The Adverse Effects of Triptolide on the Reproductive System of Caenorhabditis elegans: Oogenesis Impairment and Decreased Oocyte Quality
Abstract
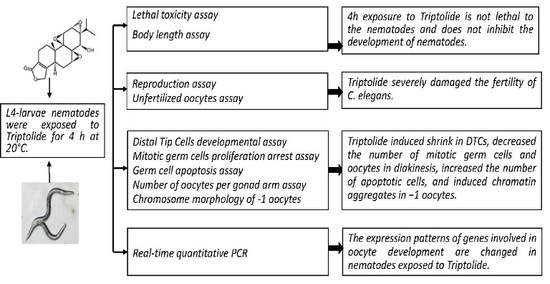
:1. Introduction
2. Results
2.1. Effects of Triptolide on Nematodes Viability and Body Length
2.2. Effects of Triptolide on Nematodes Reproduction
2.3. Effects of Triptolide on the Number of Unfertilized Oocytes
2.4. Effects of Triptolide on the Number and Morphology of Spermatids
2.5. Effects of Triptolide on Spermatids Activation
2.6. Effects of Triptolide on the Development of DTCs
2.7. Effects of Triptolide on Mitotic Germ Cell Proliferation
2.8. Effects of Triptolide on Apoptosis
2.9. Effects of Triptolide on Oocytes in Diakinesis Stage
2.10. Effects of Triptolide on the Expression Patterns of Genes Involved in the Regulation of Oocyte Properties
2.11. Effects of Triptolide on the Expression Patterns of Genes Involved in the Regulation of Spermatids Properties
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Strain Preparation
4.3. Lethal Toxicity and Measurement of Body Length
4.4. Reproduction Assays
4.5. Number of Unfertilized Oocytes
4.6. Number of Spermatids
4.7. Morphology of Spermatids
4.8. Rate of Spermatids Activation In Vitro
4.9. DTC Developmental Assays
4.10. Mitotic Germ Cell Proliferation Arrest Assays
4.11. Germ Cell Apoptosis Assays
4.12. Number of Oocytes Per Gonad Arm and Chromosome Morphology of −1 Oocytes in Diakinesis Stage
4.13. Real-Time qRT-PCR
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Caenorhabditis elegans | C. elegans |
| TP | Triptolide |
| DTC | Distal tip cell |
| DAPI | 4,6-diamidino-2-phenylindole |
References
- Chen, B.J. Triptolide, a novel immune suppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk. Lymphoma 2001, 42, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.K.; Wang, C.Z.; Qian, G.S.; Lin, K.X.; Liu, G. Regulating effect of Triptolide on expression of IL-5, IL-3 and GM-CSF receptors mRNA in BALF eosinophils of asthmatic guinea pigs. Immunol. J. 2002, 18, 102–106. [Google Scholar]
- Qiu, D.; Kao, P. Immunosuppressive and anti-inflammatory mechanisms of Triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook F. Drugs R. D. 2003, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, J.; Gu, Z.; Xu, X.M.; Wang, L.; Pei, X.F.; Yang, J.; Underhill, C.B.; Zhang, L. Triptolide inhibits the growth and metastasis of solid tumors. Mol. Cancer Ther. 2003, 2, 65–72. [Google Scholar] [PubMed]
- Phillips, P.A.; Dudeja, V.; McCarroll, J.A.; Borja-Cacho, D.; Dawra, R.K.; Grizzle, W.E.; Vickers, S.M.; Saluja, A.K. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007, 67, 9407–9416. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Xiong, M.J.; Xu, Y.F.; Su, Y.; Zou, P.; Zhou, H. Triptolide attenuates idiopathic pneumonia syndrome in a mouse bone marrow transplantation model by down-regulation of IL-17. Int. Immunopharmacol. 2012, 14, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.W.; Jiang, Z.Z.; Liu, J.; Tai, T.; Wang, T.; Zhang, L.Y. Inhibition of Triptolide on proliferation in human gastric cancer cells line SGC-7901 and its mechanism. Chin. Tradit. Herb. Drugs 2011, 42, 1174–1176. [Google Scholar]
- Wu, R.; Li, Y.; Guo, Z.; Gong, J.F.; Zhu, W.M.; Li, N.; Li, J.S. Triptolide ameliorates ileocolonic anastomosis inflammation in IL-10 deficient mice by mechanism involving suppression of miR-155/SHIP-1 signaling pathway. Mol. Immunol. 2013, 56, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.Y.; Zhang, C.F.; Huang, G.Y. Effects of Tripterygium Wilfordii Hook F. on menstruation: A clinical analysis of 83 cases. Acta Univ. Med. Tongji 1987, 5, 352–353. [Google Scholar]
- Liu, J.; Jiang, Z.; Liu, L.; Zhang, Y.; Zhang, S.; Xiao, J.; Ma, M.; Zhang, L. Triptolide induces adverse effect on reproductive parameters of female Sprague Dawley rats. Drug Chem. Toxicol. 2011, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. C. elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, E.J.A.; Greenstein, D. Introduction to the germ line. In Wormbook: The C. elegans Research Community; Wormbook: Pasadena, CA, USA, 2005. [Google Scholar]
- Greenstein, D. Control of oocyte meiotic maturation and fertilization. In Wormbook: The C. elegans Research Community; Wormbook: Pasadena, CA, USA, 2005. [Google Scholar]
- Mehlmann, L.M. Stops and starts in mammalian oocytes: The recent advanced in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 2005, 130, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O.; Ellis, R.E.; Horvitz, H.R. Caenorhabditis elegans gene CED-9 protects cells from programmed cell death. Nature 1992, 356, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M.; O’Rourke, K.; Lane, B.R.; Dixit, V.M. Interaction of CED-4 with CED-3 and CED-9: A molecular framework for cell death. Science 1997, 275, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Zhao, Y.; Wu, L.J.; Tang, M.L.; Su, C.X.; Hei, T.K.; Yu, Z.L. Induction of germline cell cycle arrest and apoptosis by sodium arsenite in Caenorhabditis elegans. Chem. Res. Toxicol. 2007, 20, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.L.; Ju, J.J.; Li, Y.H.; Li, X.B.; Liu, R.; Liang, G.Y.; Zhang, J.; Pu, Y.P.; Wang, D.Y.; Yin, L.H. Chlorpyrifos exposure reduced reproductive capacity owing to a damaging effect on gametogenesis in the nematode Caenorhabditis elegans. J. Appl. Toxicol. 2012, 32, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Tian, H.M.; Chu, H.G.; Wu, J.J.; Li, Y.Y.; Wang, Y.H. The effect of tributyltin chloride on Caenorhabditis elegans germline is mediated by a conserved DNA damage checkpoint pathway. Toxicol. Lett. 2014, 225, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, M.; Dai, H.; Hong, W.; Wang, M.D.; Wang, J.J.; Weng, N.Y.; Nie, Y.G.; Xu, A. Endosulfan isomers and sulfate metabolite induced reproductive toxicity in Caenorhabditis elegans involves genotoxic response genes. Environ. Sci. Technol. 2015, 49, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Li, Q.Q.; Shi, J.; Shi, L.L.; Li, B.Q.; Xu, A.; Zhao, G.P.; Wu, L.J. Perfluorooctane sulfonate exposure causes gonadal developmental toxicity in Caenorhabditis elegans through ROS-induced DNA damage. Chemosphere 2016, 155, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.G.; Zhong, W.; Meng, Z.L. The clinical observation of the side effect of Tripterygium wilfordii glycosides tablet. Guangxi Med. J. 2002, 24, 576–579. [Google Scholar]
- Zeng, Y.J.; Li, S.M. The effect of resveratrol on the toxicity induced by Triptolide. J. New Chin. Med. 2014, 46, 172–174. [Google Scholar]
- Wang, R.F.; Hao, L.; Yuan, L.; Yan, J.F.; Zhang, D.F. The effect of Triptolide on apoptosis of cultured rat granulose cell and the protective effect with estrogen therapy. Pharmacol. Clin. Chin. Mater. Med. 2015, 31, 26–29. [Google Scholar]
- Allard, P.; Colaiácovo, M.P. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl. Acad. Sci. USA 2010, 107, 20405–20410. [Google Scholar] [CrossRef] [PubMed]
- Allard, P.; Kleinstreuer, N.C.; Knudsen, T.B.; Colaiácovo, M.P. A C. elegans screening platform for the rapid assessment of chemical disruption of germline function. Environ. Health Perspect. 2013, 121, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsmurugan, K.; Ashokkumar, B.; Moussaif, M.; Sze, J.Y.; Said, H.M. Cloning and functional characterization of a folate transporter from the nematode Caenorhabditis elegans. Am. J. Physiol. Cell Physiol. 2007, 293, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.U.; Liau, W.S.; Balamurugan, K.; Ashokkumar, B.; Said, H.M.; LaMunyon, C.W. Knockout of the folate transporter folt-1 causes germline and somatic defects in C. elegans. BMC Dev. Biol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Shakes, D.C.; Ward, S. Mutations that disrupt the morphogenesis and localization of a sperm-specific organelle in Caenorhabditis elegans. Dev. Biol. 1989, 134, 307–316. [Google Scholar] [CrossRef]
- Gleason, E.J.; Lindsey, W.C.; Kroft, T.L.; Singson, A.W.; L’Hernault, S.W. spe-10 Encodes a DHHC-CRD zinc-finger membrane protein required for endplasmic reticulum/Golgi membrane morphogenesis during Caenorhabditis elegans spermatogenesis. Genetics 2006, 172, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, J.F.; Mandell, M.A.; Moulder, G.; Hill, K.L.; L’Hernault, S.W.; Barstead, R.; Titus, M.A. Myosin VI is required for asymmetric segregation of cellular components during C. elegans spermatogenesis. Curr. Biol. 2000, 10, 1489–1496. [Google Scholar] [CrossRef]
- Luo, S.; Kleemann, G.A.; Ashraf, J.M.; Shaw, W.M.; Murphy, C.T. TGF-β and insulin signaling regulates reproductive aging via oocyte and germline quality maintenance. Cell 2010, 143, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kawasaki, I.; Shim, Y.H. cdc-25.2, A C. elegans ortholog of cdc25, is required to promote oocyte maturation. J. Cell Sci. 2010, 123, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Teng, X.; Wang, Y.; Yu, H.Q.; Luo, X.; Xu, A.; Wu, L. Molecular control of arsenite-induced apoptosis in Caenorhabditis elegans: Roles of insulin-like growth factor-1 signaling pathway. Chemosphere 2014, 112, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Wu, L.J.; Wang, Y.; Luo, X.; Lu, Y. Copper-induced germline apoptosis in Caenorhabditis elegans: The independent roles of DNA damage response signaling and the dependent roles of MAPK cascades. Chem. Biol. Interact. 2009, 180, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Bian, P.; Liang, J.T.; Yang, Y.C.; Li, L.Z.; Wang, J.; Yuan, H.; Chen, S.P.; Xu, A.; Wu, L.J. Synergistic effects induced by a low dose of diesel particulate extract and ultraviolet-A in Caenorhabditis elegans: DNA damage-triggered germ cell apoptosis. Chem. Res. Toxicol. 2015, 27, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, M.M.; Wang, L.; Dai, H.; Wang, M.; Hong, W.; Nie, X.X.; Wu, L.J.; Xu, A. Reproductive toxicity of endosulfan: Implication from germ cell apoptosis modulated by mitochondrial dysfunction and genotoxic response genes in Caenorhabditis elegans. Toxicol. Sci. 2015, 145, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Guardia, Y.D.; Gilliat, A.F.; Hellberg, J.; Rennert, P.; Cabreiro, F.; Gems, D. Run-on of germline apoptosis promotes gonad senescence in C. elegans. Onco_target 2016, 7, 39082–39096. [Google Scholar] [CrossRef] [PubMed]
- Gumienny, T.L.; Lambie, E.; Hartwieg, E.; Horvitz, H.R.; Hengartner, M.O. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 1999, 126, 1011–1022. [Google Scholar] [PubMed]
- Gartner, A.; Milstein, S.; Ahmed, S.; Hodgkin, J.; Hengartner, M.O. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 2000, 5, 435–443. [Google Scholar] [CrossRef]
- Perrin, A.J.; Gunda, M.; Yu, B.; Yen, K.; Ito, S.; Forster, S.; Tissenbaum, H.A.; Derry, W.B. Noncanonical control of C. elegans germline apoptosis by the insulin/IGF-1 and Ras/MAPK signaling pathways. Cell Death Differ. 2013, 20, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.L.; Ragle, J.M.; Conradt, B.; Bhalla, N. Differential regulation of germline apoptosis in response to meiotic checkpoint activation. Genetics 2014, 198, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.N.; Hikim, A.P.; Wang, C.; Stefonovic, K.; Lue, Y.H.; Leung, A.; Atienza, V.; Baravarian, S.; Reutrakul, V.; Swerdloff, R.S. Long-time effects of Triptolide on spermatogenesis, epididymal sperm function, and fertility in male rats. J. Androl. 2000, 21, 689–699. [Google Scholar] [PubMed]
- Ni, B.; Jiang, Z.; Huang, X.; Xu, F.; Zhang, R. Male Reproductive toxicity and toxicokinetics of Triptolide in rats. Arzneimittelforschung 2008, 58, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.L.; Dusenbery, D.B. Aquatic toxicity testing using the nematode, Caenorhabditis elegans. Environ. Toxicol. Chem. 1990, 9, 1285–1290. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Swain, S.C.; Keusekotten, K.; Baumeister, R.; Sturzenbaum, S.R. C. elegans metallothioneins: New insights into the phenotypic effects of cadmium toxicosis. J. Mol. Biol. 2004, 341, 951–959. [Google Scholar] [CrossRef] [PubMed]
- L’Hernault, S.W. The genetics and cell biology of spermatogenesis in the nematode C. elegans. Mol. Cell. Endocrinol. 2009, 306, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, G.; Chatterjee, I.; Marcello, M.R.; Singson, A. Isolation and in vitro activation of Caenorhabditis elegans sperm. Annu. Rev. Cell Dev. Biol. 2007, 23, 405–433. [Google Scholar]
- Stergiou, L.; Doukoumetzidis, K.; Sendoel, A.; Hengartner, M.O. The nucleotide excision repair pathway is required for UV-C-induced apoptosis in C. elegans. Cell Death Differ. 2007, 14, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.O.; Dernburg, A.F.; Stanfield, G.M.; Villeneuve, A.M. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 2000, 156, 617–630. [Google Scholar] [PubMed]
- Shaham, S. Methods in cell biology. In Wormbook: The C. elegans Research Community; Wormbook: Pasadena, CA, USA, 2006. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]












| Gene Primer | Sequence | Annealing Temperature |
|---|---|---|
| mlh-1 | Forward GAGAAGACGATGATGTGGA | 50.3 °C |
| Reverse GAGATGGTAGAGGGAGGTG | ||
| cyb-3 | Forward ATGGTCTCACAATCCCGTC | 62.9 °C |
| Reverse GTGGCATCACCTCCTCTCC | ||
| cdc-25.2 | Forward GCCAGGTGCGTCTGTTCG | 54.2 °C |
| Reverse CGTTTCCGCTGCTGTAGG | ||
| glp-1 | Forward AACTGTTGTCGCTGGTGT | 50.0 °C |
| Reverse TCTCTTGGTATTGGGGTG | ||
| ced-3 | Forward ACGGGAGATCGTGAAAGC | 53.0 °C |
| Reverse AGAGTTGGCGGATGAAGG | ||
| ced-4 | Forward AGTCACTCGCAATGGCTCT | 57.5 °C |
| Reverse GCTGATGAACGACGGAAT | ||
| ced-9 | Forward AAAGGCACAGAGCCCACC | 50.0 °C |
| Reverse CGTTCCCATAACTCGCATC | ||
| folt-1 | Forward TCCATTCCTCACTCCGTTTCTA | 60.4 °C |
| Reverse GCATCTGCCATACTCCTTTACC | ||
| spe-10 | Forward TTTTATTGTCGGCGGAGTGT | 57.8 °C |
| Reverse CGATGACTGCGAACTTTGAG | ||
| spe-15 | Forward GGAGTTTTGGATGTCGCTGGTT | 60.4 °C |
| Reverse GCTCTCTGGGTGAAATGTTGGA | ||
| act-1 | Forward ATGTGTGACGACGAGGTT | 60.4 °C |
| Reverse GAAGCACTTGCGGTGAAC |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Q.; Xu, Y.; Xu, R.; Wang, J.; Hua, Y.; Wang, M.; Duan, J. The Adverse Effects of Triptolide on the Reproductive System of Caenorhabditis elegans: Oogenesis Impairment and Decreased Oocyte Quality. Int. J. Mol. Sci. 2017, 18, 464. https://doi.org/10.3390/ijms18020464
Ruan Q, Xu Y, Xu R, Wang J, Hua Y, Wang M, Duan J. The Adverse Effects of Triptolide on the Reproductive System of Caenorhabditis elegans: Oogenesis Impairment and Decreased Oocyte Quality. International Journal of Molecular Sciences. 2017; 18(2):464. https://doi.org/10.3390/ijms18020464
Chicago/Turabian StyleRuan, Qinli, Yun Xu, Rui Xu, Jiaying Wang, Yongqing Hua, Meng Wang, and Jinao Duan. 2017. "The Adverse Effects of Triptolide on the Reproductive System of Caenorhabditis elegans: Oogenesis Impairment and Decreased Oocyte Quality" International Journal of Molecular Sciences 18, no. 2: 464. https://doi.org/10.3390/ijms18020464
APA StyleRuan, Q., Xu, Y., Xu, R., Wang, J., Hua, Y., Wang, M., & Duan, J. (2017). The Adverse Effects of Triptolide on the Reproductive System of Caenorhabditis elegans: Oogenesis Impairment and Decreased Oocyte Quality. International Journal of Molecular Sciences, 18(2), 464. https://doi.org/10.3390/ijms18020464