Abstract
Purpose
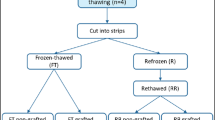
To assess follicular growth after xenografting in order to understand how freezing and/or grafting may affect follicular development.
Methods
Human ovarian biopsies were used for fresh and frozen-thawed xenografting to SCID mice. After xenotransplantation, follicular morphology and proportion, oocyte and follicle diameter, and quantitative and qualitative parameters of antral follicles were analyzed.
Results
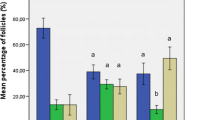
The proportion of growing follicles was significantly higher in grafted than non-grafted ovarian tissue. Follicular growth to the antral stage was observed and there was no significant difference in oocyte or follicle diameter in fresh or frozen-thawed grafts. Although no significant difference was observed in antral area or zona pellucida thickness, the theca layer in antral follicles from frozen-thawed grafted tissue was found to be significantly thinner than in fresh grafts.
Conclusion
Antral follicles obtained after grafting of frozen-thawed human ovarian tissue showed a thinner theca cell layer compared to those from fresh grafts, which could affect follicular development and function. Further studies are nevertheless warranted to confirm the identity of theca cells and assess if they retain the ability to respond to luteinizing hormone and produce androgens.





Similar content being viewed by others
References
Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–35.
Donnez J, Squifflet J, Van Eyck AS, Demylle D, Jadoul P, Van Langendonckt A, et al. Restoration of ovarian function in orthotopically transplanted cryopreserved ovarian tissue: a pilot experience. Reprod Biomed Online. 2008;16:694–704.
Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43(6):437–50.
Camboni A, Martinez-Madrid B, Dolmans MM, Amorim CA, Nottola SA, Donnez J, et al. Preservation of fertility in young cancer patients: contribution of transmission electron microscopy. Reprod Biomed Online. 2008;17:136–50.
Schubert B, Canis M, Darcha C, Artonne C, Smitz J, Grizard G. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertil Steril. 2008;89:1787–94.
Kim SS, Kang HG, Kim NH, Lee HC, Lee HH. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum Reprod. 2005;20:2502–8.
Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–46.
Dolmans MM, Donnez J, Camboni A, Demylle D, Amorim C, Van Langendonckt A, et al. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod. 2009;24:2778–87.
Gook DA, Edgar DH, Borg J, Archer J, McBain JC. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum Reprod. 2005;20:72–8.
Soleimani R, Heytens E, van den Broecke R, Rottiers I, Dhont M, Cuvelier CA, et al. Xenotransplantation of cryopreserved human ovarian tissue into murine back muscle. Hum Reprod. 2010;25:1458–70.
Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–42.
Piver P, Amiot C, Agnani G, Pech J, Rohrlich PS, Vidal E, et al. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. In: 25th Annual Meeting of ESHRE, 28 June–1 July, 2009. Amsterdam, the Netherlands: Oxford University Press, Hum Reprod 2009:i15.
Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74:122–9.
Gook DA, McCully BA, Edgar DH, McBain JC. Development of antral follicles in human cryopreserved ovarian tissue following xenografting. Hum Reprod. 2001;16:417–22.
Van den Broecke R, Liu J, Handyside A, Van der Elst JC, Krausz T, Dhont M, et al. Follicular growth in fresh and cryopreserved human ovarian cortical grafts transplanted to immunodeficient mice. Eur J Obstet Gynecol Reprod Biol. 2001;97:193–201.
David A, Dolmans MM, Van Langendonckt A, Donnez J. Andrade Amorim C. Immunohistochemical localization of growth factors after cryopreservation and 3 weeks’ xenotransplantation of human ovarian tissue. Fertil Steril. 2011;95:1241–6.
Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196°C. Hum Reprod. 1994;9:597–603.
Van Eyck AS, Jordan B, Gallez B, Heilier J, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374–81.
Dolmans MM, Yuan WY, Camboni A, Torre A, Van Langendonckt A, Martinez-Madrid B, et al. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod Biomed Online. 2008;16:705–11.
Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–7.
Gook DA, Edgar DH, Borg J, Archer J, Lutjen PJ, McBain JC. Oocyte maturation, follicle rupture and luteinization in human cryopreserved ovarian tissue following xenografting. Hum Reprod. 2003;18:1772–81.
Griffin J, Emery BR, Huang I, Peterson CM, Carrell DT. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J Exp Clin Assist Reprod. 2006;3:2.
Bangle Jr R, Alford WC. The chemical basis of the periodic acid Schiff reaction of collagen fibers with reference to periodate consumption by collagen and by insulin. J Histochem Cytochem. 1954;2:62–76.
Senou M, Khalifa C, Thimmesch M, Jouret F, Devuyst O, Col V, et al. A coherent organization of differentiation proteins is required to maintain an appropriate thyroid function in the pendred thyroid. J Clin Endocrin Metab. 2010;95:4021–30.
Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology. 1999;140:462–71.
Hernandez-Fonseca H, Bosch P, Sirisathien S, Wininger JD, Massey JB, Brackett BG. Effect of site of transplantation on follicular development of human ovarian tissue transplanted into intact or castrated immunodeficient mice. Fertil Steril. 2004;81:888–92.
Maltaris T, Kaya H, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Comparison of xenografting in SCID mice and LIVE/DEAD assay as a predictor of the developmental potential of cryopreserved ovarian tissue. In vivo. 2006;20:11–6.
Maltaris T, Koelbl H, Fischl F, Seufert R, Schmidt M, Kohl J, et al. Xenotransplantation of human ovarian tissue pieces in gonadotropin-stimulated SCID mice: the effect of ovariectomy. Anticancer Res. 2006;26:4171–6.
Maltaris T, Beckmann MW, Mueller A, Hoffmann I, Kohl J, Dittrich R. Significant loss of primordial follicles after prolonged gonadotropin stimulation in xenografts of cryopreserved human ovarian tissue in severe combined immunodeficient mice. Fertil Steril. 2007;87:195–7.
Maltaris T, Beckmann MW, Binder H, Mueller A, Hoffmann I, Koelbl H, et al. The effect of a GnRH agonist on cryopreserved human ovarian grafts in severe combined immunodeficient mice. Reproduction. 2007;133:503–9.
Nottola SA, Camboni A, Van Langendonckt A, Demylle D, Macchiarelli G, Dolmans MM, et al. Cryopreservation and xenotransplantation of human ovarian tissue: an ultrastructural study. Fertil Steril. 2008;90:23–32.
Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1,2-propanediol. Hum Reprod. 1999;14:2061–8.
Fabbri R, Pasquinelli G, Bracone G, Orrico C, Di Tommaso B, Venturoli S. Cryopreservation of human ovarian tissue. Cell Tissue Bank. 2006;7:123–33.
Camboni A, Martinez-Madrid B, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Autotransplantation of frozen-thawed ovarian tissue in a young woman: ultrastructure and viability of grafted tissue. Fertil Steril. 2008;90:1215–8.
Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freewing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83.
Siebzehnrübl E, Kohl J, Dittrich R, Wildt L. Freezing of human ovarian tissue—not the oocytes but the granulosa is the problem. Mol Cell Endocrinol. 2000;169:109–11.
Eyden B, Radford J, Shalet SM, Thomas N, Brison DR, Lieberman BA. Ultrastructural preservation of ovarian cortical tissue cryopreserved in dimethylsulfoxide for subsequent transplantation into young female cancer patients. Ultrastruct Pathol. 2004;28:239–45.
Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37:1344–9.
Orisaka M, Tajima K, Mizutani T, Miyamoto K, Tsang BK, Fukuda S, et al. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol Reprod. 2006;75:734–40.
Parrott JA, Skinner MK. Direct actions of kit-ligand on theca cell growth and differentiation during follicle development. Endocrinology. 1997;138:3819–27.
Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504.
Acknowledgments
The authors thank Mira Hryniuk for reviewing the English language of the manuscript, Maria-Dolores Gonzalez, Department of Gynecology, for the double-blind study, and Céline Bugli (SMCS—IMMAQ—UCL) for statistical advice.
Funding
The present study was supported by grants from the Commissariat General aux Relations Internationales (UCL) awarded to Anu David, the Fonds National de la Recherche Scientifique de Belgique (grant Télévie N07.4507.10, grant 3.4.590.08 awarded to Marie-Madeleine Dolmans), the Fonds Spéciaux de Recherche, the Fondation St Luc, the Région Wallonne (grant WBI 2009–2010 awarded to Alessandra Camboni) and the Foundation Against Cancer, and donations from Mr Pietro Ferrero, Baron Frère and Viscount Philippe de Spoelberch.
Author information
Authors and Affiliations
Corresponding author
Additional information
C A Amorim and A David are joint first authors
Capsule
The theca layer in antral follicles from cryopreserved grafted human ovarian tissue is thinner than in fresh grafts.
Rights and permissions
About this article
Cite this article
Amorim, C.A., David, A., Dolmans, MM. et al. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J Assist Reprod Genet 28, 1157–1165 (2011). https://doi.org/10.1007/s10815-011-9672-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9672-z