoro P, Moon H-J, Yang H-J, Kim K-A, Cha Y-S. Protective Effect of Gochujang on Inflammation in a DSS-Induced Colitis Rat Model. Foods. 2021; 10(5):1072. https://doi.org/10.3390/foods10051072
Chicago/Turabian StyleMahoro, Patience, Hye-Jung Moon, Hee-Jong Yang, Kyung-Ah Kim, and Youn-Soo Cha. 2021. "Protective Effect of Gochujang on Inflammation in a DSS-Induced Colitis Rat Model" Foods 10, no. 5: 1072. https://doi.org/10.3390/foods10051072
APA StyleMahoro, P., Moon, H. -J., Yang, H. -J., Kim, K. -A., & Cha, Y. -S. (2021). Protective Effect of Gochujang on Inflammation in a DSS-Induced Colitis Rat Model. Foods, 10(5), 1072. https://doi.org/10.3390/foods10051072