Dietary Crocin is Protective in Pancreatic Cancer while Reducing Radiation-Induced Hepatic Oxidative Damage
Abstract
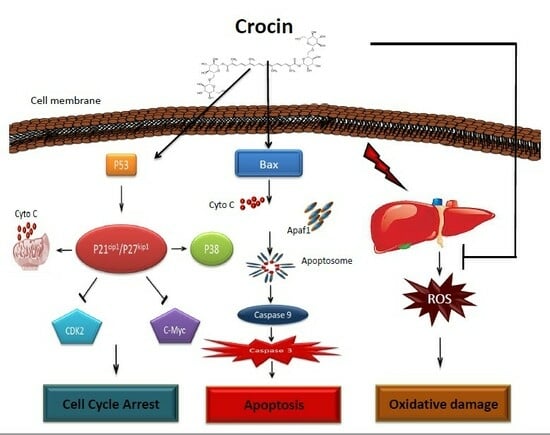
:1. Introduction
2. Materials and Methods
2.1. In Vitro Thiobarbituric Acid Reacting Substances Assay
2.2. Chemicals and Reagents
2.3. Cell Lines and Culture Method
2.4. Drug Preparation
2.5. MTT Assay
2.6. Western Blot Analysis
2.7. Gene Expression Profile Using Microarray Analysis
2.8. In Vivo Radiation-Induced Liver Toxicity
2.9. In Vivo Tumor Xenograft
3. Histology of Liver Tissue
Densitometry and Statistical Analysis
4. Results
4.1. Inhibition of Lipid Peroxidation by Crocin (In Vitro)
4.2. Cytotoxicity of Crocin on BXPC3 Cells and Capan-2 Cells
4.3. Role of Crocin on Apoptosis in BXPC3 Cells and Capan-2 Cells
4.4. Effect of Crocin on Cytochrome C Release in BXPC3 Cells
4.5. P53 and Cell Cycle Proteins Expression in Crocin Treated BXPC3 Cells and Capan-2 Cells
4.6. Identification of Crocin Treated BXPC3 Cells Gene Signature
4.7. In Vivo Pancreatic Tumor Remission by Crocin
4.8. Restoration of Radiation Induced Liver Toxicity by Crocin (In Vivo)
5. Results
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- National Cancer Institute. M. SEER Cancer Statistics Factsheets; Pancreas Cancer (National Cancer Institute): Bethesda, MD, USA, 2012. [Google Scholar]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takamori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef]
- Groot, V.P.; Gemenetzis, G.; Blair, A.B.; Rivero-Soto, R.J.; Yu, J.; Javed, A.A.; Burkhart, R.A.; Rinkes, I.H.M.B.; Molenaar, I.Q.; Cameron, J.L.; et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 1154–1162. [Google Scholar] [CrossRef]
- Ghosn, M.; Ibrahim, T.; Assi, T.; Rassy, E.E.; Kourie, H.R.; Kattan, J. Dilemma of first-line regimens in metastatic pancreatic adenocarcinoma. World J. Gastroenterol. 2016, 22, 10124–10130. [Google Scholar] [CrossRef] [PubMed]
- Hajatdoost, L.; Sedaghat, K.; Walker, E.J.; Thomas, J.; Kosari, S. Chemotherapy in Pancreatic Cancer: A Systematic Review. Medicina 2018, 54, 48. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Kubota, K.; Shimizu, T.; Katoh, M. Randomized clinical trial of adjuvant chemotherapy with S-1 versus gemcitabine after pancreatic cancer resection. Br. J. Surg. 2015, 102, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Lewin, K.; Millis, R.R. Human radiation hepatitis A morphologic study with emphasis on the late change. Arch. Pathol. 1973, 96, 21–26. [Google Scholar] [PubMed]
- Christiansen, H.; Saile, B.; Neubauer-Saile, K.; Tippelt, S.; Rave-Fränk, M.; Hermann, R.M.; Dudas, J.; Hess, C.F.; Schmidberger, H.; Ramadori, G. Irradiation leads to susceptibility of hepatocytes to TNF-alpha mediated apoptosis. Radiother. Oncol. 2004, 72, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Du, S.S.; Qiang, M.; Zeng, Z.C.; Zhou, J.; Tan, Y.S.; Zhang, Z.Y.; Zeng, H.Y.; Liu, Z.S. Radiation-induced liver fibrosis is mitigated by gene therapy inhibiting transforming growth factor-β signaling in the rat. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1513–1523. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Zhou, H.; Roy-Chowdhury, N.; Macaluso, F.; Liu, L.; Yamamoto, T.; Liping, L.; Rao, G.V.; Charles, E.; Solberg, T.D.; et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009, 49, 258–267. [Google Scholar] [CrossRef]
- Robbins, M.E.; Zhao, W. Chronic oxidative stress and radiation-induced late normal tissue injury: A review. Int. J. Radiat. Biol. 2004, 80, 251–259. [Google Scholar] [CrossRef]
- Argyropoulou, A.; Aligiannis, N.; Trougakos, I.P.; Skaltsounis, A.-L. Natural compounds with anti-ageing activity. Nat. Prod. Rep. 2013, 30, 1412–1437. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Miura, M.; Matsuda, N.; Alharrasi, A.S.; Guillemin, G.; Yamauchi, M.; Arivazhagan, G.; Song, H. An in vitro evidence for caffeic acid, rosmarinic acid and trans-cinnamic acid as a skin protectant against γ-radiation. Int. J. Low Radiat. 2014, 9, 305–316. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Ziaee, T.; Danaee, A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J. Pharm. Pharm. Sci. 2005, 8, 387–393. [Google Scholar]
- Tamaddonfard, E.; Farshid, A.-A.; Eghdami, K.; Samadi, F.; Erfanparast, A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol. Rep. 2013, 65, 1272–1280. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H.; Javadpour, Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 2010, 24, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Hosseinzadeh, H.; Abnous, K.; Motamedshariaty, V.S.; Imenshahidi, M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran. J. Basic Med. Sci. 2013, 16, 64–72. [Google Scholar] [PubMed]
- Bakshi, H.A.; Hakkim, F.L.; Sam, S.; Javid, F.; Rashan, L. Dietary crocin reverses melanoma metastasis. J. Biomed. Res. 2018, 32, 39–50. [Google Scholar]
- Bakshi, H.A.; Hakkim, F.L.; Sam, S.; Javid, F. Role of Dietary Crocin in In Vivo Melanoma Tumor Remission. Asian Pac. J. Cancer Prev. 2017, 18, 841–846. [Google Scholar]
- Bakshi, H.A.; Touseef, T.; Fassal, G. Crocus sativus L. prevents progression of cell growth and enhances cell toxicity in human breast cancer and lung cancer cell lines. Int. J. Pharma Life Sci. 2012, 2, 120–124. [Google Scholar]
- Bakshi, H.A.; Hakkim, F.L.; Sam, S. Molecular Mechanism of Dietary Crocin Induced Caspase Mediated MCF-7 Cell Death: In Vivo Toxicity Profiling and Ex Vivo Macrophage Activation. Asian Pac. J. Cancer Prev. 2016, 17, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.; Sam, S.; Rozati, R.; Sultan, P.; Islam, T.; Rathore, B.; Lone, Z.; Sharma, M.; Triphati, J.; Saxena, R.C. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by crocin from kashmiri saffron in a human pancreatic cancer cell line. Asian Pac. J. Cancer Prev. 2010, 11, 675–679. [Google Scholar] [PubMed]
- Bakshi, H.A.; Sam, S.; Feroz, A.; Ravesh, Z.; Shah, G.A.; Sharma, M. Crocin from Kashmiri saffron (Crocus sativus) induces in vitro and in vivo xenograft growth inhibition of Dalton’s lymphoma (DLA) in mice. Asian Pac. J. Cancer Prev. 2009, 10, 887–890. [Google Scholar]
- Hoshyar, R.; Bathaie, S.Z.; Sadeghizadeh, M. Crocin triggers the apoptosisthrough increasing the Bax/Bcl-2 ratio and caspase activation in human gastricadenocarcinoma: AGS, cells. DNA Cell Biol. 2013, 32, 50–57. [Google Scholar] [CrossRef]
- Yang, J.Y.; Della-Fera, M.A.; Baile, C.A. Esculetin induces mitochondria-mediated apoptosis in 3T3-L1 adipocytes. Apoptosis 2006, 11, 1371–1378. [Google Scholar] [CrossRef]
- Hsu, C.L.; Yen, G.C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases 2, 3, 6, 7, 8, and 10 in a caspase-9-dependent manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef]
- Keane, M.M.; Ettenberg, S.A.; Nau, M.M.; Russell, E.K.; Lipkowitz, S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999, 59, 734–741. [Google Scholar]
- Bellarosa, D.; Ciucci, A.; Bullo, A.; Nardelli, F.; Manzini, S.; Maggi, C.A.; Goso, C. Apoptotic events in a human ovarian cancer cell line exposed to anthracyclines. J. Pharmacol. Exp. Ther. 2001, 296, 276–283. [Google Scholar]
- Kottke, T.J.; Blajeski, A.L.; Martins, L.M.; Mesner, P.W., Jr.; Davidson, N.E.; Earnshaw, W.C.; Armstrong, D.K.; Kaufmann, S.H. Comparison of paclitaxel-, 5-fluoro-2′-deoxyuridine-, and epidermal growth factor (EGF)-induced apoptosis. Evidence for EGF-induced anoikis. J. Biol. Chem. 1999, 274, 15927–15936. [Google Scholar] [CrossRef]
- Shamas-Din, A.; Brahmbhatt, H.; Leber, B.; Andrews, D.W. BH3-only proteins: Orchestrators of apoptosis. Biochim. Biophys. Acta. 2011, 1813, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.-J.; Shi, F.; Zheng, X.-L.; Wang, Q.; Yang, L.; Sun, H.; He, F.; Zhang, L.; Lin, Y.; Qin, Y.; et al. Crocetin induces cytotoxicity and enhances vincristine induced cancer cell death via p53-dependent and -independent mechanisms. Acta Pharmacol. Sin. 2011, 32, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F.; Borji, A. Protective Effects of Carvacrol against Oxidative Stress Induced by ChronicStress in Rat’s Brain, Liver, and Kidney. Biochem. Res. Int. 2016, 2016, 2645237. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Abraham, S.K. Efficacy of crocin and safranal as protective agents against genotoxic stressinduced by gamma radiation, urethane and procarbazine in mice. Hum. Exp. Toxicol. 2018, 37, 13–20. [Google Scholar] [CrossRef]
- Ohkawa, H.; Onishi, N.; Yagi, K. Assay of lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Revathi, S.; Hakkim, F.L.; Kumar, N.R.; Bakshi, H.A.; Rashan, L.; Al-Buloshi, M.; Hasson, S.S.A.A.; Krishnan, M.; Javid, F.; Nagarajan, K. Induction of HT-29 Colon Cancer Cells Apoptosis by Pyrogallol with Growth Inhibiting Efficacy Against Drug-Resistant Helicobacter pylori. Anticancer Agents Med Chem. 2018, 18, 1875–1884. [Google Scholar] [CrossRef]
- Tung, B.T.; Rodríguez-Bies, E.; Ballesteros-Simarro, M.; Motilva, V.; Navas, P.; López-Lluch, G. Modulation of endogenous antioxidant activity by resveratrol and exercise in mouse liver is age dependent. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 398–409. [Google Scholar] [CrossRef]
- Swinney, D.C.; Anthony, J. How were new medicines discovered? Nat. Rev. Drug Discov. 2011, 10, 507–519. [Google Scholar] [CrossRef]
- Bajbouj, K.; Schulze-Luehrmann, J.; Diermeier, S.; Amin, A.; Schneider-Stock, R. The anticancer effect of saffron in two p53 isogenic colorectal cancer celllines. BMC Complement. Altern. Med. 2012, 12, 69–77. [Google Scholar] [CrossRef]
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F. Design and statistical methods in studies using animal models of development. ILAR J. 2006, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, X.; Wang, S.; Ou, Y.; Zheng, X.; Wang, Q. The relationship between mitochondrial Fusion/fission and apoptosis in the process of adipose-derived stromal cells differentiation into astrocytes. Neurosci. Lett. 2014, 575, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Narita, M.; Shimizu, S.; Ito, T.; Chittenden, T.; Lutz, R.J.; Matsuda, H.; Tsujimoto, Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 14681–14686. [Google Scholar] [CrossRef]
- D’Alessandro, A.M.; Mancini, A.; Lizzi, A.R.; De-Simone, A.; Marroccella, C.E.; Gravina, G.L.; Tatone, C.; Festuccia, C. Crocus sativus stigma extract and its major constituent crocin possess significant anti-proliferative properties against human prostate cancer. Nutr. Cancer 2013, 65, 930–942. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, S.; Wang, X.; Zhang, L.; Jiang, E.; Gu, Y.; Shangguan, A.J.; Zhao, H.; Lv, T.; Yu, Z. Crocin inhibits cell proliferation and enhances cisplatin andpemetrexed chemosensitivity in lung cancer cells. Transl. Lung Cancer Res. 2015, 4, 775–783. [Google Scholar]
- Sun, Y.; Wang, Z.; Xu, H.J.; Zhao, Y.X.; Wang, L.Z.; Sun, L.R.; Sun, X.F. Crocinexhibits antitumor effects on human leukemia HL-60Cells in vitro and In vivo.Evid.-Based Compl. Altern. Med. 2013, 2013, 690164. [Google Scholar]
- Aljabali, A.A.A.; Bakshi, H.A.; Hakkim, F.L.; Haggag, Y.A.; Al-Batanyeh, K.M.; Zoubi, M.S.A.; Al-Trad, B.; Nasef, M.M.; Satija, S.; Mehta, M. Albumin Nano-Encapsulation of Piceatannol Enhances Its Anticancer Potential in Colon Cancer Via Downregulation of Nuclear p65 and HIF-1α. Cancers 2020, 12, 113. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Bakshi, H.A.; Khan, S.; Nasef, M.; Farzand, R.; Sam, S.; Rashan, L.; Al-Baloshi, M.S.; Abdo Hasson, S.S.A.; Jabri, A.A.; et al. Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes. Onco_target 2019, 37, 3472–3490. [Google Scholar] [CrossRef]
- Khan, M.N.; Haggag, Y.A.; Lane, M.E.; McCarron, P.A.; Tambuwala, M.M. Polymeric Nano-Encapsulation of Curcumin Enhances its Anti-Cancer Activity in Breast (MDA-MB231) and Lung (A549) Cancer Cells Through Reduction in Expression of HIF-1α and Nuclear p65 (Rel A). Curr. Drug Deliv. 2018, 15, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.A.; Mishra, V.; Satija, S.; Mehta, M.; Hakkim, F.L.; Kesharwani, P.; Dua, K.; Chellappan, D.K.; Charbe, N.B.; Shrivastava, G.; et al. Dynamics of Prolyl Hydroxylases Levels During Disease Progression in Experimental Colitis. Inflammation 2019, 42, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.A.; Hakkim, F.L.; Smitha, S. Assessment of in vitro cytotoxicity of saffron (Crocus sativus L.) on cervical cancer cells (HEp-2) and their in vivo pre-clinical toxicity in normal swiss albino mice. Int. J. Herbal Med. 2016, 4, 80–83. [Google Scholar]
- Skemiene, K.; Rakauskaite, G.; Trumbeckaite, S.; Liobikas, J.; Brown, G.C.; Borutaite, V. Anthocyanins block ischemia-induced apoptosis in the perfused heart and support mitochondrial respiration potentially by reducing cytosolic cytochrome c. Int. J. Biochem. Cell Biol. 2013, 45, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Grochola, L.F.; Taubert, H.; Greither, T.; Bhanot, U.; Udelnow, A.; Wurl, P. Elevated transcript levels from the MDM2 p1 promoter and low p53 transcript levels are associated with poor prognosis in human pancreatic ductal adenocarcinoma. Pancreas 2011, 40, 265–270. [Google Scholar] [CrossRef]
- Vali, F.; Changizi, V.; Safa, M. Synergistic Apoptotic Effect of Crocin and Paclitaxel or Crocin and Radiation on MCF-7 Cells, a Type of Breast Cancer Cell Line. Int. J. Breast Cancer 2015, 2015, 139349. [Google Scholar] [CrossRef]
- Mollaei, H.; Safaralizadeh, R.; Babaei, E.; Abedini, M.R.; Hoshyar, R. The anti-proliferative and apoptotic effects of crocin on chemosensitive and chemoresistant cervical cancer cells. Biomed. Pharmacother. 2017, 94, 307–316. [Google Scholar] [CrossRef]
- Balkhi, H.M.; Sana, S.; Haq, E. Crocin induced apoptosis through p53-dependent pathway in C6 glioma Cells. Int. J. Adv. Res. Sci. Eng. 2017, 6, 910–915. [Google Scholar]
- Jin, Y.H.; Choi, J.; Shin, S.; Lee, K.Y.; Park, J.H.; Lee, S.K.; Yoo, K.J. Panaxadiol selectively inhibits cyclin A-associated Cdk2 activity by elevating p21WAF1/CIP1 protein levels in mammalian cells. Carcinogenesis 2013, 24, 1767–1772. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising _targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Grana, X.; Reddy, E.P. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11, 211–219. [Google Scholar] [PubMed]
- Ellinger-Ziegelbauer, H.; Kelly, K.; Siebenlist, U. Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol. Cell Biol. 1999, 19, 3857–3868. [Google Scholar] [CrossRef]
- Bulavin, D.V.; Saito, S.; Hollander, M.C.; Sakaguchi, K.; Anderson, C.W.; Appella, E.; Fornace, A.J., Jr. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999, 18, 6845–6854. [Google Scholar] [CrossRef]
- Keller, D.; Zeng, X.; Li, X.; Kapoor, M.; Iordanov, M.S.; Taya, Y.; Lozano, G.; Magun, B.; Lu, H. The p38MAPK inhibitor SB203580 alleviates ultraviolet-induced phosphorylation at serine 389 but not serine 15 and activation of p53. Biochem. Biophys. Res. Commun. 1999, 261, 464–471. [Google Scholar] [CrossRef]
- Bretones, G.; Delgado, M.D.; Leon, J. Myc and cell cycle control. Biochim. Biophys. Acta 2015, 1849, 506–516. [Google Scholar] [CrossRef]
- Shachaf, C.M.; Felsher, D.W. Tumor dormancy and MYC inactivation: Pushing cancer to the brink of normalcy. Cancer Res. 2005, 65, 4471–4474. [Google Scholar] [CrossRef]
- Zheng, F.M.; Long, Z.J.; Hou, Z.J.; Luo, Y.; Xu, L.Z.; Xia, J.L.; Liu, J.W.; Wang, X.; Kamran, M.; Yan, M.; et al. A novel small molecule aurora kinase inhibitor attenuates breast tumor-initiating cells and overcomes drug resistance. Mol. Cancer Ther. 2014, 13, 1991–2003. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Kamei, D.; Murakami, M.; Sasaki, Y.; Nakatani, Y.; Majima, M.; Ishikawa, Y.; Ishii, T.; Uematsu, S.; Akira, S.; Hara, S.; et al. Microsomal prostaglandin E synthase-1 in both cancer cells and hosts contributes to tumour growth, invasion and metastasis. Biochem. J. 2009, 425, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van-De-Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Capdevilla, J.; Marnett, L.J.; Chacos, N.; Prough, R.A.; Estabrook, R.W. Cytochrome P-450-dependent oxygenation of arachidonic acid to hydroxyeicosatetraenoic acids. Proc. Natl. Acad. Sci. USA 1982, 79, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Koc, M.; Buyukokuroglu, M.E.; Altinkaynak, K.; Sahin, Y.N. Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J. Pineal Res. 2003, 34, 173–177. [Google Scholar] [CrossRef]
- Blum, J.; Fridovich, I. Inactivation of glutathione peroxidase by superoxide radicals. Arch. Biochem. Biophys. 1985, 240, 500–508. [Google Scholar] [CrossRef]







| GSH (µM/mg Tissue) | 30 min | 1 h | 2 h | 4 h |
|---|---|---|---|---|
| PBS | 56.6 ± 0.05 | 50 ± 0.20 | 46.6 ± 0.05 | 50 ± 0.18 |
| Crocin (100 mg/kg bwt) | 63.6 ± 0.03 a | 56.6 ± 0.1 a | 56.6 ± 0.1 a | 53.3 ± 0.20 a |
| PBS + RT (4 Gy.) | 16.6 ± 0.11 | 13.3 ± 0.08 | 6.6 ± 0.05 | 3.33 ± 0.03 |
| Crocin (100 mg/kg bwt) + RT(4Gy) | 63.3 ± 0.08 1 | 60 ± 0.18 1 | 56.6 ± 0.1 1 | 46.6 ± 0.05 1 |
| GPx (µM/min/mg/protein) | ||||
| PBS | 2.4 ± 0.05 | 2.5 ± 0.12 | 2.8 ± 0.05 | 2.83 ± 0.06 |
| Crocin (100 mg/kg bwt) | 2.8 ± 0.05 c | 2.9 ± 0.05 b | 2.9 ± 0.1 | 3.06 ± 0.12 |
| PBS + RT (4 Gy.) | 2.4 ± 0.1 | 2.3 ± 0.02 | 2.0 ± 0.12 | 1.8 ± 0.05 |
| Crocin (100 mg/kg bwt) + RT(4Gy) | 2.7 ± 0.11 | 2.6 ± 0.11 2 | 2.5 ± 0.05 2 | 2.33 ± 0.08 3 |
| GR(µM/min./mg/protein) | ||||
| PBS | 0.3 ± 0.05 | 0.25 ± 0.05 | 0.2 ± 0.006 | 0.25 ± 0.05 |
| Crocin (100 mg/kg bwt) | 0.3 ± 0.005 | 0.4 ± 0.02 b | 0.4 ± 0.01 a | 0.5 ± 0.0066 b |
| PBS + RT (4 Gy.) | 0.65 ± 0.02 | 0.5 ± 0.003 | 0.4 ± 0.008 | 0.15 ± 0.02 |
| Crocin (100 mg/kg bwt) + RT(4Gy) | 1.1 ± 0.04 1 | 0.9 ± 0.04 1 | 0.7 ± 0.01 1 | 0.6 ± 0.01 1 |
| GT(µM/min/mg/Protein) | 30 min | 1h | 2 h | 4 h |
|---|---|---|---|---|
| PBS | 1.4 ± 0.05 | 1.2 ± 0.05 | 1.3 ± 0.05 | 1.1 ± 0.1 |
| Crocin (100 mg/kg bwt) | 1.5 ± 0.05 | 1.5 ± 0.11b | 1.3 ± 0.05 | 1.4 ± 0.11 |
| PBS + RT (4Gy.) | 1.75 ± 0.02 | 1.04 ± 0.02 | 1.02 ± 0.01 | 1.0 ± 0.006 |
| Crocin (100 mg/kg bwt) + RT (4 GY) | 1.9 ± 0.05 2 | 1.6 ± 0.113 | 1.4 ± 0.05 3 | 1.2 ± 0.11 |
| MDA (µg/mg protein) | ||||
| PBS | 0.05 ± 0.003 | 0.1 ± 0.008 | 0.1 ± 0.01 | 0.03 ± 0.003 |
| Crocin (100 mg/kg bwt) | 0.02 ± 0.01 c | 0.03 ± 0.003 c | 0.1 ± 0.008 | 0.02 ± 0.001 b |
| PBS + RT (4 Gy.) | 0.1 ± 0.006 | 0.2 ± 0.005 | 0.2 ± 0.01 | 0.3 ± 0.01 |
| Crocin (100 mg/kg bwt) + RT(4Gy) | 0.1 ± 0.01 | 0.1 ± 0.006 1 | 0.1 ± 0.003 2 | 0.15 ± 0.015 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakshi, H.A.; Zoubi, M.S.A.; Faruck, H.L.; Aljabali, A.A.A.; Rabi, F.A.; Hafiz, A.A.; Al-Batanyeh, K.M.; Al-Trad, B.; Ansari, P.; Nasef, M.M.; et al. Dietary Crocin is Protective in Pancreatic Cancer while Reducing Radiation-Induced Hepatic Oxidative Damage. Nutrients 2020, 12, 1901. https://doi.org/10.3390/nu12061901
Bakshi HA, Zoubi MSA, Faruck HL, Aljabali AAA, Rabi FA, Hafiz AA, Al-Batanyeh KM, Al-Trad B, Ansari P, Nasef MM, et al. Dietary Crocin is Protective in Pancreatic Cancer while Reducing Radiation-Induced Hepatic Oxidative Damage. Nutrients. 2020; 12(6):1901. https://doi.org/10.3390/nu12061901
Chicago/Turabian StyleBakshi, Hamid A., Mazhar S Al Zoubi, Hakkim L. Faruck, Alaa A A Aljabali, Firas A. Rabi, Amin A. Hafiz, Khalid M Al-Batanyeh, Bahaa Al-Trad, Prawej Ansari, Mohamed M. Nasef, and et al. 2020. "Dietary Crocin is Protective in Pancreatic Cancer while Reducing Radiation-Induced Hepatic Oxidative Damage" Nutrients 12, no. 6: 1901. https://doi.org/10.3390/nu12061901
APA StyleBakshi, H. A., Zoubi, M. S. A., Faruck, H. L., Aljabali, A. A. A., Rabi, F. A., Hafiz, A. A., Al-Batanyeh, K. M., Al-Trad, B., Ansari, P., Nasef, M. M., Charbe, N. B., Satija, S., Mehta, M., Mishra, V., Gupta, G., Abobaker, S., Negi, P., Azzouz, I. M., Dardouri, A. A. K., ... Tambuwala, M. M. (2020). Dietary Crocin is Protective in Pancreatic Cancer while Reducing Radiation-Induced Hepatic Oxidative Damage. Nutrients, 12(6), 1901. https://doi.org/10.3390/nu12061901