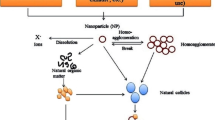
Abstract
Nanomaterials (NMs) are known to have at least one dimension between about 1 and 100 nm. These nanomaterials can be categorized into nanoparticles (NPs), nanoplates, and nanofibers. In the last few decades, nanomaterial has got attention due to their wide applications in various sectors (such as environmental, pharmaceutical, personal care products, disease diagnosis, and treatment) based on their physicochemical properties. However, there are more concerns among environmentalists and scientists about the release of nanoparticles into the environment through different sources, which can cause environmental problems, human health hazard, and ecotoxicological issues. Aquatic biota is predominantly affected by the pollution caused by NPs. The fate, transport, and toxicity of NPs in aquatic ecosystem are affected by the transformation, which is dependent upon the initial NM properties, and its surrounding chemical and biological environment. Numerous research investigations have explored different types of NPs and their properties, applications, and hazards, but only a few have focused on the effect of NPs on aquatic ecosystem. In this chapter, ecotoxicological effects of NPs on aquatic ecosystem have been discussed based on the physiochemical properties by exploiting the latest research reports and future directions are highlighted.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abd Ellah NH, Abouelmagd SA (2017) Surface functionalization of polymeric nanoparticles for tumor drug delivery: approaches and challenges. Expert Opin Drug Deliv 14(2):201–214. https://doi.org/10.1080/17425247.2016.1213238
Abouelmagd SA, Meng F, Kim BK, Hyun H, Yeo Y (2016) Tannic acid-mediated surface functionalization of polymeric nanoparticles. ACS Biomater Sci Eng 2(12):2294–2303. https://doi.org/10.1021/acsbiomaterials.6b00497
Adams-Haduch JM, Paterson DL, Sidjabat HE, Pasculle AW, Potoski BA, Muto CA et al (2008) Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob Agents Chemother 52(11):3837–3843
Aitken RJ, Chaudhry MQ, Boxall AB, Hull M (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med (Lond) 56(5):300–306. doi:56/5/300 [pii]10.1093/occmed/kql051
Alexander DE (1999) Encyclopedia of environmental science. Springer. ISBN 0-412-74050-8
Ali A, Zafar H, Zia M, Ul Haq I, Phull AR, Ali JS et al (2016) Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 9:49–67
Allen BL, Kichambare PD, Gou P, Vlasova II, Kapralov AA, Konduru N et al (2008) Biodegradation of single-walled carbon nanotubes through enzymatic catalysis 8(11):3899–3903. https://doi.org/10.1021/nl802315h
Aruoja V, Pokhrel S, Sihtma¨e M, Mortimer M, Ma¨dler L, Kahru A (2015) Toxicity of 12 metalbased nanoparticles to algae, bacteria and protozoa. Environ Sci Nano 630(2)
Asharani PV, Lian WY, Gong Z, Valiyaveettil S (2008) Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 19(25):255102. doi:S0957-4484(08)71001-8 [pii]10.1088/0957-4484/19/25/255102
Ates M, Daniels J, Arslan Z, Farah IO, Rivera HF (2013) Comparative evaluation of impact of Zn and ZnO nanoparticles on brine shrimp (Artemia salina) larvae: effects of particle size and solubility on toxicity. Environ Sci Process Impacts 15(1):225–233. https://doi.org/10.1039/c2em30540b
Avasare V, Zhang Z, Avasare D, Khan I, Qurashi A (2015) Room-temperature synthesis of TiO2 nanospheres and their solar driven photoelectrochemical hydrogen production. Int J Energy Res 39:1714–1719
Baptista MS, Miller RJ, Halewood ER, Hanna SK, Almeida CM, Vasconcelos VM et al (2015) Impacts of silver nanoparticles on a natural estuarine plankton community. Environ Sci Technol 49(21):12968–12974. https://doi.org/10.1021/acs.est.5b03285
Bian SW, Mudunkotuwa IA, Rupasinghe T, Grassian VH (2011) Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueousenvironments: influence of ph, ionic strength, size, and adsorption ofhumic acid. Langmuir 27:6059–6068
Burchardt AD, Carvalho RN, Valente A, Nativo P, Gilliland D, Garcia CP et al (2012) Effects of silver nanoparticles in diatom Thalassiosira pseudonana and cyanobacterium Synechococcus sp. Environ Sci Technol 46(20):11336–11344. https://doi.org/10.1021/es300989e
Buseck PR, Posfai M (1999) Airborne minerals and related aerosol particles: effects on climate and the environment. Proc Natl Acad Sci U S A 96(7):3372–3379. https://doi.org/10.1073/pnas.96.7.3372
Calvo P, Remuoon-Lopez C, Vila-Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63:125–132
Champion JA, Mitragotri S (2006) Role of _target geometry in phagocytosis. Proc Natl Acad Sci USA 103(13):4930–4934. doi:0600997103 [pii]10.1073/pnas.0600997103
Chen Z, Meng H, Xing G, Chen C, Zhao Y, Jia G et al (2006) Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett 163(2):109–120. doi:S0378-4274(05)00317-6 [pii]10.1016/j.toxlet.2005.10.003
Chithrani BD, Ghazani AA, Chan WC (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6(4):662–668. https://doi.org/10.1021/nl052396o
Das P, Williams CJ, Fulthorpe RR, Hoque ME, Metcalfe CD, Xenopoulos MA (2012) Changes in bacterial community structure after exposure to silver nanoparticles in natural waters. Environ Sci Technol 46(16):9120–9128. https://doi.org/10.1021/es3019918
Domingo G, Bracale M, Vannini C (2019). Phytotoxicity of silver nanoparticles to aquatic plants, algae, and microorganisms
Donaldson K, Stone V (2003) Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann Ist Super Sanita 39(3):405–410
Dong H, Wen B, Melnik R (2014) Relative importance of grain boundaries and size effects in thermal conductivity of nanocrystalline materials. Sci Rep 4:7037. doi:srep07037 [pii]10.1038/srep07037
Donia DT, Carbone M (2019) Fate of the nanoparticles in environmental cycles. Int J Environ Sci Technol 16:583–600
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA (2012) The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 41(7):2740–2779. https://doi.org/10.1039/c1cs15237h
Duong TT, Le TS, Huong Tran TT, Nguyen TK, Ho CT, Dao TH, Dang DK, Ha PT (2016) Inhibition effect of engineered silver nanoparticles to bloom forming cyanobacteria. Adv Nat Sci Nanosci Nanotechnol 7(3)
El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM (2011) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45(1):283–287. https://doi.org/10.1021/es1034188
Elzey S, Grassian V (2010) Agglomeration, isolation and dissolution of commercially manufactured silver nanoparticles in aqueous environments. J Nanopart Res 12(5):1945–1958
Fabrega J, Fawcett SR, Renshaw JC, Lead JR (2009) Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ Sci Technol 43(19):7285–7290. https://doi.org/10.1021/es803259g
Fabrega J, Tantra R, Amer A, Stolpe B, Tomkins J, Fry T et al (2012) Sequestration of zinc from zinc oxide nanoparticles and life cycle effects in the sediment dweller amphipod Corophium volutator. Environ Sci Technol 46(2):1128–1135. https://doi.org/10.1021/es202570g
Fang XQ, Liu JX, Gupta V (2013) Fundamental formulations and recent achievements in piezoelectric nano-structures: a review. Nanoscale 5(5):1716–1726. https://doi.org/10.1039/c2nr33531j
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281(5374):237–240. https://doi.org/10.1126/science.281.5374.237
Gatoo MA, Naseem S, Arfat MY, Dar AM, Qasim K, Zubair S (2014) Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int 2014:498420. https://doi.org/10.1155/2014/498420
Gilbert B, Lu G, Kim CS (2007) Stable cluster formation in aqueous suspensions of iron oxyhydroxide nanoparticles. J Colloid Interface Sci 313(1):152–159. doi:S0021-9797(07)00479-1 [pii]10.1016/j.jcis.2007.04.038
Goodman CM, McCusker CD, Yilmaz T, Rotello VM (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem 15(4):897–900. https://doi.org/10.1021/bc049951i
Greeley J, Markovic NM (2012) The road from animal electricity to green energy: combining experiment and theory in electrocatalysis. Energy Environ Sci:5
Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27(9):1972–1978. doi:08-002 [pii]10.1897/08-002.1
Gubbins EJ, Batty LC, Lead JR (2011) Phytotoxicity of silver nanoparticles to Lemna minor L. Environ Pollut 159(6):1551–1559. doi:S0269-7491(11)00131-X [pii]10.1016/j.envpol.2011.03.002
Gujrati M, Malamas A, Shin T, Jin E, Sun Y, Lu ZR (2014) Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol Pharm 11(8):2734–2744. https://doi.org/10.1021/mp400787s
Guo D, Xie G, Luo J (2014) Mechanical properties of nanoparticles: basics and applications. J Phys D Appl Phys 47(13001)
Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A (2009) Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol 6:35. doi:1743-8977-6-35 [pii]10.1186/1743-8977-6-35
He D, Dorantes-Aranda JJ, Waite TD (2012) Silver nanoparticle-algae interactions: oxidative dissolution, reactive oxygen species generation and synergistic toxic effects. Environ Sci Technol 46(16):8731–8738. https://doi.org/10.1021/es300588a
Holden PA, Klaessig F, Turco RF, Priester JH, Rico CM, Avila-Arias H et al (2014) Evaluation of exposure concentrations used in assessing manufactured nanomaterial environmental hazards: are they relevant? Environ Sci Technol 48(18):10541–10551. https://doi.org/10.1021/es502440s
Holgate ST (2010) Exposure, uptake, distribution and toxicity of nanomaterials in humans. J Biomed Nanotechnol 6(1):1–19. https://doi.org/10.1166/jbn.2010.1098
Hsiao IL, Huang YJ (2011) Effects of various physicochemical characteristics on the toxicities of ZnO and TiO nanoparticles toward human lung epithelial cells. Sci Total Environ 409(7):1219–1228. doi:S0048-9697(10)01364-1 [pii]10.1016/j.scitotenv.2010.12.033
Huang J, Cheng J, Yi J (2016) Impact of silver nanoparticles on marine diatom Skeletonema costatum. J Appl Toxicol 36(10):1343–1354. https://doi.org/10.1002/jat.3325
Iavicoli I, Leso V, Ricciardi W, Hodson LL, Hoover MD (2014) Opportunities and challenges of nanotechnology in the green economy. Environ Health 13:78. doi:1476-069X-13-78 [pii]10.1186/1476-069X-13-78
Jahan S, Yusoff IB, Alias YB, Bakar A (2017) Reviews of the toxicity behavior of five potential engineered nanomaterials (ENMs) into the aquatic ecosystem. Toxicol Rep 4:211–220. https://doi.org/10.1016/j.toxrep.2017.04.001S2214-7500(17)30017-3[pii]
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Jiang HS, Li M, Chang FY, Li W, Yin LY (2012) Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem 31(8):1880–1886. https://doi.org/10.1002/etc.1899
Jiang HS, Qiu XN, Li GB, Li W, Yin LY (2014) Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ Toxicol Chem 33(6):1398–1405. https://doi.org/10.1002/etc.2577
Kang S, Mauter MS, Elimelech M (2008) Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ Sci Technol 42(19):7528–7534. https://doi.org/10.1021/es8010173
Karnik BS, Davies SH, Baumann MJ, Masten SJ (2005) Fabrication of catalytic membranes for the treatment of drinking water using combined ozonation and ultrafiltration. Environ Sci Technol 39(19):7656–7661. https://doi.org/10.1021/es0503938
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ et al (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44(6):1962–1967. https://doi.org/10.1021/es902987d
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1–17
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Kim J, Kim S, Lee S (2011) Differentiation of the toxicities of silver nanoparticles and silver ions to the Japanese medaka (Oryzias latipes) and the cladoceran Daphnia magna. Nanotoxicology 5(2):208–214. https://doi.org/10.3109/17435390.2010.508137
Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY et al (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27(9):1825–1851. https://doi.org/10.1897/08-090.1
Kohli AK, Alpar HO (2004) Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int J Pharm 275(1–2):13–17
Kot M, Major Ł, Lackner JM, Chronowska-Przywara K, Janusz M, Rakowski W (2016) Mechanical and tribological properties of carbon-based graded coatings. J Nanomater:1–14
Kraas M, Schlich K, Knopf B, Wege F, Kagi R, Terytze K et al (2017) Long-term effects of sulfidized silver nanoparticles in sewage sludge on soil microflora. Environ Toxicol Chem 36(12):3305–3313. https://doi.org/10.1002/etc.3904
Lankveld DP, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, Noorlander CW et al (2010) The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 31(32):8350–8361. doi:S0142-9612(10)00888-4 [pii]10.1016/j.biomaterials.2010.07.045
Larner F, Dogra Y, Dybowska A, Fabrega J, Stolpe B, Bridgestock LJ et al (2012) Tracing bioavailability of ZnO nanoparticles using stable isotope labeling. Environ Sci Technol 46(21):12137–12145. https://doi.org/10.1021/es302602j
Laurent S, Bridot JL, Elst LV, Muller RN (2010) Magnetic iron oxide nanoparticles for biomedical applications. Future Med Chem 2(3):427–449. https://doi.org/10.4155/fmc.09.164
Lee R (2001) Bioavailability, biotransformation and fate of organic contaminants in estuarine animals. CRC Press
Lee JE, Lee N, Kim T, Kim J, Hyeon T (2011) Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc Chem Res 44(10):893–902. https://doi.org/10.1021/ar2000259
Lei YM, Huang WX, Zhao M, Chai YQ, Yuan R, Zhuo Y (2015) Electrochemiluminescence resonance energy transfer system: mechanism and application in Ratiometric Aptasensor for lead ion. Anal Chem 87(15):7787–7794. https://doi.org/10.1021/acs.analchem.5b01445
Levard C, Hotze EM, Lowry GV, Brown GE Jr (2012) Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 46(13):6900–6914. https://doi.org/10.1021/es2037405
Li X, Schirmer K, Bernard L, Sigg L, Pillai P, Behra R (2015) Silver nanoparticle toxicity and association with the alga Euglena gracilis. Environ Sci Nano 2(6):594e602
Liu J, Hurt RH (2010) Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol 44(6):2169–2175. https://doi.org/10.1021/es9035557
Liu D, Li C, Zhou F, Zhang T, Zhang H, Li X et al (2015) Rapid synthesis of monodisperse Au nanospheres through a laser irradiation-induced shape conversion, self-assembly and their electromagnetic coupling SERS enhancement. Sci Rep 5:7686. doi:srep07686 [pii]10.1038/srep07686
Loureiro A, Azoia NG, Gomes AC, Cavaco-Paulo A (2016) Albumin-based Nanodevices as drug carriers. Curr Pharm Des 22(10):1371–1390. doi:CPD-EPUB-73211 [pii]10.2174/1381612822666160125114900
Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. Environ Sci Technol 46(13):6893–6899. https://doi.org/10.1021/es300839e
Mansha M, Khan I, Ullah N, Qurashi A (2017) Synthesis, characterization and visible-light-driven photoelectrochemical hydrogen evolution reaction of carbazole-containing conjugated polymers. Int J Hydrog Energy. https://doi.org/10.1016/j
Martis E, Badve R, Degwekar M (2012) Nanotechnology based devices and applications in medicine: an overview. Chron Young Sci 3(68)
Miller RJ, Lenihan HS, Muller EB, Tseng N, Hanna SK, Keller AA (2010) Impact of metal oxidenanoparticles on marine phytoplankton. Environ Sci Technol 44(19):7329–7334
Monteiro-Riviere NA, Wiench K, Landsiedel R, Schulte S, Inman AO, Riviere JE (2011) Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: an in vitro and in vivo study. Toxicol Sci 123(1):264–280. doi:kfr148 [pii]10.1093/toxsci/kfr148
Montes MO, Hanna SK, Lenihan HS, Keller AA (2012) Uptake, accumulation, and biotransformation of metal oxide nanoparticles by a marine suspension-feeder. J Hazard Mater 225-226:139–145. doi:S0304-3894(12)00491-8 [pii]10.1016/j.jhazmat.2012.05.009
Movafeghi A, Khataee A, Abedi M, Tarrahi R, Dadpour M, Vafaei F (2018) Effects of TiO2 nanoparticles on the aquatic plant Spirodela polyrrhiza: evaluation of growth parameters, pigment contents and antioxidant enzyme activities. J Environ Sci (China) 64:130–138. doi:S1001-0742(17)30321-2 [pii]10.1016/j.jes.2016.12.020
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42(12):4447–4453. https://doi.org/10.1021/es7029637
Nikalje AP (2015) Nanotechnology and its applications in medicine. Med Chem 5
Ning Z, Cheung CS, Fu J, Liu MA, Schnell MA (2006) Experimental study of environmental tobacco smoke particles under actual indoor environment. Sci Total Environ 367(2–3):822–830. doi:S0048-9697(06)00155-0 [pii]10.1016/j.scitotenv.2006.02.017
OECD OfEC, Development. Guidelines for testing of chemicals, section 2: effects on biotic systems, procedure 201. Algal Growth Inhibition Test 1984; Organisation for Economic Cooperation and Development, Paris
Oukarroum A, Barhoumi L, Pirastru L, Dewez D (2013) Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemnagibba. Environ Toxicol Chem 32(902e907)
Park KH, Chhowalla M, Iqbal Z, Sesti F (2003) Single-walled carbon nanotubes are a new class of ion channel blockers. J Biol Chem 278(50):50212–50216. https://doi.org/10.1074/jbc.M310216200M310216200[pii]
Peng X, Palma S, Fisher NS, Wong SS (2011) Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat Toxicol 102(3–4):186–196. doi:S0166-445X(11)00021-X [pii]10.1016/j.aquatox.2011.01.014
Petersen EJ, Nelson BC (2010) Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal Bioanal Chem 398(2):613–650. https://doi.org/10.1007/s00216-010-3881-7
Powers KW, Palazuelos M, Moudgil BM, Roberts SM (2007) Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology 1(1):42–51
Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E et al (2009) Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst 26(6):523–580. doi:7a0c3bee026fb778,313cd9ee34e9e406 [pii]10.1615/critrevtherdrugcarriersyst.v26.i6.10
Rao JP, Geckeler KE (2011) Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci 36:887–913
Risom L, Moller P, Loft S (2005) Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res 592(1–2):119–137. doi:S0027-5107(05)00246-0 [pii]10.1016/j.mrfmmm.2005.06.012
Rogozea EA, Petcu AR, Olteanu NL, Lazar CA, Cadar D, Mihaly M (2017) Tandem adsorption-photodegradation activity induced by light on NiO-ZnO p–n couple modified silica nanomaterials. Mater Sci Semicond Process 57:1–11
Saeed K, Khan I (2014) Preparation and properties of single-walled carbon nanotubes/poly(butylene terephthalate) nanocomposites. Iran Polym J 23:53–58
Saeed K, Khan I (2016) Preparation and characterization of singlewalled carbon nanotube/nylon 6,6 nanocomposites. Instrum Sci Technol 44:435–444
Sani-Kast N, Labille J, Ollivier P, Slomberg D, Hungerbuhler K, Scheringer M (2017) A network perspective reveals decreasing material diversity in studies on nanoparticle interactions with dissolved organic matter. Proc Natl Acad Sci U S A 114(10):E1756–E1E65. doi:1608106114 [pii]10.1073/pnas.1608106114
Sapkota A, Symons JM, Kleissl J, Wang L, Parlange MB, Ondov J et al (2005) Impact of the 2002 Canadian forest fires on particulate matter air quality in Baltimore city. Environ Sci Technol 39(1):24–32. https://doi.org/10.1021/es035311z
Scown TM, Santos EM, Johnston BD, Gaiser B, Baalousha M, Mitov S et al (2010) Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol Sci 115(2):521–534. doi:kfq076 [pii]10.1093/toxsci/kfq076
Shao W, Nabb D, Renevier N, Sherrington I, Luo JK (2012) Mechanical and corrosion resistance properties of TiO2 nanoparticles reinforced Ni coating by electrodeposition. IOP Conf Ser Mater Sci Eng 40(12042)
Shin WK, Cho J, Kannan AG, Lee YS, Kim DW (2016) Cross-linked composite gel polymer electrolyte using mesoporous methacrylate-functionalized SiO2 nanoparticles for lithium-ion polymer batteries. Sci Rep 6:26332. doi:srep26332 [pii]10.1038/srep26332
Sioutas C, Delfino RJ, Singh M (2005) Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ Health Perspect 113(8):947–955. https://doi.org/10.1289/ehp.7939
Sohm B, Immel F, Bauda P, Pagnout C (2015) Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics 15(1):98–113. https://doi.org/10.1002/pmic.201400101
Stefani D, Wardman D, Lambert T (2005) The implosion of the Calgary general hospital: ambient air quality issues. J Air Waste Manag Assoc 55(1):52–59. https://doi.org/10.1080/10473289.2005.10464605
Sun S, Murray CB, Weller D, Folks L, Moser A (2000) Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 287(5460):1989–1992. doi:8349 [pii]10.1126/science.287.5460.1989
Taylor DA (2002) Dust in the wind. Environ Health Perspect 110(2):A80–A87. doi:sc271_5_1835 [pii]10.1289/ehp.110-a80
Taylor C, Matzke M, Kroll A, Read DS, Svendsen C, Crossley A (2016) Toxic interactions of different silver forms with freshwater green algae and cyanobacteria and their effects on mechanistic endpoints and the production of extracellular polymeric substances. Environ Sci Nano 3(396e408)
Thalmann B, Voegelin A, Morgenroth E, Kaegi R (2016) Effect of humic acid on the kinetics of silver nanoparticle sulfidation. Environ Sci Nano 3(1):203–212
Thomas SC, Harshita, Mishra PK, Talegaonkar S (2015) Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des 21(42):6165–6188. doi:CPD-EPUB-71344 [pii]10.2174/1381612821666151027153246
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nano Today 1:44–48
Turan NB, Erkan HS, Engin GO, Bilgili MS (2019) Nanoparticles in the aquatic environment: usage, properties,transformation and toxicity—a review. Process Saf Environ Prot 130:238–249
Turner A, Brice D, Brown MT (2012) Interactions of silver nanoparticles with the marine macroalga. Ulva lactuca Ecotoxicology 21(1):148–154. https://doi.org/10.1007/s10646-011-0774-2
Ullah H, Khan I, Yamani ZH, Qurashi A (2017) Sonochemical-driven ultrafast facile synthesis of SnO2 nanoparticles: growth mechanism structural electrical and hydrogen gas sensing properties. Ultrason Sonochem 34:484–490. doi:S1350-4177(16)30223-1 [pii]10.1016/j.ultsonch.2016.06.025
Unser S, Bruzas I, He J, Sagle L (2015) Localized surface Plasmon resonance biosensing: current challenges and approaches. Sensors (Basel) 15(7):15684–15716. doi:s150715684 [pii]10.3390/s150715684
USEPA UEPA (1996) Ecological effect test guidelines. OPPTS 850.5400. Algal Toxicity, Tiers I and II
Vaccari DA (2005) Environmental biology for engineers and scientists. Wiley-Interscience. ISBN 0-471-74178-7
Walters C, Pool E, Somerset V (2013) Aggregation and dissolution of silver nanoparticles in alaboratory-based freshwater microcosm under simulated environmental conditions. Toxicol Environ Chem 95(10):1690–1701
Walters C, Pool E, Somerset V (2016) Nanotoxicology: a review, toxicology – new aspects to this scientific conundrum. Larramendy. IntechOpen. https://doi.org/10.5772/64754
Weinberg H, Galyean A, Leopold M (2011) Evaluating engineered nanoparticlesin natural waters. TrAC Trends Anal Chem 30:72–83
Wong SW, Leung PT, Djurisic AB, Leung KM (2010) Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal Bioanal Chem 396(2):609–618. https://doi.org/10.1007/s00216-009-3249-z
Wu J, Lu H, Zhu G, Chen L, Chang Y, Yu R (2017) Regulation of membrane fixation and energy production/conversion for adaptation and recovery of ZnO nanoparticle impacted Nitrosomonas europaea. Appl Microbiol Biotechnol 101(7):2953–2965. https://doi.org/10.1007/s00253-017-8092-010.1007/s00253-017-8092-0[pii]
Xiong D, Fang T, Yu L, Sima X, Zhu W (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ 409(8):1444–1452
Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12(8):4271–4275. https://doi.org/10.1021/nl301934w
Yang ST, Wang X, Jia G, Gu Y, Wang T, Nie H et al (2008) Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol Lett 181(3):182–189. doi:S0378-4274(08)01199-5 [pii]10.1016/j.toxlet.2008.07.020
Young KJ, Martini LA, Milot RL, Snoeberger RC III, Batista VS, Schmuttenmaer CA et al (2012) Light-driven water oxidation for solar fuels. Coord Chem Rev 256(21–22):2503–2520. https://doi.org/10.1016/j.ccr.2012.03.031
Zhao J, Wang Z, White JC, Xing B (2014) Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ Sci Technol 48(17):9995–10009. https://doi.org/10.1021/es5022679
Zhu X, Wang J, Zhang X, Chang Y, Chen Y (2009) The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 20(19):195103. doi:S0957-4484(09)03849-5 [pii]10.1088/0957-4484/20/19/195103
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Appendices
Questions
-
1.
What type of nanoparticles are 100 times stronger than steel and can be used for the structural reinforcement?
-
A.
Carbon-based nanoparticles.
-
B.
Semiconductor nanoparticles.
-
C.
Ceramic nanoparticles.
-
D.
None of the above.
-
A.
-
2.
How elevated temperature affects the aggregation property in relevance to toxicity of nanoparticles?
-
3.
How does size and surface area affect the toxicity of nanoparticles?
-
4.
What are the different categories of environmental applications of nanoparticles?
-
5.
What are the different sources of nanoparticles release into the environment?
-
6.
What is transformation of nanoparticles? What are the different forms of nanoparticle transformation occurring in natural systems?
-
7.
What are the ecotoxicological effects of NPs on phytoplankton, which are the primary producers of aquatic food web.
Answers
-
1.
(A) Carbon-based nanoparticles
-
2.
With increase in temperature, smaller aggregates will be formed, which further increases toxicity of nanoparticles.
-
3.
Size affects the toxicity of NPs as it leads to change in surface area. As the size of the material decreases, the surface area increases exponentially, which enhances the reactivity of nanoparticle surface. For example, with decrease in size of copper nanoparticles, the oral toxicity also increases.
-
4.
Three categories:
-
A.
Environmentally benign and/or sustainable products (e.g., green chemistry or pollution prevention).
-
B.
Remediation of materials contaminated with hazardous substances.
-
C.
Sensors for environmental agents.
-
A.
-
5.
There are three main sources, which include: incidental, engineered, and naturally released nanoparticles.
-
6.
The process of converting the nanoparticles into different reactive forms has potential impacts on their behavior, and effects in the environment are called as transformation of nanoparticles. The chemical, physical, and biological were different forms of nanoparticle transformation occurring in natural systems.
-
7.
NPs affect the phytoplankton in numerous ways, such as the growth rate of phytoplankton gets suppressed by NPs. They also decline the equilibrium densities of phytoplankton as well as zooplankton, as the contact with NPs and phytoplankton increases. Photosynthetic efficiency of them was also reduced by the toxicity of NPs.
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Choudhary, R., Kumar, S., Sethi, P. (2023). New Perspective Application and Hazards of Nanomaterial in Aquatic Environment. In: Kumar, R., Kumar, R., Chaudhary, S. (eds) Advanced Functional Nanoparticles "Boon or Bane" for Environment Remediation Applications. Environmental Contamination Remediation and Management. Springer, Cham. https://doi.org/10.1007/978-3-031-24416-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-24416-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24415-5
Online ISBN: 978-3-031-24416-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)