Erythropoietin Intensifies the Proapoptotic Activity of LFM-A13 in Cells and in a Mouse Model of Colorectal Cancer
Abstract
:1. Introduction
2. Results
2.1. Erythropoietin in Combination with LFM-A13 Decreases Colon Cancer Viability
2.2. Erythropoietin in Combination with LFM-A13 Induces Apoptosis through Intracellular Signaling Pathway Attenuation
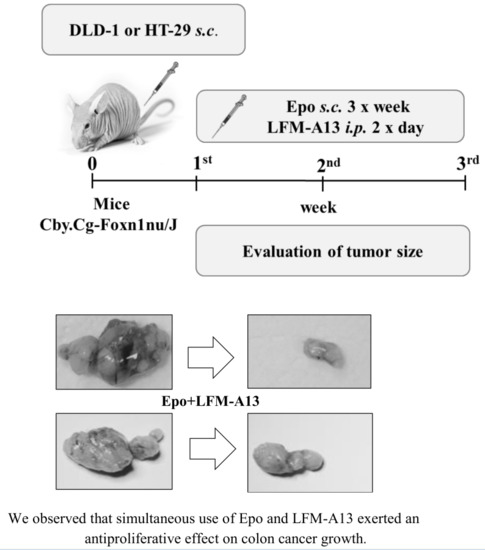
2.3. Anti-Colon Cancer Activity of the Simultaneous Use of Epo and LFM-A13 in Mouse Xenografts
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Cultures
4.3. Exogenous Erythropoietin and LFM-A13 Administration
4.4. Cell Viability Assay
4.5. Western Blotting
4.6. Flow Cytometry Assessment of Annexin V Binding
4.7. Analysis of Mitochondrial Membrane Potential
4.8. Establishment of Xenograft
4.9. Immunocytochemistry
4.10. Statistical Analysis
5. Conclusions
6. Patents
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| BAX | apoptotic protein |
| BCL-2 | antiapoptotic protein |
| βcR β | β common receptor |
| BTK | Bruton’s tyrosine kinase |
| BT474 | cell line of human ductal carcinoma |
| DLD-1 | cell line of human colorectal adenocarcinoma |
| EphB4 | epinephrine B4 receptor |
| Epo | erythropoietin |
| EpoR | erythropoietin receptor |
| FAS | Fas-associated protein with death domain |
| FADD | FLICE inhibitory proteins: regulators of death receptor-mediated apoptosis |
| GM-CSF | granulocyte–macrophage colony stimulation factor |
| HER2 | human epidermal growth factor 2 |
| HT-29 | cell line of human colorectal adenocarcinoma |
| IL | interleukin |
| JAK2 | Janus kinase 2 |
| LFM-A13 | Bruton’s tyrosine kinase inhibitor |
| MAPK | mitogen-activated protein kinases |
| MCF-7 | cell line of human breast cancer |
| MMP | mitochondrial membrane potential |
| MYC | a regulator gene that codes for a transcription factor |
| NF-κB | nuclear factor κ-light-chain-enhancer of activated B cells |
| p-Akt | phosphorylated protein kinase B |
| PH | pleckstrin homology |
| PI3K | phosphoinositide 3-kinase |
| VEGF | vascular endothelial growth factor |
References
- Watson, A.J. Apoptosis and colorectal cancer. Gut 2004, 53, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, J. Role of apoptosis in colon cancer biology, therapy, and prevention. Curr. Colorectal Cancer Rep. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, O.J.; Uckun, F.M. Novel Bruton’s tyrosine kinase inhibitors currently in development. Onco-_targets Ther. 2013, 6, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, A.; Ozer, Z.; Navara, C.; Mahajan, S.; Uckun, F.M. Bruton’s tyrosine kinase as an inhibitor of the Fas/CD95 death-inducing signaling complex. J. Biol. Chem. 1999, 274, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M.; Zheng, Y.; Cetkovic-Cvrlje, M.; Vassilev, A.; Lisowski, E.; Waurzyniak, B.; Chen, H.; Carpenter, R.; Chen, C.L. In vivo pharmacokinetic features, toxicity profile, and chemosensitizing activity of α-cyano-β-hydroxy-β-methyl-N-(2,5-dibromophenyl)propenamide (LFM-A13), a novel antileukemic agent _targeting Bruton’s tyrosine kinase. Clin. Cancer Res. 2002, 8, 1224–1233. [Google Scholar] [PubMed]
- Hata, D.; Kitaura, J.; Hartman, S.E.; Kawakami, Y.; Yokota, T.; Kawakami, T. Bruton’s tyrosine kinase-mediated interleukin-2 gene activation in mast cells. Dependence on the c-Jun N-terminal kinase activation pathway. J. Biol. Chem. 1998, 273, 10979–10987. [Google Scholar] [CrossRef] [PubMed]
- Kokabee, L.; Wang, X.; Sevinsky, C.J.; Wang, W.L.; Cheu, L.; Chittur, S.V.; Karimipoor, M.; Tenniswood, M.; Conklin, D.S. Bruton’s tyrosine kinase is a potential therapeutic _target in prostate cancer. Cancer Biol. Ther. 2015, 16, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bouchlaka, M.N.; Wolff, J.; Grindle, K.M.; Lu, L.; Qian, S.; Zhong, X.; Pflum, N.; Jobin, P.; Kahl, B.S.; et al. FBXO10 deficiency and BTK activation upregulate BCL2 expression in mantle cell lymphoma. Oncogene 2016, 35, 6223–6234. [Google Scholar] [CrossRef] [PubMed]
- Cinar, M.; Hamedani, F.; Mo, Z.; Cinar, B.; Amin, H.M.; Alkan, S. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by Ibrutinib induces apoptosis. Leuk. Res. 2013, 37, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Van den Akker, E.; van Dijk, T.B.; Schmidt, U.; Felida, L.; Beug, H.; Löwenberg, B.; von Lindern, M. The Btk inhibitor LFM-A13 is a potent inhibitor of Jak2 kinase activity. Biol. Chem. 2004, 385, 409–413. [Google Scholar] [PubMed]
- Schmidt, U.; van den Akker, E.; Parren-van Amelsvoort, M.; Litos, G.; de Bruijn, M.; Gutiérrez, L.; Hendriks, R.W.; Ellmeier, W.; Löwenberg, B.; Beug, H.; et al. Btk is required for an efficient response to erythropoietin and for SCF-controlled protection against TRAIL in erythroid progenitors. J. Exp. Med. 2004, 199, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Acs, G.; Acs, P.; Beckwith, S.M.; Pitts, R.L.; Clements, E.; Wong, K.; Verma, A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001, 61, 3561–3565. [Google Scholar] [PubMed]
- Dicato, M.; Plawny, L.; Diederich, M. Anemia in cancer. Ann. Oncol. 2010, 21, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz-Kwedlo, A.; Hermanowicz, J.M.; Domaniewski, T.; Pawlak, K.; Rusak, M.; Pryczynicz, A.; Surazynski, A.; Kaminski, T.; Kazberuk, A.; Pawlak, D. Simultaneous use of erythropoietin and LFM-A13 as a new therapeutic approach for colorectal cancer. Br. J. Pharmacol. 2018, 175, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kim, J. PLK-1 _targeted Inhibitors and Their Potential against Tumorigenesis. BioMed Res. Int. 2015, 2015, 705745. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M.; Dibirdik, I.; Qazi, S.; Vassilev, A.; Ma, H.; Mao, C.; Benyumov, A.; Emami, K.H. Anti-breast cancer activity of LFM-A13, a potent inhibitor of Polo-like kinase (PLK). Bioorg. Med. Chem. 2007, 15, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, R.; Bhardwaj, G.; Yang, J.C.; Changou, C.; Ma, A.H.; Mazloom, A.; Chintapalli, S.; Xiao, K.; Xiao, W.; et al. _targeting BTK/Etk of prostate cancer cells by a novel dual inhibitor. Cell Death Dis. 2014, 5, e1409. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz-Kwedlo, A.; Pawlak, D.; Domaniewski, T.; Buczko, W. Effect of erythropoietin, 5-fluorouracil and SN-38 on the growth of DLD-1 cells. Pharmacol. Rep. 2010, 62, 926–937. [Google Scholar] [CrossRef]
- Brunelle, J.K.; Zhang, B. Apoptosis assays for quantifying the bioactivity of anticancer drug products. Drug Resist. Updates 2010, 13, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Kałuża, Z.; Bielawska, A.; Gabryel-Porowska, H.; Czarnomysy, R.; Bielawski, K. Cytotoxic efficacy of a novel dinuclear platinum(II) complex used with anti-MUC1 in human breast cancer cells. Mol. Cell. Biochem. 2014, 392, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Teijido, O.; Dejean, L. Upregulation of Bcl2 inhibits apoptosis-driven BAX insertion but favors BAX relocalization in mitochondria. FEBS Lett. 2010, 584, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Boersma, A.W.; Nooter, K.; Burger, H.; Kortland, C.J.; Stoter, G. BAX upregulation is an early event in cisplatin-induced apoptosis in human testicular germ-cell tumor cell line NT2, as quantitated by flow cytometry. Cytometry 1997, 27, 275–282. [Google Scholar] [CrossRef]
- Novero, A.; Ravella, P.M.; Chen, Y.; Dous, G.; Liu, D. Ibrutinib for B cell malignancies. Exp. Hematol. Oncol. 2014, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.E.; Gordon, A.L.; Hertlein, E.; Ramanunni, A.; Zhang, X.; Jaglowski, S.; Flynn, J.; Jones, J.; Blum, K.A.; Buggy, J.J.; et al. Bruton tyrosine kinase represents a promising therapeutic _target for treatment of chronic lymphocytic leukemia and is effectively _targeted by PCI-32765. Blood 2011, 117, 6287–6296. [Google Scholar] [CrossRef] [PubMed]
- Trost, N.; Stepisnik, T.; Berne, S.; Pucer, A.; Petan, T.; Komel, R.; Debeljak, N. Recombinant human erythropoietin alters gene expression and stimulates proliferation of MCF-7 breast cancer cells. Radiol. Oncol. 2013, 47, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Perelman, N.; Luc, L.R.; Shimada, H.; Malik, P. Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Lab. Investig. 2003, 83, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Spets, H.; Strömberg, T.; Georgii-Hemming, P.; Siljason, J.; Nilsson, K.; Jernberg-Wiklund, H. Expression of the BCL-2 family of pro- and anti-apoptotic genes in multiple myeloma and normal plasma cells: Regulation during interleukin-6(IL-6)-induced growth and survival. Eur. J. Haematol. 2002, 69, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz-Kwedlo, A.; Hermanowicz, J.M.; Surażyński, A.; Kwedlo, W.; Rożkiewicz, D.; Pawlak, K.; Domaniewski, T.; Pawlak, D. Erythropoietin enhances the cytotoxic effect of hydrogen peroxide on colon cancer cells. Curr. Pharm. Biotechnol. 2017, 18, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Chen, X.Y.; Ji, K.W.; Tao, F. _targeting Btk with ibrutinib inhibit gastric carcinoma cells growth. Am. J. Transl. Res. 2016, 8, 3003–3012. [Google Scholar] [PubMed]
- Qiu, Y.; Kung, H.J. Signaling network of the BTK family kinases. Oncogene 2000, 19, 5651–5661. [Google Scholar] [CrossRef] [PubMed]
- Eifert, C.; Wang, X.; Kokabee, L.; Kourtidis, A.; Jain, R.; Gerdes, M.J.; Conklin, D.S. A novel isoform of the B cell tyrosine kinase BTK protects breast cancer cells from apoptosis. Genes Chromosomes Cancer 2013, 52, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Grassilli, E.; Pisano, F.; Cialdella, A.; Bonomo, S.; Missaglia, C.; Cerrito, M.G.; Masiero, L.; Ianzano, L.; Giordano, F.; Cicirelli, V.; et al. A novel oncogenic BTK isoform is overexpressed in colon cancers and required for RAS-mediated transformation. Oncogene 2016, 35, 4368–4378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althubiti, M.; Rada, M.; Samuel, J.; Escorsa, J.M.; Najeeb, H.; Lee, K.G.; Lam, K.P.; Jones, G.D.; Barlev, N.A.; Macip, S. BTK Modulates p53 Activity to Enhance Apoptotic and Senescent Responses. Cancer Res. 2016, 76, 5405–5414. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Althubiti, M.; Ekpenyong-Akiba, A.E.; Lee, K.G.; Lam, K.P.; Fedorova, O.; Barlev, N.A.; Macip, S. BTK blocks the inhibitory effects of MDM2 on p53 activity. Onco_target 2017, 8, 106639–106647. [Google Scholar] [CrossRef] [PubMed]
- Arcasoy, M.O.; Amin, K.; Karayal, A.F.; Chou, S.C.; Raleigh, J.A.; Varia, M.A.; Haroon, Z.A. Functional significance of erythropoietin receptor expression in breast cancer. Lab. Investig. 2002, 82, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W.; Bohlius, J.; Hallek, M.; Sytkowski, A.J. The erythropoietin receptor in normal and cancer tissues. Crit. Rev. Oncol. Hematol. 2008, 67, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Su, K.H.; Shyue, S.K.; Kou, Y.R.; Ching, L.C.; Chiang, A.N.; Yu, Y.B.; Chen, C.Y.; Pan, C.C.; Lee, T.S. β Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J. Cell. Physiol. 2011, 226, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Sautina, L.; Sautin, Y.; Beem, E.; Zhou, Z.; Schuler, A.; Brennan, J.; Zharikov, S.I.; Diao, Y.; Bungert, J.; Segal, M.S. Induction of nitric oxide by erythropoietin is mediated by the {β} common receptor and requires interaction with VEGF receptor 2. Blood 2010, 115, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Balleari, E.; Clavio, M.; Arboscello, E.; Bellodi, A.; Bruzzone, A.; Del Corso, L.; Lucchetti, M.V.; Miglino, M.; Passalia, C.; Pierri, I.; et al. Weekly standard doses of rh-EPO are highly effective for the treatment of anemic patients with low-intermediate 1 risk myelodysplastic syndromes. Leuk. Res. 2011, 35, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Baumgart-Vogt, E.; Naidu, S.; Qian, G.; Immenschuh, S. Bruton’s tyrosine kinase is required for TLR-dependent heme oxygenase-1 gene activation via Nrf2 in macrophages. J. Immunol. 2011, 187, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M. Chemosensitizing anti-cancer activity of LFM-A13, a leflunomide metabolite analog _targeting polo-like kinases. Cell Cycle 2007, 6, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.; Wang, Y.; Rhim, J.S.; Bhattacharya, N.; Loda, M.; Sytkowski, A.J. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate 2006, 66, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Westenfelder, C.; Baranowski, R.L. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000, 58, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Törnqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Öberg, M. Strategic Focus on 3R Principles Reveals Major Reductions in the Use of Animals in Pharmaceutical Toxicity Testing. PLoS ONE 2014, 9, e101638. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz-Kwedlo, A.; Hermanowicz, J.; Surażynski, A.; Rożkiewicz, D.; Pryczynicz, A.; Domaniewski, T.; Pawlak, K.; Kemona, A.; Pawlak, D. Erythropoietin accelerates tumor growth through increase of erythropoietin receptor (EpoR) as well as by the stimulation of angiogenesis in DLD-1 and Ht-29 xenografts. Mol. Cell. Biochem. 2016, 421, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tankiewicz-Kwedlo, A.; Hermanowicz, J.M.; Pawlak, K.; Czarnomysy, R.; Bielawski, K.; Prokop, I.; Pawlak, D. Erythropoietin Intensifies the Proapoptotic Activity of LFM-A13 in Cells and in a Mouse Model of Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 1262. https://doi.org/10.3390/ijms19041262
Tankiewicz-Kwedlo A, Hermanowicz JM, Pawlak K, Czarnomysy R, Bielawski K, Prokop I, Pawlak D. Erythropoietin Intensifies the Proapoptotic Activity of LFM-A13 in Cells and in a Mouse Model of Colorectal Cancer. International Journal of Molecular Sciences. 2018; 19(4):1262. https://doi.org/10.3390/ijms19041262
Chicago/Turabian StyleTankiewicz-Kwedlo, Anna, Justyna Magdalena Hermanowicz, Krystyna Pawlak, Robert Czarnomysy, Krzysztof Bielawski, Izabela Prokop, and Dariusz Pawlak. 2018. "Erythropoietin Intensifies the Proapoptotic Activity of LFM-A13 in Cells and in a Mouse Model of Colorectal Cancer" International Journal of Molecular Sciences 19, no. 4: 1262. https://doi.org/10.3390/ijms19041262
APA StyleTankiewicz-Kwedlo, A., Hermanowicz, J. M., Pawlak, K., Czarnomysy, R., Bielawski, K., Prokop, I., & Pawlak, D. (2018). Erythropoietin Intensifies the Proapoptotic Activity of LFM-A13 in Cells and in a Mouse Model of Colorectal Cancer. International Journal of Molecular Sciences, 19(4), 1262. https://doi.org/10.3390/ijms19041262