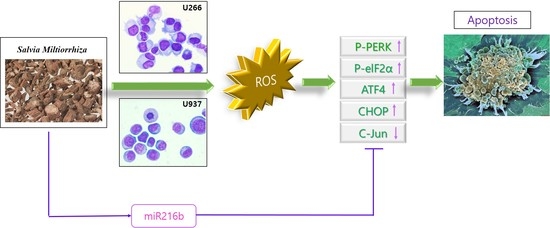
Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salvia miltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells
Abstract
:1. Introduction
2. Results
2.1. Salvia miltiorrhiza (SM) Suppresses the Growth of U266 and U937 Cells in a Concentration-Dependent Manner
2.2. SM Increases Reactive Oxygen Species (ROS) Generation and Cytotoxic Effect Is Dependent on ROS
2.3. SM-Induced ER Stress Mediates Apoptosis
2.4. SM Increases Expression of miR-216b and Decreases Its _target, c-Jun, in U266 and U937 Cells
2.5. Inhibition of miR-216b Reverses SM-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. Western Blot Analysis
4.5. Measurement of ROS Generation
4.6. Real-Time Polymerase Chain Reaction
4.7. SiRNA Transfection
4.8. Statistical Analysis
5. Conclusion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sant, M.; Allemani, C.; Tereanu, C.; de Angelis, R.; Capocaccia, R.; Visser, O.; Marcos-Gragera, R.; Maynadié, M.; Simonetti, A.; Lutz, J.-M. Incidence of hematological malignancies in Europe by morphological subtype: Results of the HAEMACARE project. Blood 2010, 116, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Incidence of haematological malignancy by sub-type: A report from the Haematological Malignancy Research Network. Br. J. Cancer 2011, 105, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.; Rajkumar, S.V. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Oortgiesen, B.E.; Azad, R.; Hemmelder, M.H.; Kibbelaar, R.E.; Veeger, N.J.G.M.; de Vries, J.C.; Roon, E.N.; Hoogendoorn, M. The impact of the introduction of bortezomib on dialysis independence in multiple myeloma patients with renal impairment: a nationwide Dutch population-based study. Haematologica 2018, 15, 184754. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.; Berg, J.W.; Cutler, S.J. Occurrence and prognosis of extranodal lymphomas. Cancer 1972, 29, 252–260. [Google Scholar] [CrossRef]
- Kamdar, M.K.; Smith, S.M. Extranodal Marginal Zone Lymphoma: No Longer Just a Sidekick. J. Clin. Oncol. 2017, 35, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Reed, J.C.; Pellecchia, M. Apoptosis-based therapies for hematologic malignancies. Blood 2005, 106, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Bcl-2–family proteins and hematologic malignancies: History and future prospects. Blood 2008, 111, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Fusetti, L.; Pruneri, G.; Gobbi, A.; Rabascio, C.; Carboni, N.; Peccatori, F.; Martinelli, G.; Bertolini, F. Human myeloid and lymphoid malignancies in the non-obese diabetic/severe combined immunodeficiency mouse model: Frequency of apoptotic cells in solid tumors and efficiency and speed of engraftment correlate with vascular endothelial growth factor production. Cancer Res. 2000, 60, 2527–2534. [Google Scholar] [PubMed]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Debatin, K.-M. Cellular stress response and apoptosis in cancer therapy. Blood 2001, 98, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Czuczman, M. Enhancing activity and overcoming chemoresistance in hematologic malignancies with bortezomib: Preclinical mechanistic studies. Ann. Oncol. 2010, 21, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Miyazawa, K.; Moriya, S.; Ohtomo, T.; Che, X.-F.; Naito, M.; Itoh, M.; Tomoda, A. Combined treatment with bortezomib plus bafilomycin A1 enhances the cytocidal effect and induces endoplasmic reticulum stress in U266 myeloma cells: Crosstalk among proteasome, autophagy-lysosome and ER stress. Int. J. Oncol. 2011, 38, 643–654. [Google Scholar] [PubMed]
- Du, Y.; Wang, K.; Fang, H.; Li, J.; Xiao, D.; Zheng, P.; Chen, Y.; Fan, H.; Pan, X.; Zhao, C. Coordination of intrinsic, extrinsic, and endoplasmic reticulum-mediated apoptosis by imatinib mesylate combined with arsenic trioxide in chronic myeloid leukemia. Blood 2006, 107, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Rouault-Pierre, K.; Lopez-Onieva, L.; Foster, K.; Anjos-Afonso, F.; Lamrissi-Garcia, I.; Serrano-Sanchez, M.; Mitter, R.; Ivanovic, Z.; de Verneuil, H.; Gribben, J. HIF-2α protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell Stem Cell 2013, 13, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Z.; Lawson, B.; Brewer, J.W.; Zinszner, H.; Sanjay, A.; Mi, L.-J.; Boorstein, R.; Kreibich, G.; Hendershot, L.M.; Ron, D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol. 1996, 16, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell. Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kaufman, R. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006, 13, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Moenner, M.; Pluquet, O.; Bouchecareilh, M.; Chevet, E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007, 67, 10631–10634. [Google Scholar] [CrossRef] [PubMed]
- Belmont, P.J.; Chen, W.J.; Thuerauf, D.J.; Glembotski, C.C. Regulation of microRNA expression in the heart by the ATF6 branch of the ER stress response. J. Mol. Cell. Cardiol. 2012, 52, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.-P.; Wang, L.; Han, D.; Wang, E.S.; Huskey, N.E.; Lim, L.; Truitt, M.; McManus, M.T.; Ruggero, D.; Goga, A. IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 2012, 338, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Tharun, S. Roles of eukaryotic Lsm proteins in the regulation of mRNA function. Int. Rev. Cell Mol. Biol. 2008, 272, 149–189. [Google Scholar]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Ann. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ying, H.-Q.; He, B.-S.; Pan, Y.-Q.; Deng, Q.-W.; Sun, H.-L.; Chen, J.; Liu, X.; Wang, S.-K. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Onco_target 2015, 6, 7899. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, Y.-H.; Bae, Y.-S. MiR-186, miR-216b, miR-337-3p, and miR-760 cooperatively induce cellular senescence by _targeting α subunit of protein kinase CKII in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2012, 429, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bu, Y.; Chitnis, N.; Koumenis, C.; Fuchs, S.Y.; Diehl, J.A. MiR-216b regulation of c-Jun mediates GADD153/CHOP-dependent apoptosis. Nat. Commun. 2016, 7, 11422. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, J.; Bao, J.; Lu, J.; Wang, Y. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): A systematic review. Med. Res. Rev. 2014, 34, 768–794. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Jung, J.H.; Jeong, S.J.; Sohn, E.J.; Kim, B.; Kim, S.H. Tanshinone IIA Induces Autophagic Cell Death via Activation of AMPK and ERK and Inhibition of mTOR and p70 S6K in KBM-5 Leukemia Cells. Phytother. Res. 2014, 28, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, S.; Zhang, M.; Wang, W.W.; Zhang, Y.F.; Rehman, K.; Naranmandura, H.; Chen, Z. Anticancer activity in human multiple myeloma U266 cells: Synergy between cryptotanshinone and arsenic trioxide. Metallomics 2013, 5, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Liu, W.-D.; Yang, H.-Z.; Zhang, Y.; Fang, Z.-G.; Liu, P.-Q.; Lin, D.-J.; Xiao, R.-Z.; Hu, Y.; Wang, C.-Z. Inactivation of PI3k/Akt signaling pathway and activation of caspase-3 are involved in tanshinone I-induced apoptosis in myeloid leukemia cells in vitro. Ann. Hematol. 2010, 89, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Blade, J.; Samson, D.; Reece, D.; Apperley, J.; Bjorkstrand, B.; Gahrton, G.; Gertz, M.; Giralt, S.; Jagannath, S.; Vesole, D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br. J. Haematol. 1998, 102, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Gunn, E.J.; Williams, J.T.; Huynh, D.T.; Iannotti, M.J.; Han, C.; Barrios, F.J.; Kendall, S.; Glackin, C.A.; Colby, D.A.; Kirshner, J. The natural products parthenolide and andrographolide exhibit anti-cancer stem cell activity in multiple myeloma. Leuk. Lymphoma 2011, 52, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Suh, N.; Luyengi, L.; Fong, H.H.; Kinghorn, A.D.; Pezzuto, J.M. Discovery of natural product chemopreventive agents utilizing HL-60 cell differentiation as a model. Anticancer Res. 1995, 15, 233–239. [Google Scholar] [PubMed]
- Akaberi, M.; Mehri, S.; Iranshahi, M. Multiple pro-apoptotic _targets of abietane diterpenoids from Salvia species. Fitoterapia 2015, 100, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, S.-H.; Jeong, S.-J.; Sohn, E.J.; Jung, J.H.; Lee, M.H.; Kim, S.-H. Brazilin induces apoptosis and G2/M arrest via inactivation of histone deacetylase in multiple myeloma U266 cells. J. Agric. Food Chem. 2012, 60, 9882–9889. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Ito, T.; Yoshimura, H.; Hotta, M.; Nakanishi, T.; Fujita, S.; Nakaya, A.; Satake, A.; Ishii, K. Evaluation of thrombosis-related biomarkers before and after therapy in patients with multiple myeloma. J. Blood Med. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.A.; Song, H.S.; Kang, B.; Park, M.N.; Park, K.S.; Kim, S.H.; Shim, B.S.; Kim, B. miR-211 Plays a Critical Role in Cnidium officinale Makino Extract-Induced, ROS/ER Stress-Mediated Apoptosis in U937 and U266 Cells. Int. J. Mol. Sci. 2018, 19, 865. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, F.; Wang, Y.; Yao, L.; Qiu, Z.; Wang, T.; Wang, Z.; Yang, F.; Peng, D.; Chen, W. Comparison of the Chromatographic Fingerprint, multi-component quantitation and antioxidant activity of Salvia miltiorrhiza Bge. between sweating and non-sweating. Biomed. Chromatogr. 2018, e4203. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, J.; Song, P.; Wang, X.; Shi, W. Effects of Salvia miltiorrhiza Polysaccharides on Lipopolysaccharide-Induced Inflammatory Factor Release in RAW264.7 Cells. J. Interferon Cytokine Res. 2018, 38, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lobina, C.; Colombo, G.; Gessa, G.L.; Carai, M.A.M.; Allegrini, P.; Morazzoni, P.; Riva, A. Anxiolytic effect of an extract of Salvia miltiorrhiza roots in rats. JCMA 2017, 81, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, A.; Jiao, Y.; Zhao, Y.; Yang, X. Antitumor effect and molecular mechanism of antioxidant polysaccharides from Salvia miltiorrhiza Bunge in human colorectal carcinoma LoVo cells. Int. J. Biol. Macromol. 2018, 108, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Yan, X.; Yang, Y.; Qian, L.; Tian, Y.; Mao, J.H.; Chen, W. The component formula of Salvia miltiorrhiza and Panax ginseng induces apoptosis and inhibits cell invasion and migration through _targeting PTEN in lung cancer cells. Onco_target 2017, 8, 101599–101613. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, L.M. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. N. Y. 2004, 71, 289–297. [Google Scholar]
- Cheng, C.-Y.; Su, C.-C. Tanshinone IIA may inhibit the growth of small cell lung cancer H146 cells by up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial membrane potential. Mol. Med. Rep. 2010, 3, 645–650. [Google Scholar] [PubMed]
- Rouschop, K.M.; Dubois, L.J.; Keulers, T.G.; van den Beucken, T.; Lambin, P.; Bussink, J.; van der Kogel, A.J.; Koritzinsky, M.; Wouters, B.G. PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc. Natl. Acad. Sci. USA 2013, 110, 4622–4627. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; GuiLian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.E.; Cleary, P.; Saveljeva, S.; Samali, A. New directions in ER stress-induced cell death. Apoptosis 2013, 18, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Tang, H.; Zhou, Y.; Zhou, M.; Xiong, W.; Zheng, Y.; Ye, Q.; Zeng, X.; Liao, Q.; Guo, X.; et al. miR-216b suppresses tumor growth and invasion by _targeting KRAS in nasopharyngeal carcinoma. J. Cell Sci. 2011, 124 Pt 17, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.A.; Zhang, Y.; Zheng, Z.; Li, K.; Wu, X.H.; Du, Q.G.; Ye, X.; Wang, L.; Zhu, L. MicroRNA-216b reduces growth, migration and invasion of pancreatic ductal adenocarcinoma cells by directly _targeting ρ-associated coiled-coil containing protein kinase 1. Oncol. Lett. 2018, 15, 6745–6751. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, R.; Johnson, R.S.; Moore, C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999, 18, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Baumann, B.; Cotten, M.; Angel, P.; Wagner, E. Fos is an essential component of the mammalian UV response. EMBO J. 1995, 14, 5338. [Google Scholar] [PubMed]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Spencer, J.P.; Catherine, R.-E.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Olson, D.; Cheng, B.; Guo, X.; Wang, K. Sanguis Draconis resin stimulates osteoblast alkaline phosphatase activity and mineralization in MC3T3-E1 cells. J. Ethnopharmacol. 2012, 142, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhang, G.; Su, Z.; Ouyang, F. Identification of major active constituents in the fingerprint of Salvia miltiorrhiza Bunge developed by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1041, 239–243. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Song, H.-S.; Park, H.; Kim, B. Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salvia miltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells. Int. J. Mol. Sci. 2018, 19, 1240. https://doi.org/10.3390/ijms19041240
Kim C, Song H-S, Park H, Kim B. Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salvia miltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells. International Journal of Molecular Sciences. 2018; 19(4):1240. https://doi.org/10.3390/ijms19041240
Chicago/Turabian StyleKim, Changmin, Hyo-Sook Song, Hojung Park, and Bonglee Kim. 2018. "Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salvia miltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells" International Journal of Molecular Sciences 19, no. 4: 1240. https://doi.org/10.3390/ijms19041240
APA StyleKim, C., Song, H.-S., Park, H., & Kim, B. (2018). Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salvia miltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells. International Journal of Molecular Sciences, 19(4), 1240. https://doi.org/10.3390/ijms19041240