Abstract
Purpose of Review Experimental evidence suggests that flavonoids prevent neurodegeneration and improves cognitive function. In this study, we systematically reviewed the effect of hesperidin on cognitive deficits and neurobehavioural outcomes in in vivo studies.
Recent Findings: A systematic search of PubMed, EBSCOhost, Web of Science, Scopus and ProQuest was conducted. Meta-analysis was performed on the effect of hesperidin on cognitive and neurobehavioural parameters (Morris Water Maze, Y-Maze, elevated plus maze, rotarod test, locomotion activity, passive avoidance test, open field test and forced swimming test). The mixed effect model was used to compute the standard mean difference (SMD). A total of 1069 documents were retrieved. However, 46 studies were included in the systematic review and meta-analysis. Our findings revealed that hesperidin did not significantly affect cognitive performance in normal rats compared with placebo. Moreover, hesperidin improved memory and learning, sensorimotor function and locomotion activity in cognitive impaired rats. Hesperidin did not show any significant effect on anxiety-related outcomes in the diseased model.
Summary: Hesperidin improved cognitive function and neurocognitive effects could be associated with its neuroprotective effects against neuroinflammation, oxidative stress-induced neuronal damage, inhibition of cholinergic deficit and mitochondrial dysfunction. These results correlate with available scientific evidence on the effect of hesperidin on cognitive dysfunction and neurobehavioural deficits in cognitive-impaired rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hesperidin is a natural flavanone glycoside and one of the most biologically active flavonoids in citrus fruit [1]. Apart from being identified in Citrus sp., hesperidin is also present in banana peel, bergamot fruit, lemon fruit and peels. It is lipophilic and can easily cross the blood-brain barrier. Structurally, hesperidin has an aglycone known as hesperetin, which binds to rutinose [6-O-(α-l-Rhamnopyranosyl)-D-glucopyranose] and/or [6-O-(α-l-Rhamnosyl)-D-glucose]. It is also referred to as β-7-rutinoside of hesperetin [2].
Previous investigations revealed that hesperidin possesses several biological activities, including anti-inflammatory, anti-cancer and antioxidant activities [3]. It also exhibits beneficial effects on hypercholesterolemia, infertility and hypertension [4,5,6]. Several studies have shown the impact of hesperidin on central nervous system disorders. Diet supplemented with hesperidin improved cerebral blood flow, cognitive function and memory performance. Hesperidin reduced depressive symptoms in Parkinson’s model via modulation of serotonergic and kappa-opioid receptors and enhancement of dopamine, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid levels [7]. Also, hesperidin improved learning and memory and neurobehavioural functions in Alzheimer’s disease mouse model [8]. Hesperidin suppressed amyloid precursor protein expression and inhibited accumulation of β-amyloid protein and expression of γ-secretase. There are previous reviews on the neuroprotective mechanisms of hesperidin and its beneficial effects on central nervous system disorders [9]. However, currently, there has been no attempt to systematically synthesize available evidence on the effect of hesperidin on cognitive and neurobehavioural function. This systematic review and meta analysis evaluates current pre-clinical evidence of the effect of hesperidin on cognitive performance and neurobehavioural function. Studies that investigated the impact of hesperidin on learning and memory, anxiety, motor coordination and locomotion were included, and meta-analysis was performed to evaluate its effect quantitatively.
Materials and methods
Study Design
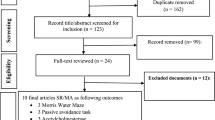
Primary animal-based studies that assayed hesperidin as therapeutic against cognitive and neurobehavioural disorders were rigorously searched in Scopus, EBSCOhost (including MEDLINE, CINAHL, APA PsycInfo etc.), Web of Science (WoS), Proquest, and PubMed via topic-based query-algorithm without regional, temporal or language confinements using the “Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines” [10] (Fig. 1). The topical algorithm was defined with the key elements as “hesperidin AND (memor* OR cogniti* OR *behav* OR neuroinflammati* OR neurodegenerat* OR Alzheim* OR Parkinso*)” and where necessary, adapted to different databases requirements for optimum retrieval. New study(ies) was(were) thereafter sought via email-alert recording until final analysis. The data querying and acquisition coupled with preprocessing involving merging and duplicate removal of multiple occurrences of a document in Endnote version 20 and Excel 2016 was done by E.T.C. Then, the initial screening of the studies for inclusion and exclusion decision-making was title and abstract oriented as per the screening protocol and criteria pre-set for this study. Full-text of the studies that passed the abstract/title screening was downloaded for _targeted variables extraction in predesigned data collection forms created as per _targeted data criteria. Finally, external records were further sought from manual review of citations in the eligible studies to ensure the non-missing of relevant studies. The workflow is presented in Figure S1.
Inclusion Criteria
The chief criterion for any study included in the current investigation was to report in vivo models that assayed hesperidin effects on cognitive performance and neurobehaviour of pathologically induced neurodegenerative disease in rats. Only studies that evaluated metrics of learning and memory, anxiety, sensorimotor and locomotion in rats were selected. The study design must include a placebo group (untreated groups) and experimental groups involving untreated pathologic (induced neurogenerative disease) control group and intervention group (hesperidin-treated induced neurodegenerative disease group). All studies were included irrespective of hesperidin doses used. Reasonable outcomes (provided in measurable metrics) related to cognitive performance and neurobehavioural function and an adequate description of the corresponding assay must be presented.
Exclusion Criteria
Articles other than primary studies reporting the use of hesperidin in combination with other substances or intervention, were excluded in this study.
Data Extraction
The _targeted data extracted for the present study from the final database of the eligible studies include the study characteristics (authors’ name, publication year, sample size from each group), outcome variables (mean and standard deviation) in the hesperidin-treated group, untreated pathologic group, and placebo group, dose, and route of administration. Data were executed and validated by OTA and ETC. Disagreement was resolved by discussion in any case.
Statistical Analysis
Outcome variables (effect sizes) datasets related to the intervention (hesperidin treated) group versus untreated (pathologic) group and intervention group versus placebo group were fitted in a mixed-effects model to compute the SMD (bias-corrected standardised mean difference) using the inverse variance technique based on the exact formulae [11]. The SMD confidence intervals were adjusted using the Knapp-Hartung protocol [12]. The between-studies variance (τ2) estimate was achieved via the restricted maximum likelihood estimator [13]. Assessment of heterogeneity between studies was based on I2-statistic acceptable limit set as I² ≯75% [14]. Outcome variables with ≥ 3 studies were meta-analysed. Finally, the sensitivity of the model was tested via leave-one-study-out mixed-effects meta-analytic cross-validation (Viechtbauer and Cheung, 2010). The final outcomes were summarized in forest plots. In all cases, results from the random-effects model were interpreted in lieu of common-effects model. Small study effects or publication bias were assessed using limit meta-analysis (Rücker et al. 2011). Models were fitted in R version 4.3.0 (2023-04-21 ucrt) based on metasens v.1.5-2, metafor v.4.0–0, meta v.6.2-1, dmetar v.0.0.9000, and performance analytics v.2.0.4 packages [13, 15,16,17].
Results
Selection of Articles and Study Characteristics
The search results revealed that 1069 documents were retrieved from 5 databases: PubMed (n = 150), Web of Science (n = 257), Scopus (n – 339), ProQuest (n = 90) and EBSCOhost (n = 233), as shown in Fig. 1. Out of the 1069 articles retrieved, 46 studies were included in the systematic and meta-analysis review based on the inclusion and exclusion criteria. The characteristics of the included studies are shown in Table 1. A total of 35 studies presented in Table 1 used Sprague Dawley rats, while 11 studies used mice. Six studies used the aluminium chloride and diabetes–induced cognitive impairment model while 5 studies employed stress-induced model. Two studies used valproic acid model, 6-hydroxydopamine model, lipopolysaccharide model, APPswedish transgenic model, ketamine and 3-nitropropionic acid model. One study each used scopolamine, sodium fluoride, D-galactose, methothrexate, traumatic brain, nitric oxide, formaldehyde, thioacetamide, febrile seizure, ischemia, post-partum, neuropathic pain, methionine, quinolinic acid, emamectin benzoate, homocysteine, olfactory bulbectomy, paclitaxel, pentylenetetrazole, rotenone, 5xFAD models. The study duration varied in the included studies from 5 to 112 days as shown in Table 1.
Hesperidin (Sample Control) vs. Placebo (Normal Control)
Figure 2 revealed the analysis of 16 studies that reported elevated plus maze in normal rats treated with hesperidin and placebo. The result shows no significant difference after treatment with hesperidin when compared to the placebo group with SMD = 0.04 and 95% CI -2.66;2.75 and I2 = 84%. The pooled mean difference from the Y-Maze test between hesperidin-treated groups (Sample control group) and placebo (normal control) also revealed no significant difference (SMD = 0.27, 95% CI [-0.79;1.32], P < 0.01) in the fixed and random effect models with I2 = 76% as shown in Fig. 3. Similar results were obtained for Morris Water Maze (SMD = 0.50, 95% CI = -1.69;2.69, I2 = 83) and object recognition tests (SMD = -1.32), 95% CI = -4.72–2.01), I2 = 91%) measured in hesperidin (sample control) and placebo groups as shown in Figs. 4 and 5. Figure 6 shows the forest plot of pooled SMD data from the passive avoidance test between the treated group and placebo. No significant difference was observed between the groups tested, as revealed by the SMD (5.07) and 95% CI [-17.51; 27.65] with I2 = 89%.
The result of the open field test of 16 eligible studies is shown in Fig. 7. The forest plot revealed no significant difference between the hesperidin (Sample control) and placebo groups with SMD = 0.49 and 95% CI [-0.17;1.16] and I2 = 59%. The forest plot for pooled standard mean difference (SMD) from the rotarod test measured between hesperidin-treated rats (sample control) and placebo (normal control) is shown in Fig. 8. The result showed a significant difference between hesperidin (Sample control) and placebo groups with SMD = -4.10 and 95% CI [-7.92;-0.29] and I2 = 89%. In the forced swimming test, no significant difference was observed between the hesperidin-treated group and placebo as shown in the forest plot (SMD = -1.33; 95% CI [-3.41; 0.42]; I2 = 86%) in Fig. 9. The result of the meta-analysis of locomotor activity between hesperidin-treated rats and placebo showed no significant difference (SMD = -2.31, 95% CI = -7.64;3.02, I2 = 78%) as shown in Fig. 10.
Hesperidin (Intervention Group) vs. Cognitive Impaired Group
Our findings from the forest plot pooled data from 16 included studies that reported elevated plus maze test showed no significant difference (SMD = -0.21, 95%CI = -3.12; 2.69, I2 = 93%) after intervention with hesperidin as shown in Supplementary Fig. 1. However, results from the Y-Maze test showed a significant difference between the hesperidin-treated cognitively impaired rats and the untreated group (SMD = 8.31; 95%CI = 4.55–12.6; I2 = 93%), as revealed by the forest plot in Supplementary Fig. 2. Similarly, a significant difference was also observed between the hesperidin-treated group and untreated cognitive impaired rats (SMD = -5.90, 95% CI = − 11.18; -0.61, I2 = 83%) from the Morris Water Maze test of 13 included studies as shown in the forest plot in Supplementary Fig. 3. The result of the novel recognition test showed a significant difference (SMD = -3.59, 95% CI = -6.82; -0.35, I2 = 92%) between the hesperidin-treated and untreated cognitively impaired rats, respectively, as shown in Supplemented Fig. 4. For the passive avoidance test, there was no significant difference (SMD = 8.66, 95% CI = -0.20; 17.53) between hesperidin-treated and untreated cognitively impaired rats, as shown in Supplementary Fig. 5.
The forest plot from the rotarod test also revealed a significant difference between hesperidin-treated (intervention) and untreated cognitive impaired rats (pathological) (SMD = -9.72, 95% CI = -13.71;-5.73 and I2 = 83%) (Supplementary Fig. 6). The result of the open field test in Supplementary Fig. 7 showed a significant difference (SMD = 3.47 95% CI = 0.51; 6.43 I2 = 94%) between the hesperidin-treated and untreated cognitively impaired rats. The forest plot of the forced swimming test presented in Supplementary Fig. 8 also revealed a significant difference between hesperidin-treated and untreated cognitive impaired rats (SMD = -3.86, 95% CI = − 6.24; -1.47, I2 = 94%). Also, a significant difference was observed in the locomotion activity of hesperidin-treated groups compared to the untreated cognitive impaired group (SMD = 5.85, 95% CI = 0.64; 11.07, I2 = 94%) as shown in Supplementary Fig. 9.
Discussion
This is the first systematic review and meta-analysis on the effect of hesperidin on cognitive function and neurobehavioural outcomes in animal studies. The efficacy of hesperidin was assessed in two different studies. First, we assessed the effect of hesperidin on normal animals (sample control vs. placebo). In the second study, a meta-analysis was conducted on the effect of hesperidin on cognitively impaired animals (hesperidin-treated group (intervention) vs. disease (pathological) group).
Results from the first meta-analytic study revealed that treatment with hesperidin did not significantly affect cognitive and neurobehavioural outcomes in normal rats except in the studies on rotarod tests. This result suggests that hesperidin may improve muscular coordination and motor function in normal rats.
The overall outcome of the second study revealed that hesperidin showed a significant effect on cognitive performance and neurohavioural function in cognitively impaired rats. Using the eligible studies, we investigated the effect of hesperidin on neurobehavioural impairments. Some of the parameters used for the investigation include Y-Maze, Morris Water Maze Passive avoidance test and novel object recognition tests (Learning and memory tests), elevated plus maze (anxiety-related behavioural test), open field and rotarod tests (sensorimotor tests) as well as forced swimming test and locomotor activity (locomotor test).
Effect of Hesperidin on Anxiety-Related Outcomes
The meta-analytic results showed that hesperidin did not improve anxiety-related behaviour in rats. It was observed from the forest plot data pooled from 16 included studies that hesperidin did not exhibit an effect on anxiety, which is contrary to individual studies. Furthermore, data pooled from 4 studies showed that hesperidin did not improve cognitive performance associated with aversive stimuli, as revealed by results from the passive avoidance test.
Hesperidin Improved Learning and Memory Function
Our findings revealed that hesperidin showed significant learning and memory-enhancing effects as revealed by the pooled data from the Y-Maze, Morris water maze and novel object recognition test. The meta-regression data confirmed a significant association between treatment with hesperidin and memory function in cognitively impaired rats. These results suggest that hesperidin specifically improved spatial learning and memory, spatial memory and long-term memory, as well as recognition memory in cognitive impaired rats, which correlates with the results of the individual studies included in this study. The ability of hesperidin to improve cognitive performance in memory-impaired rats may be due to different biochemical mechanisms, especially its antioxidant and anti-neuroinflammatory effects. Memory and learning-enhancing effects of hesperidin have also been linked to the improvement of cholinergic function in cognitive impaired rats. The degeneration of cholinergic neurons and depletion of acetylcholine levels in the brain is associated with cognitive impairment. Hesperidin inhibited acetylcholinesterase activity and improved cholinergic function in cognitive-impaired rats [20, 38]. Oxidative stress also contributes to poor neurobehavioural outcomes. The brain is susceptible to oxidative damage and may cause radical-induced neuronal damage, leading to loss of synaptic connections, accumulation of protein aggregates, neurodegeneration and ultimately memory impairment [61, 62]. Impaired neurogenesis may contribute to memory impairment because it involves the development of neuronal cells from neural stem cells that are progenitor cells.
The neural progenitor cells resident in the dentate gyrus of the brain’s hippocampal region are important for neurogenesis during the embryonic stage and adulthood. It has been shown that they also contribute to maintaining memory and learning functions. The work of Aranarochana [8] and Naewla et al. [47] revealed that hesperidin attenuated memory impairment caused by valproic acid and methotrexate–induced impaired neurogenesis.
Hesperidin Improved Sensorimotor Function
The effect of hesperidin on sensorimotor function was examined as shown by the pooled data from 23 eligible studies that reported open field (15) and rotarod (7) tests. Our findings suggest that hesperidin improved sensorimotor coordination and motor learning in cognitive-impaired rats. In the open field tests, cognitive-impaired animals were characterized by decreased movement and activities [20, 63]. Hesperidin improved motor function, locomotive and exploratory activities of cognitively impaired rats [22, 34, 64]. Hesperidin exhibited good behavioural outcomes via attenuation of sensorimotor deficit, motor dysfunction, impaired grip strength, and muscular coordination skills in the rotarod test [38, 49, 57].
Effect of Hesperidin on Locomotor Activity
Our findings also revealed that hesperidin improved locomotive activity in cognitively impaired rats, as shown by the results from the forced swimming and locomotion activity tests. This result correlates with previous findings [29, 25, 27, 50]. Kumar et al. [25] reported that impairment in motor function was triggered by degeneration of dopaminergic neurons in the striatum, impaired antioxidant defense mechanism in the neurons and microglia activation. However, hesperidin showed significant inhibition of neuroinflammation and oxidative damage to neurons, contributing to improved locomotive behaviour. Hesperidin also reduced immobility and improved cognition in memory-impaired rats [27].
Evidence Gaps and Future Directions
One of the limitations of this study is the variation in the doses of hesperidin observed in the included studies. Different doses were observed in the included studies, though some authors reported the same hesperidin dose in the investigation. Furthermore, different study designs were combined from the identified studies in the meta-analysis. There were variations in the study design, which involved the use of different chemical compounds to induce cognitive and behavioural impairments in the animals. Furthermore, species effects may also affect the study as we combined results from investigations that used rats and mice in the meta-analysis. Hence, this study did not consider variation in doses, specificity in the study design, route of administration of hesperidin and chemical compounds used for the study design. A strength of the study was that we examined the effects of hesperidin on cognitive performance and behavioural function in preclinical models using different learning and memory, sensorimotor and locomotive paradigms. This study provided the opportunity to quantitatively examine the consistency of the memory-enhancing effects of hesperidin in mice and rats. The role of hesperidin in cognitive performance and neurobehaviour outcomes requires further investigation, especially in human studies. Studies on the nootropic effects of hesperidin in childhood and adulthood and sensitivity to sexes should also be explored. More studies are required to determine the dose range at which hesperidin can exert positive cognitive and neurobehaviour outcomes in neurologically impaired subjects.
Conclusion
This study highlighted that hesperidin improved learning and memory, sensorimotor and locomotive functions in cognitive-impaired rats. These results correlate with previous findings from individual studies. However, our findings did not reveal a positive effect on the anxiety-related behavioural outcome, which contradicts reports from individual studies. These reported actions of hesperidin have been linked to its neuroprotective effects via inhibition of acetylcholinesterase activity and cholinergic impairment, attenuation of neuroinflammation, inhibition of oxidative stress-induced neuronal damage and mitochondrial dysfunction. These findings suggest that hesperidin treatment could be a potentially suitable neuroprotective therapy capable of improving cognitive performance and neurobehavioural outcomes. Further studies are suggested especially n the effect of hesperidin on anxiety-like behaviour. These results will provide scientific information with potential translational value from animal studies to clinical trials.
Data Availability
Data are contained in the manuscript. Any further data request should be directed to the corresponding author.
References
de Souza P, da Silva RCV, Mariano LNB, Dick SL, Ventura GC, Cechinel-Filho V. Diuretic and natriuretic effects of hesperidin, a flavanone glycoside, in female and male hypertensive rats. Plants (Basel). 2022;12(1):25.
Hajialyani M, Farzaei MH, Echeverría J, Nabavi SM, Uriarte E, Eduardo SS. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules. 2019;24(3):648.
Choi SS, Lee SH, Lee KA. A comparative study of hesperetin, hesperidin and hesperidin glucoside: antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants (Basel). 2022;11(8):1618.
Khamis T, Hegazy AA, El-Fatah SSA, Abdelfattah ER, Abdelfattah MMM, Fericean LM, Arisha AH. Hesperidin mitigates cyclophosphamide-induced testicular dysfunction via altering the hypothalamic pituitary gonadal axis and testicular steroidogenesis, inflammation, and apoptosis in male rats. Pharmaceuticals (Basel). 2023;16(2):301.
Mas-Capdevila A, Teichenne J, Domenech-Coca C, Caimari A, Del Bas JM, Escoté X, Crescenti A. Effect of hesperidin on cardiovascular disease risk factors: the role of intestinal microbiota on hesperidin bioavailability. Nutrients. 2020;12(5):1488.
Valls RM, Pedret A, Calderon-Perez L, Llaurado E, Pla-Paga L, Companys J, Moragas A, Martin-Lujan F, Ortega Y, Giralt M, Romeu M, Rubio L, Mayneris-Perxachs J, Canela N, Puiggros F, Caimari A, Del Bas JM, Arola L, Sola R. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: a randomized controlled trial (Citrus study). Eur J Nutr. 2021;60:1277–88.
Antunes MS, Goes AT, Boeira SP, Prigol M, Jesse CR. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition. 2014;30:1415–22.
Aranarochana A, Kaewngam S, Anosri T, Sirichoat A, Pannangrong W, Wigmore P, Welbat JU. Hesperidin reduces memory impairment associated with adult rat hippocampal neurogenesis triggered by valproic acid. Nutrients. 2021;13(12):4364.
Li X, Huang W, Tan R, Xu C, Chen X, Li S, Liu Y, Qiu H, Cao H, Cheng Q. The benefits of hesperidin in central nervous system disorders, based on the neuroprotective effect. Biomed Pharmacother. 2023b;159:114222.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–2535.
Schwarzer GC, Rücker JR G. Meta-analysis in R. Systematic Reviews in Health Research. edn. Springer; 2015. p. 1.
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Harrer M, Cuijpers P, Furukawa T, Ebert DD. (2019) dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’. R package version 0.0.9000.
Peterson BG, Carl P, Boudt K, Bennett R, Ulrich J, Zivot E, Cornilly D, Hung E, Lestel M, Balkissoon K. (2020) PerformanceAnalytics: Econometric tools for performance and risk analysis. R package version 2.0. 4.
Ahmed AS, Mona MM, Elsisy RA, Hantash EM. Hesperidin preserves cognitive functions and Hippocampus histological architecture in albino wistar rats subjected to stress through enhancement of brain-derived neurotrophic factor. Neurotoxicity Res. 2022;40(1):179–85.
Antunes MS, Cattelan Souza L, Ladd FVL, Ladd AABL, Moreira AL, Bortolotto VC, et al. Hesperidin ameliorates anxiety-depressive-like behavior in 6-OHDA model of Parkinson’s disease by regulating striatal cytokine and neurotrophic factors levels and dopaminergic innervation loss in the striatum of mice. Mol Neurobiol. 2020;57:3027–41.
Antunes MS, Jesse CR, Ruff JR, de Oliveira Espinosa D, Gomes NS, Altvater EET, Donato F, Giacomeli R, Boeira SP. Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity. Eur J Pharmacol. 2016;789:411–20.
Hemanth Kumar B, Dinesh Kumar B, Diwan PV. Hesperidin, a citrus flavonoid, protects against l-methionine-induced hyperhomocysteinemia by abrogation of oxidative stress, endothelial dysfunction and neurotoxicity in Wistar rats. Pharm Biol. 2017;55:146–55.
Bensaoula DA, Bouhali IE, Boukhris N, Tahraoui A. Hesperidin, a natural polyphenol, alleviates Hyperglycaemic State and mitigates anxiety-like Behavior in Diabetic Male Wistar Rat. Middle-East J Sci Res. 2015;27:1276–9.
Boyina HK, Jerald MK, Bharatraj DK, Diwan PV. Influence of fisetin combined with hesperidin on chronic mild hyperhomocysteinemia induced cognitive dysfunction and oxidative stress in wistar rats. PharmaNutrition. 2018;6(3):125–136
Pradhan SP, Sahoo S, Behera A, Sahoo R, Sahu PK. Memory amelioration by hesperidin conjugated gold nanoparticles in diabetes induced cognitive impaired rats. J Drug Deliv Sci Technol. 2022;69:103145.
Kumar A, Chaudhary T, Mishra J. Minocycline modulates neuroprotective effect of hesperidin against quinolinic acid induced Huntington’s disease like symptoms in rats: behavioral, biochemical, cellular and histological evidences. Eur J Pharmacol. 2013;720:16–28.
Noshy PA, Azouz RA. Neuroprotective effect of hesperidin against emamectin benzoate-induced neurobehavioral toxicity in rats. Neurotoxicol Teratol. 2021;86:106981.
Kumar N, Yadav M, Kumar A, Kadian M, Kumar S. (2022) Neuroprotective effect of hesperidin and its combination with coenzyme Q10 on an animal model of ketamine-induced psychosis: behavioral changes, mitochondrial dysfunctions, and oxidative stress. Future Journal of Pharmaceutical Sciences 8.
Thenmozhi AJ, Raja TRW, Janakiraman U, Manivasagam T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem Res. 2015;40:767–76.
Habibyar AF, Sharma N, Khurana N. PASS assisted prediction and pharmacological evaluation of hesperidin against scopolamine induced amnesia in mice. Eur J Pharmacol. 2016;789:385–94.
Menze ET, Tadros MG, Abdel-Tawab AM, Khalifa AE. Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. Neurotoxicology. 2012;33(5):1265–75.
Ishola IO, Ben-Azu B, Adebayo OA, Ajayi AM, Omorodion IL, Edje KE, Adeyemi OO. Prevention and reversal of ketamine-induced experimental psychosis in mice by the neuroactive flavonoid, hesperidin: the role of oxidative and cholinergic mechanisms. Brain Res Bull. 2021;177:239–51.
Kumar P, Kumar A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington’s like symptoms in rats: possible role of nitric oxide. Behav Brain Res. 2010;206(1):38–46.
Antunes MS, Goes AT, Boeira SP, Prigol M, Jesse CR. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition. 2014;30(11-12):1415–22.
Fu H, Liu L, Tong Y, Li Y, Zhang X, Gao X, Yong J, Zhao J, Xiao D, Wen K, Wang H. The antidepressant effects of hesperidin on chronic unpredictable mild stress-induced mice. Eur J Pharmacol. 2019;853:236–46.
Zhu X, Liu H, Liu Y, Chen Y, Liu Y, Yin X. The antidepressant-like effects of hesperidin in streptozotocin-induced diabetic rats by activating Nrf2/ARE/Glyoxalase 1 pathway. Front Pharmacol. 2020;11:1325.
Xie L, Gu Z, Liu H, Jia B, Wang Y, Cao M, et al. The Anti-Depressive Effects of Hesperidin and the Relative Mechanisms Based on the NLRP3 Inflammatory Signaling Pathway. Front Pharmacol. 2020;11:1251.
Lee B, Choi GM, Sur B. Antidepressant-like effects of hesperidin in animal model of post-traumatic stress disorder. Chin J Integr Med. 2021;27(1):39–46.
Justin-Thenmozhi A, Dhivya Bharathi M, Kiruthika R, Manivasagam T, Borah A, Essa MM. Attenuation of Aluminum Chloride-Induced Neuroinflammation and Caspase Activation through the AKT/GSK-3β pathway by Hesperidin in Wistar rats. Neurotox Res. 2018;34:463–76.
Banji OJ, Banji D, Ch K. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem Toxicol. 2014;74:51–9.
Jaiswal P, Mandal M, Mishra A. Effect of hesperidin on fluoride-induced neurobehavioral and biochemical changes in rats. J Biochem Mol Toxicol. 2020;34(11):e22575.
Khodadadeh A, Hassanpour S, Akbari G. Prenatal exposure to hesperidin improves reflexive motor behaviors in mice offspring. Int J Dev Neurosci. 2020;80(7):648–56.
Abou Baker DH, Ibrahim BMM, Hassan NS, Yousuf AF, Gengaihi SE. Exploiting Citrus aurantium seeds and their secondary metabolites in the management of Alzheimer disease. Toxicol Rep. 2020;7:723–29.
Ekundayo BE, Obafemi TO, Afolabi BA, Adewale OB, Onasanya A, Osukoya OA, et al. Gallic acid and hesperidin elevate neurotransmitters level and protect against oxidative stress, inflammation and apoptosis in aluminum chloride-induced Alzheimer–s disease in rats. Pharm Res Modern Chin Med. 2022;5:100193.
Kwatra M, Ahmed S, Gawali B, Panda SR, Naidu V. Hesperidin alleviates chronic restraint stress and lipopolysaccharide-induced Hippocampus and Frontal cortex damage in mice: Role of TLR4/NF-κB, p38 MAPK/JNK, Nrf2/ARE signaling. Neurochem Int. 2020;140:104835.
Wang D, Liu L, Zhu X, Wu W, Wang Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer–s disease. Cell Mol Neurobiol. 2014;34(8):1209–21.
Li H, Ren J, Li Y, Wu Q, Wei J. Oxidative stress: the nexus of obesity and cognitive dysfunction in diabetes. Front Endocrinol (Lausanne). 2023;14:1134025.
Naewla S, Sirichoat A, Pannangrong W, Chaisawang P, Wigmore P, Welbat JU. Hesperidin alleviates methotrexate-induced memory deficits via hippocampal neurogenesis in adult rats. Nutrients. 2019;11(4):936.
Thenmozhi JA, William Raja TR, Manivasagam T, Janakiraman U, Essa MM. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer–s disease. Nutr Neurosci. 2017;20(6):360–68.
Raza SS, Khan MM, Ahmad A, Ashafaq M, Khuwaja G, Tabassum R, Javed H, Siddiqui MS, Safhi MM, Islam F. Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Res. 2011;1420:93–105.
Viswanatha GL, Shylaja H, Sandeep Rao KS, Santhosh Kumar VR, Jagadeesh M. (2012) Hesperidin ameliorates immobilization-stress-induced behavioral and biochemical alterations and mitochondrial dysfunction in mice by modulating nitrergic pathway. ISRN Pharmacol. 2012:479570.
Jangra A, Kasbe P, Pandey SN, Dwivedi S, Gurjar SS, Kwatra M, et al. Hesperidin and silibinin ameliorate aluminum-induced neurotoxicity: modulation of antioxidants and inflammatory cytokines level in mice hippocampus. Biol Trace Elem Res. 2015;168:462–71.
Kosari-Nasab M, Shokouhi G, Ghorbanihaghjo A, Abbasi MM, Salari AA. Hesperidin attenuates depression-related symptoms in mice with mild traumatic brain injury. Life Sci. 2018;213:198–205.
Hong Y, An Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch Pharm Res. 2018;41:655–63.
Bensaoula DA, Bouhali IE, Boukhris N, Tahraoui A. Hesperidin, a natural polyphenol, alleviates hyperglycaemic state and mitigates anxiety-like behavior in diabetic male Wistar rat. Middle-East J Sci Res. 2015;23(7):1276–79.
Zhu X, Zhang YM, Zhang MY, Chen YJ, Liu YW. Hesperetin ameliorates diabetes-associated anxiety and depression-like behaviors in rats via activating Nrf2/ARE pathway. Metab Brain Dis. 2021;36:1969–83.
Lee D, Kim N, Jeon SH, Gee MS, Ju YJ, Jung MJ, et al. Hesperidin improves memory function by enhancing neurogenesis in a mouse model of Alzheimer’s disease. Nutrients. 2022;14(15):3125.
Gaur V, Kumar A. Hesperidin pre-treatment attenuates NO-mediated cerebral ischemic reperfusion injury and memory dysfunction. Pharmacol Rep. 2010;62:635–48.
Atabaki R, Roohbakhsh A, Moghimi A, Mehri S. Protective effects of maternal administration of curcumin and hesperidin in the rat offspring following repeated febrile seizure: role of inflammation and TLR4. Int Immunopharmacol. 2020;86:106720.
Merzoug S, Toumi ML. Effects of hesperidin on formaldehyde-induced toxicity in pregnant rats. EXCLI J. 2017;16:400.
El-Magd NFA, El-Kashef DH, El-Sherbiny M, Eraky SM. Hepatoprotective and cognitive-enhancing effects of hesperidin against thioacetamide-induced hepatic encephalopathy in rats. Life Sci. 2023;313:121280.
Kandlur A, Satyamoorthy K, Gangadharan G. Oxidative stress in cognitive and epigenetic aging: a retrospective glance. Front Mol Neurosci. 2020;13:41.
Singh P, Barman B, Thakur MK. Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front Aging Neurosci. 2022;14:944697.
Antunes MS, Ladd FVL, Ladd A, Moreira AL, Boeira SP, Cattelan Souza L. Hesperidin protects against behavioral alterations and loss of dopaminergic neurons in 6-OHDA-lesioned mice: the role of mitochondrial dysfunction and apoptosis. Metab Brain Dis. 2021;36:153–67.
Li M, Shao H, Zhang X, Qin B. Hesperidin alleviates Lipopolysaccharide-Induced Neuroinflammation in mice by promoting the miRNA-132 pathway. Inflammation. 2016;39:1681–9.
Acknowledgements
Not applicable.
Funding
Open access funding provided by University of KwaZulu-Natal.
Author information
Authors and Affiliations
Contributions
TAO conceptualised, designed the study and wrote the first draft. TCE initiated the search for relevant articles and carried our meta analysis. TAO and TCE carried out screening of aricles, inclusion and exclusion decision-making. OI and AOO critically proofread the manuscript. All the authors read, proof checked, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olasehinde, T.A., Ekundayo, T.C., Ijabadeniyi, O.A. et al. The Impact of Hesperidin on Cognitive Deficit and Neurobehavioural Disorders: A Systematic Review and Meta-Analysis of Preclinical Individual Studies. Curr Behav Neurosci Rep 11, 246–259 (2024). https://doi.org/10.1007/s40473-024-00284-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40473-024-00284-9