Comparative Computational Studies of 3,4-Dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitrile Derivatives as Potential Antinociceptive Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antinociceptive Activity
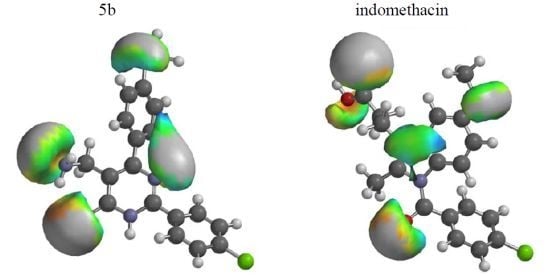
2.2. Molecular Modeling Studies
3. Experimental
3.1. 3,4-Dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitriles
3.2. Animals
3.3. Antinociceptive Activity
3.4. Computational Methods
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Brown, D.J. Pyrimidines and their benzoderivatives. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 3, pp. 57–155. [Google Scholar]
- Singh, A.K. Analytical Reactions of Substituted Pyrimidines. Talanta 1982, 29, 95–102. [Google Scholar] [CrossRef]
- Stringfellow, D.A. Antineoplastic properties of pyrimidinone interferon inducers. Adv. Enzyme Regul. 1981, 19, 335–348. [Google Scholar] [CrossRef]
- Marquet, R.L.; Eggermont, A.M.M.; de Bruin, R.W.F.; Fiers, W.; Jeekel, J. Combined treatment of colon adenocarcinoma in rats with tumor necrosis factor and the interferon inducer ABPP. J. Interferon Res. 1988, 8, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Wallace, T.L.; Wierenga, W.; Skulnick, H.I.; DeKoning, T.F. Antitumor activity of pyrimidinones, a class of small-molecule biological response modifiers. J. Biol. Response Mod. 1987, 6, 44–55. [Google Scholar] [PubMed]
- Scheringa, M.; Ijzermans, J.N.; Jeekel, J.; Marquet, R.L. The antitumor activity of the interferon inducer bropirimine is partially mediated by endogenous tumor necrosis factor α. Cancer Immunol. Immunother. 1990, 32, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Oh-Hashi, F.; Tsukagoshi, S.; Iwaguchi, T.; Kataoka, T. In vitro and in vivo antitumor activity of the interferon inducer bropirimine. Anticancer Drugs 1995, 6, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Vroegop, S.M.; Chapman, D.L.; Decker, D.E.; Galinet, L.A.; Brideau, R.J.; Ready, K.A.; Dunn, C.J.; Buxser, S.E. Pharmacokinetic properties, induction of interferon, and efficacy of selected 5-halo-6-phenyl pyrimidinones, bropirimine analogues, in a model of severe experimental autoimmune encephalomyelitis. Int. J. Immunopharmacol. 1999, 21, 647–662. [Google Scholar] [CrossRef]
- Saladino, R.; Ciambecchini, U.; Maga, G.; Mastromarino, P.; Conti, C.; Botta, M.A. A new and efficient synthesis of substituted 6-[(2′-dialkylamino)ethyl] pyrimidine and 4-N,N-dialkyl-6-vinyl-cytosine derivatives and evaluation of their anti-rubella activity. Bioorg. Med. Chem. Lett. 2002, 10, 2143–2153. [Google Scholar] [CrossRef]
- de Lucca, G.V.; Liang, J.; de Lucca, I. Stereospecific synthesis, structure-activity relationship, and oral bioavailability of tetrahydropyrimidin-2-one HIV protease inhibitors. J. Med. Chem. 1999, 42, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Bernhart, C.A.; Haudricourt, F.B.; Assens, J.L.; Gougat, J.; Lacour, C.; Roccon, A.; Cazaubon, C.; Brelière, J.C.; Le Fur, G.; Nisato, D. Cyclopentanespiro-3H-dihydro-pyrimidinones as Angiotensin II AT1 receptor antagonists. Bioorg. Med. Chem. Lett. 1994, 4, 157–162. [Google Scholar] [CrossRef]
- Salimbeni, A.; Canevotti, R.; Paleari, F.; Poma, D.; Caliari, S.; Fici, F.; Cirillo, R.; Renzetti, A.R.; Subissi, A.; Belvisi, L.; Bravi, G.; Scolastico, C.; Giachetti, A. N-3-substituted pyrimidinones as potent, orally active, AT1 selective angiotensin II receptor antagonists. J. Med. Chem. 1995, 38, 4806–4820. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, G.R.; Chakrabarti, R.; Vikramadithyan, R.K.; Mamidi, R.N.; Balraju, V.; Rajesh, B.M.; Misra, P.; Kumar, S.K.; Lohray, B.B.; Lohray, V.B.; Rajagopalan, R. Synthesis and biological activity of novel pyrimidinone containing thiazolidinedione derivatives. Bioorg. Med. Chem. 2002, 10, 2671–2680. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Wakasugi, K.; Saito, R.; Adachi, Y.; Yoshikawa, Y.; Sakurai, H.; Katoh, A. Syntheses of vanadyl and zinc(II) complexes of 1-hydroxy-4,5,6-substituted 2(1H)-pyrimidinones and their insulin-mimetic activities. J. Inorg. Biochem. 2006, 100, 260–269. [Google Scholar] [CrossRef] [PubMed]
- White, D.C.; Greenwood, T.D.; Downey, A.L.; Bloomquist, J.R.; Wolfe, J.F. Synthesis and anticonvulsant evaluation of some new 2-substituted-3-arylpyrido[2,3-d]pyrimidinones. Bioorg. Med. Chem. 2004, 12, 5711–5717. [Google Scholar] [CrossRef] [PubMed]
- Temple, D.L.; Yevich, J.P.; Covington, R.R.; Hanning, C.A.; Seidehamel, R.J.; Mackey, H.K.; Bartek, M.J. Synthesis of 3,4-dihydro-4-oxothieno[2,3-d]pyrimidine-2-carboxylates, a new series of orally active antiallergy agents. J. Med. Chem. 1979, 22, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ranise, A.; Bruno, O.; Bondavalli, F.; Schenone, S.; D’Amico, M.; Falciani, M.; Filippelli, W.; Rossi, F. 5-Substituted 2,3-dihydro-6-mercapto-1,3-diphenyl-2-thioxo-4(3H)-pyrimidinones and their 6-(acylthio) derivatives with platelet antiaggregating, antiinflammatory, antiarrhythmic, antihyperlipidemic and other activities. Farmaco 1994, 49, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Abignente, E.; Sacchi, A.; Laneri, S.; Rossi, F.; D’Amico, M.; Berrino, L.; Calderaro, V.; Parrillo, C. Research on heterocyclic compounds. XXXII. Synthesis and cyclooxygenase-independent antiinflammatory and analgesic activity of imidazo[1,2-a]pyrimidine derivatives. Eur. J. Med. Chem. 1994, 29, 279–286. [Google Scholar] [CrossRef]
- dos Anjos, J.V.; Mendonça, F.J.B., Jr.; Costa-Silva, J.H.; de Souza, I.A.; de Melo, S.J. Estudo Preliminar da Toxicidade e das Atividades Anti-edematogênica e Anti-nociceptiva da 3,4-diidro-2-fenil-6-para-flúor-fenil-4-oxo-pirimidina-5-carbonitrila. Lat. Am. J. Pharm. 2008, 27, 343. [Google Scholar]
- Skulnick, H.I.; Ludens, J.H.; Wendling, M.G.; Glenn, E.M.; Rohloff, N.A.; Smith, R.J.; Wierenga, W. Pyrimidinones. 3. N-substituted 6-phenylpyrimidinones and pyrimidinediones with diuretic/hypotensive and antiinflammatory activity. J. Med. Chem. 1986, 29, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Ranise, A.; Bruno, O.; Schenone, S.; Bondavalli, F.; Falcone, G.; Filippelli, W.; Sorrentino, S. Synthesis of 6-thiosubstituted 5-ethoxycarbonyl-1,3-diphenyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-ones, 6-substituted 5-hydroxy-1,3-diphenyl-2,3-dihydrothieno[2,3-d]pyrimidin-4(1H)-ones and their esters with local anesthetic, antiarrhythmic, antiinflammatory and analgesic activities. Farmaco 1997, 52, 547–555. [Google Scholar] [PubMed]
- Modica, M.; Santagati, M.; Santagati, A.; Cutuli, V.; Mangano, N.; Caruso, A. Synthesis of new [1,3,4]thiadiazolo[3,2-a]thieno[2,3-d]pyrimidinone derivatives with antiinflammatory activity. Pharmazie 2000, 55, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.G.E.; Sabry, N.M.; Abdulla, M.M. Synthesis, Reactions, and Anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh. Chem. 2007, 138, 699–707. [Google Scholar] [CrossRef]
- Mendonça, F.J.B., Jr.; dos Anjos, J.V.; Falcão, E.P.S.; Yamamoto, A.P.; de Melo, S.J.; Srivastava, R.M. A simple approach for the synthesis of 2,6-diaryl-4-oxo-3,4-dihydropyrimidine-5-carbonitriles. Heterocycl. Commun. 2005, 11, 479–484. [Google Scholar] [CrossRef]
- Wierenga, W. Antiviral and other bioactivities of pyrimidinones. Pharmacol. Ther. 1985, 30, 67–89. [Google Scholar] [CrossRef]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- de Melo, S.J.; Luu-Duc, C.; Thomasson, F.; Narcisse, G.; Gaultier, C. 5-Fluoro (3H) pyrimidine-4-ones: synthesis, reactivity and pharmacological properties. Ann. Pharm. Fr. 1992, 50, 39–51. [Google Scholar] [PubMed]
- Agarwal, S.K.; Tadiparthi, R.; Aggarwal, P.; Shivakumar, S.; Dey, D.; Nag, B. New diaryl pyrimidinone derivatives are tumor necrosis factor alpha inhibitors, useful for the treatement of rheumatoid arthritis, osteoporosis, multiple myeloma and ischemic heart disease. International Patent WO/2003/084935, 10 April 2003. [Google Scholar]
- Devadas, B.; Hartmann, S.J.; Heier, R.F.; Jerome, K.D.; Kolodziej, S.A.; Mathias, J.P.; Norton, M.B.; Promo, M.A.; Rucker, P.V.; Selness, S.R. New substituted pyridinone pyrazole urea and pyrimidinone pyrazole urea compounds useful for treating e.g. astma, inflammation and rheumatoid arthritis. International Patent WO/2007/091176, 5 February 2007. [Google Scholar]
- Liang, C.; Koenig, M. New pyrimidinones derivatives for treating disordes related to abnormal protein kinase activities e.g., cancer and inflammation. International Patent WO/2007/081901, 5 January 2007. [Google Scholar]
- Lu, Y.; Xiang, T.; Bartberger, M.D.; Bernard, C.; Bostick, T.; Huang, L.; Liu, L.; Siegmund, A.; Sukay, G.; Guo, G.; et al. An efficient one-pot construction of substituted pyrimidinones. Tetrahedron 2006, 62, 11714–11723. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, B.; Lin, A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol. Sci. 2007, 28, 286–295. [Google Scholar] [CrossRef] [PubMed]
- de Melo, S.J.; dos Santos, L.C.; Falcão, E.P.S.; Srivastava, R.M.; Luu-Duc, C. Synthesis of new 4-amino-2,6-diarylpyrimidine-5-carbonitriles. J. Chem. Res. 2002, 5, 216–217. [Google Scholar] [CrossRef]
- Falcão, E.P.S.; de Melo, S.J.; Srivastava, R.M.; Catanho, M.T.J.A.; do Nascimento, S.C. Synthesis and antiinflammatory activity of 4-amino-2-aryl-5-cyano-6-{3- and 4-(N-phthalimidophenyl)} pyrimidines. Eur. J. Med. Chem. 2006, 41, 276–282. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.B.P.; Ramos, M.N.; Barros Neto, B.; de Melo, S.J.; Falcão, E.P.S.; Catanho, M.T.J.A. Quantitative Structure Activity Relationships (QSAR) of 4-Amino-2,6-Diarylpyrimidine-5-Carbonitriles Having Anti-inflammatory Activity. J. Braz. Chem. Soc. 2008, 19, 337. [Google Scholar] [CrossRef]
- Braggio, M.M.; Lima, M.E.L.; Veasey, E.A.; Haraguchi, M. Atividades farmacológicas das folhas de Sesbania virgata (CAV.) PERS. Arq. Inst. Biol. (Sao Paulo) 2002, 69, 49–53. [Google Scholar]
- Dannhardt, G.; Kiefer, W. Cyclooxygenase inhibitors-current status and future prospects. Eur. J. Med. Chem. 2001, 36, 109–126. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, M.; de Beer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–416. [Google Scholar]
- Kruger, L. Methods in Pain Research; CRC Press: Los Angeles, CA, USA, 2001; pp. 11–39. [Google Scholar]
- Hyperchem Program Release 8.0 for Windows; Hybercube, Inc.: Gainesville, FL, USA, 2009.
- Allinger, N.L. A hydrocarbon force-field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Dewar, M.J.S.E.; Zoebisch, G.; Healy, E.F.; Stewart, J.J.P. AM1: A new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Cohen, N.C. Guidebook on Molecular Modeling in Drug Design; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Leach, A.R. Molecular Modeling: Principles and Applications; Prentice Hall: London, UK, 2001. [Google Scholar]
- SpartanModel; Wavefunction, Inc.: Irvine, CA, USA, 2009; Available online: http://www.wavefun.com/products/windows/SpartanModel/win_model.html (accessed on 10 May 2011).
Sample Availability: Samples of the compounds 5a–i are available from the authors. |



| Compd. | Dose (mg/kg) | Antinociceptive activity (%) |
|---|---|---|
| 5a | 25 | - |
| 50 | 71.5 ± 6.9 | |
| 5b | 25 | 49.5 ± 9.9 |
| 50 | 88.6 ± 3.4 | |
| 5c | 25 | - |
| 50 | 75.1 ± 5.8 | |
| 5d | 25 | 68.8 ± 8.2 |
| 50 | 86.0 ± 4.1 | |
| 5e | 25 | 56.4 ± 11.9 |
| 50 | 73.9 ± 10.0 | |
| 5f | 25 | 22.1 ± 16.1 |
| 50 | 71.4 ± 8.5 | |
| 5g | 25 | 57.7 ± 9.9 |
| 50 | 70.0 ± 5.4 | |
| 5h | 25 | 47.4 ± 13.0 |
| 50 | 61.0 ± 13.7 | |
| 5i | 25 | 85.4 ± 4.6 |
| 50 | 88.0 ± 4.0 | |
| Indomethacin | 10 | 76.3 ± 4.8 |
© 2012 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anjos, J.V.d.; Srivastava, R.M.; Costa-Silva, J.H.; Scotti, L.; Scotti, M.T.; Wanderley, A.G.; Leite, E.S.; Melo, S.J.d.; Junior, F.J.B.M. Comparative Computational Studies of 3,4-Dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitrile Derivatives as Potential Antinociceptive Agents. Molecules 2012, 17, 809-819. https://doi.org/10.3390/molecules17010809
Anjos JVd, Srivastava RM, Costa-Silva JH, Scotti L, Scotti MT, Wanderley AG, Leite ES, Melo SJd, Junior FJBM. Comparative Computational Studies of 3,4-Dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitrile Derivatives as Potential Antinociceptive Agents. Molecules. 2012; 17(1):809-819. https://doi.org/10.3390/molecules17010809
Chicago/Turabian StyleAnjos, Janaína V. dos, Rajendra M. Srivastava, João H. Costa-Silva, Luciana Scotti, Marcus T. Scotti, Almir G. Wanderley, Elisa Soares Leite, Sebastião J. de Melo, and Francisco J. B. Mendonça Junior. 2012. "Comparative Computational Studies of 3,4-Dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitrile Derivatives as Potential Antinociceptive Agents" Molecules 17, no. 1: 809-819. https://doi.org/10.3390/molecules17010809
APA StyleAnjos, J. V. d., Srivastava, R. M., Costa-Silva, J. H., Scotti, L., Scotti, M. T., Wanderley, A. G., Leite, E. S., Melo, S. J. d., & Junior, F. J. B. M. (2012). Comparative Computational Studies of 3,4-Dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitrile Derivatives as Potential Antinociceptive Agents. Molecules, 17(1), 809-819. https://doi.org/10.3390/molecules17010809