Abstract
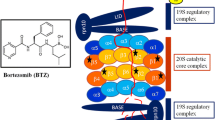
The development of proteasome inhibitors for treatment of multiple myeloma and probably other cancers has followed an unusual course but is clearly linked to recent basic advances in our understanding of intracellular protein breakdown. After the discovery of the ATP-dependent pathway for protein degradation in the 1970s, ATP was shown necessary for the conjugation of ubiquitin to cell proteins, which marks them for degradation by the 26S proteasome. Its 19S regulatory complex uses ATP to unfold proteins and to inject them into the 20S core proteasome where proteins are digested to small peptides. The active sites in the 20S proteasome function by a novel threonine-based mechanism which allows their selective inhibition (e.g., by the boronate, Velcade). Surprisingly, we initially organized a biotechnology company to develop inhibitors of the ubiquitin—proteasome pathway not for cancer therapy, but with the goal of reducing the excessive proteolysis seen in atrophying muscle or cachexia, as well as inhibition of MHC class I antigen presentation, both of which depend on proteasome function. The availability of proteasome inhibitors has greatly advanced our understanding of the many functions of the proteasome, such as its key role in the activation of the transcription factor NF-κB, which led to a recognition that proteasome inhibitors might have anti-inflammatory and antineoplastic actions. The unexpected discovery that these inhibitors cause apoptosis selectively in neoplastic cells led to systematic studies and clinical trials against cancer. Amongst their multiple actions, proteasome inhibitors (1) cause the accumulation of abnormal proteins, which can trigger apoptosis; (2) stabilize tumor suppressors (p53, p27); (3) inhibit production of NF-κB, which is antiapoptic and generates important growth factors and cell adhesion molecules. However, the actual importance of these mechanisms in vivo in combating cancer remains uncertain.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Mitch WE, et al. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 1996;335:1897–1905.
Lecker SH, et al. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 1999;129(1S suppl):227S–237S.
Goldberg AL. Protein turnover in skeletal muscle II: Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem 1969;244:3223–3229.
Goldberg AL, et al. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem 1990.
Goldberg AL, et al. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem 1976;45:747–803.
Goldberg AL. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci USA 1971;68:362–366.
Goldberg AL. Degradation of abnormal proteins in E. coli. Proc Nall Acad Sci USA 1972;69:422–426.
Seemüller E, et al. Proteasome from Thermoplasma acidophilum-a threonine protease. Science 1995;268:579–582.
Etlinger JD, et al. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Nat/ Acad Sci USA 1977;74:54–58.
Hershko A, et al. The ubiquitin system. Annu Rev Biochem 1998;67:425–479.
Glickman M, et al. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 2002;82:373–428.
Goldberg AL. The mechanism and functions of ATP-dependent proteases in bacterial and animal cells. Eur J Biochem 1992;203:9–23.
Wickner S, et al. Posttranslational quality control: folding, refolding, and degrading proteins. Science 1999;286:801–847.
Coux O, et al. Structure and functions of the 20S and 26S proteasomes. Ann Rev Biochem 1996;65:801–847.
DeMartino GN, et al. Identification and partial purification of an ATP-stimulated alkaline protease in rat liver. J Biol Chem 1979;254:3712–3715.
Hough R, et al. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem 1987;261:2400–2408.
Waxman L, et al. A soluble ATP-dependent system for protein degradation from murine erythroleukemia cells: evidence for a protease which requires ATP hydrolysis but not ubiquitin. J Biol Chem 1985;260:11994–12000.
Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry 1990;29:10289–10297.
Arrigo A, et al. Identity of the 19S ‘prosome’ particle with the large multifunctional protease complex of mammalian cells (the proteasome). Nature 1988;331:192–194.
Matthews W, et al. Involvement of the proteasome in various degradative processes in mammalian cells. Proc Nat/ Acad Sci USA 1989;86:2597–2601.
Eytan E, et al. ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc Nall Acad Sci USA 1989;86:7751–7755.
Driscoll J, et al. The proteasome (multicatalytic protease) is a component of the 1500kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem 1990;265:4789–4792.
Bodine SC, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001;294:1704–1708.
Gomes M, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 2001;98:14440–14445.
Tawa, NE, et al. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest 1997;100:197–203.
Rock KL, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class 1 molecules. Cell 1994;78:761–771.
Silverman N, et al. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dey 2001;15:2321–2342.
Lee DH, et al. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol 1998;8:397–403.
Kisselev AF, et al. Proteasome inhibitors: from research tools to drug candidates. Chem Biol 2001;8:739–758.
Lee DH, et al. The proteasome inhibitors and their uses. In: Proteasomes: The World of Regulatory Proteolysis. (Wolf DH and Hilt W, eds.). Georgetown, TX: Landes Bioscience, 1999.
Voges D, et al. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 1999;68:1015–1068.
Palombella VJ, et al. The ubiquitin-proteasome pathway is required for processing the NF-kappa-B1 precursor protein and the activation of NF-kappa-B. Cell 1994;78:773–785.
Nussbaum AK, et al. Cleavage motifs of the yeast 20S proteasome beta subunits deduced from digests of enolase 1. Proc Natl Acade Sci USA 1998;95:12504–12509.
Kisselev AF, et al. The sizes of peptides generated from protein by mammalian 26S and 20S proteasomes: implications for understanding the degradative mechanism and antigen presentation. J Biol Chem 1999;274:3363–3371.
Holzl H, et al. The regulatory complex of Drosophila melanogaster 26S proteasomes: subunit composition and localization of a deubiquitylating enzyme. J Cell Biol 2000;150:119–129.
Tanahashi N, et al. Hybrid proteasomes. Induction by interferon-gamma and contribution to ATP-dependent proteolysis. J Biol Chem 2000 275:14336–14345.
Yang Y, et al. In vivo assembly of the proteasomal complexes, implications for antigen processing. J Biol Chem 1995;270:27687–27694.
Gronostajski RM, et al. The ATP dependence of the degradation of short- and long-lived proteins in growing fibroblasts. J Biol Chem 1985;260:3344–3349.
Whitby FG, et al. Structural basis for the activation of 20S proteasomes by 11S regulators. Nature 2000;408:115–120.
Cascio P, et al. Properties of the hybrid form of the 26S proteasome containing both 19S and PA28 complexes. EMBO J 2002;21:2636–2645.
Rechsteiner M, et al. The proteasome activator 11 S REG (PA28) and class I antigen presentation. Biochem J 2000;345:1–15.
Goldberg AL, et al. Not just research tools-proteasome inhibitors offer therapeutic promise. Nat Med 2002;8:338–440.
Goldberg AL, et al. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol 2002;1169:1–17.
Baumeister W, et al. The proteasome: paradigm of a self-compartmentalizing protease. Cell 1998;92:367–380.
Groll M, et al. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 1997;386:463–471.
Groll M, et al. A gated channel into the proteasome core particle. Nat Struc Biol 2000;7:1062–1067.
Wenzel T, et al. Conformational constraints in protein degradation by the 20S proteasome. Nat Struc Biol 1995;2:199–204.
Kohler A, et al. The axial channel of the proteasome core particle is gated by the Rpt2 ATPase and controls both substrate entry and product release. Mol Cell 2001;7:1143–1152.
Cascio P, et al. 26S proteasomes and immunoproteasomes produce mainly N-extended versions of an antigenic peptide. EMBO J 2001;20:2357–2366.
Kisselev AF, et al. Binding of hydrophobic peptides to several non-catalytic sites promotes peptide hydrolysis by all active sites of 20S proteasomes. Evidence for peptide-induced channel opening in the alpha-rings. J Biol Chem 2002;277:22260–22270.
Glickman MH, et al. A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell 1998;94:615–623.
Hochstrasser M. New proteases in a ubiquitin stew. Science 2002;298:549–552.
Verma R, et al. Role of Rpn 1 1 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 2002;298:611–615.
Benaroudj N, et al. ATP hydrolysis by the proteasome regulatory complex PAN serves multiple functions in protein degradation. Mol Cell 2003;11:69–78.
Zwickl P, et al Proteasomes in prokaryotes. In: Proteasomes: The World of Regulatory Protcolysis (Wolf DH and Hilt W, eds.). Georgetown, TX: Landes Bioscience, 1999:8–20.
Zwickl P, et al. An archaebacterial ATPase, homologous to ATPases in the eukaryotic 26 S proteasome, activates protein breakdown by 20 S proteasomes. J Biol Chem 1999;274:26008–26014.
Wilson HL, et al. Biochemical and physical properties of the Methanococcus jannaschii 20S proteasome and PAN, a homolog of the ATPase (Rpt) subunits of the eucaryal 26S proteasome. J Bacteriol 2000;182:1680–1692.
Ogura T, et al. AAA+ superfamily ATPases: common structure-diverse function. Genes Cells 2001;6:575–597.
Gottesman S, et al. Regulatory subunits of energy-dependent proteases. Cell 1997;91:435–438.
Benaroudj N, et al. PAN, the proteasome activating nucleotidase from archaebacteria, is a molecular chaperone which unfolds protein substrate. Nat Cell Biol 2000;2:833–839.
Navon A, et al. Proteins are unfolded on the surface of the ATPase ring before transport into the proteasome. Mol Cell 2001;8:1339–1349.
Brannigan JA, et al. A protein catalytic framework with an N-terminal nucleophile is capable of selfactivation. Nature 1995;378:416–419.
Dick T, et al. Contribution of proteasomal beta-subunits to the cleavage of peptide substrates analyzed with yeast mutants. J Biol Chem 1998;273:25637–25646.
Löwe J, et al. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 Å resolution. Science 1995;268:533–539.
Kisselev AF, et al. Why does threonine, and not serine, function as the active site nucleophile in proteasomes? J Biol Chem 2000;275:14831–14837.
Fenteany G, et al. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 1995;268:726–731.
Bogyo M, et al. Covalent modification of the active site Thr of proteasome beta-subunits and the E. coli homologue Hs1V by a new class of inhibitors. Proc Natl Acad Sci USA 1997;94:6629–6634.
Meng L, et al. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci USA 1999;96:10403–10408.
Sherman M, et al. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases [Review]. Neuron 2001;29:15–32.
Gronostajski R, et al. The ATP-dependence of the degradation of short- and long-lived proteins in growing fibroblasts. J Biol Chem 1985;260:3344–3349.
Rock KL, et al. Degradation of cell proteins and generation of MHC class I-presented peptides. Annu Rev Immunol 1999;17:739–779.
Gaczynska M, et al. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature 1993;365:264–267.
Michalek MT, et al. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature 1993;363:552–554.
Beninga J, et al. Interferon-gamma can stimulate post-proteasomal trimming of the N-termini of an antigenic peptide by inducing leucine aminopeptidase. J Biol Chem 1998;273:18734–18742.
Saric T, et al. ERAP1, an interferon-gamma-induced aminopeptidase in the endoplasmic reticulum, that trims precursors to MHC class I-presented peptides. Nat Immunol 2002;3:1169–1176.
York IA, et al. Endoplasmic reticulum aminopeptidasel (ERAP1) generates antigenic peptides in interferon-y-stimulated cells. Nat Immunol 2002;3:1177–1184.
Goff SA, et al. Production of abnormal proteins in E. coli stimulates transcription of lon and other heatshock genes. Cell 1985;41:587–595.
Ananthan J, et al. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heatshock genes. Science 1986;232:522–524
Lopes UG, et al. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem 1997;272:12893–12896.
Bush KT, et al Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem 1997;272:9086–9092.
Wang CY, et al. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med 1999;5:412–417.
Sadoul R, et al. Involvement of the proteasome in the programmed cell death of NGF-deprived sympathetic neurons. EMBO J 1996;15:3845–3852.
Berenson JR, et al. The role of nuclear factor-kappaB in the biology and treatment of multiple myeloma. Semin Oncol 2001;28:626–633.
Jensen TJ, et al. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 1995;83:129–135.
Ward CL, et al. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 1995;83:121–127.
Qu D, et al. Degradation of a mutant secretory protein, alphal-antitrypsin Z, in the endoplasmic reticulum requires proteasome activity. J Biol Chem 1996;271:22791–22795.
Fisher EA, et al. The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J Biol Chem 1997:20427–20434.
Hughes EA, et al. Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Nall Acad Sci USA 1997;94:1896–1901.
Sommer T, et al. Endoplasmic reticulum degradation: reverse protein flow of no return. FASEB J 1997;11:1227–1233.
Ye Y, et al. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 2001;414:652–656.
Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest 2002;110:1389–1398
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2004 Springer Science+Business Media New York
About this chapter
Cite this chapter
Goldberg, A.L. (2004). Introduction to the Proteasome and its Inhibitors. In: Adams, J. (eds) Proteasome Inhibitors in Cancer Therapy. Cancer Drug Discovery and Development. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-59259-794-9_2
Download citation
DOI: https://doi.org/10.1007/978-1-59259-794-9_2
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-61737-452-4
Online ISBN: 978-1-59259-794-9
eBook Packages: Springer Book Archive