Abstract
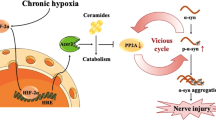
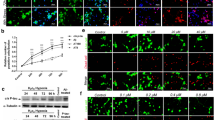
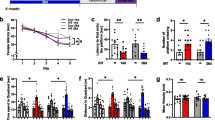
Chronic hypoxia has been reported to contribute to the development of Alzheimer’s disease (AD). However, the mechanism of hypoxia in the pathogenesis of AD remains unclear. The purpose of this study was to investigate the effects of chronic hypoxia treatment on β-amyloid, tau pathologies, and the behavioral consequences in the double transgenic (APP/PS1) mice. Double transgenic mice (APP/PS1 mice) were treated with hypoxia, and spatial learning and memory abilities of mice were assessed in the Morris water maze. β-amyloid level and plaque level in APP/PS1 double transgenic mice were detected by immunohistochemistry. Protein tau, p35/p25, cyclin-dependent kinase 5 (CDK5), and calpain were detected by western blotting analysis. Chronic hypoxia treatment decreased memory and cognitive function in AD mice. In addition, chronic hypoxia treatment resulted in increased senile plaques, accompanying with increased tau phosphorylation. The hypoxia-induced increase in the tau phosphorylation was associated with a significant increase in the production of p35 and p25 and upregulation of calpain, suggesting that hypoxia induced aberrant CDK5/p25 activation via upregulation of calpain. Our results showed that chronic hypoxia exposure accelerates not only amyloid pathology but also tau pathology via calpain-mediated tau hyperphosphorylation in an AD mouse model. These pathological changes possibly contribute to the hypoxia-induced behavioral change in AD mice.







Similar content being viewed by others
References
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY (2006) Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169(2):566–583
Alvarez A, Munoz JP, Maccioni RB (2001) A CDK5-p35 stable complex is involved in the beta-amyloid-induced deregulation of CDK5 activity in hippocampal neurons. Exp Cell Res 264(2):266–274
Ballabh P, Braun A, Nedergaard M (2004) The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16(1):1–13
Bazan NG, Palacios-Pelaez R, Lukiw WJ (2002) Hypoxia signaling to genes: significance in Alzheimer’s disease. Mol Neurobiol 26(2–3):283–298
Borenstein AR, Wu Y, Mortimer JA, Schellenberg GD, McCormick WC, Bowen JD, McCurry S, Larson EB (2005) Developmental and vascular risk factors for Alzheimer’s disease. Neurobiol Aging 26(3):325–334
Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med 10(Suppl):S2–S9
Busciglio J, Lorenzo A, Yankner BA (1995) Beta-myloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14(4):879–888
Cancino GI, Perez de Arce K, Castro PU, Toledo EM, von Bernhardi R, Alvarez AR (2009) c-Abl tyrosine kinase modulates tau pathology and CDK5 phosphorylation in AD transgenic mice. Neurobiol Aging 32(7):1249–1261
Chaudhary P, Suryakumar G, Prasad R, Singh SN, Ali S, Liavazhagan G (2012) Chronic hypobaric hypoxia mediated skeletal muscle atrophy: role of ubiquitin-proteasome pathway and calpains. Mol Cell Biochem 364(1–2):101–113
Chen X, Huang T, Zhang J, Song J, Chen L, Zhu Y (2008) Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1’s attenuation of beta-amyloid peptide25-35-induced tau hyperphosphorylation in cortical neurons. Brain Res 1200:99–106
Engmann O, Giese KP (2009) Crosstalk between CDK5 and GSK3beta: implications for Alzheimer’s disease. Front Mol Neurosci 2:2
Grammer M, Li D, Arunthavasothy N, Lipski J (2008) Contribution of calpain activation to early stages of hippocampal damage during oxygen–glucose deprivation. Brain Res 1196:121–130
Higuchi M, Iwata N, Matsuba Y, Takano J, Suemoto T, Maeda J, Ji B, Ono M, Staufenbiel M, Suhara T, Saido TC (2012) Mechanistic involvement of the calpain-calpastatin system in Alzheimer neuropathology. FASEB J 26(3):1204–1217
Iqbal K, Alonso AC, Chen S, Chohan MO, EI-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Igbal I (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739(2–3):198–210
Iqbal K, Grundke-Iqbal I (2006) Discoveries of tau, abnormally hyperphosphorylated tau and others of neurofibrillary degeneration: a personal historical perspective. J Alzheimers Dis 9(3 Suppl):219–242
Kim MJ, Oh SJ, Park SH, Kang HJ, Won MH, Kang TC, Hwang IK, Park JB, Kim JI, Kim J, Lee JY (2007) Hypoxia-induced cell death of HepG2 cells involves a necrotic cell death mediated by calpain. Apoptosis 12(4):707–718
Kitazawa M, Cheng D, Laferla FM (2009) Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates CDK5 in a mouse model of AD. J Neurochem 108(6):1550–1560
Koh SH, Noh MY, Kim SH (2008) Amyloid-beta-induced neurotoxicity is reduced by inhibition of glycogen synthase kinase-3. Brain Res 1188:254–262
Lahiri DK, Maloney B, Basha MR, Ge YW, Zawia NH (2007) How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr Alzheimer Res 4(2):219–228
Lee VM, Balin BJ, Otvos L, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 251(4994):675–678
Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405(6784):360–364
Li L, Zhang X, Yang D, Luo G, Chen S, Le W (2009) Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging 30(7):1091–1098
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79(4):1431–1568
Lopes JP, Oliveira CR, Agostinho P (2010) Neurodegeneration in an Abeta-induced model of Alzheimer’s disease: the role of CDK5. Aging Cell 9(1):64–77
Mandelkow EM, Biernat J, Drewes G, Steiner B, Lichtenberg-Kraag B, White H, Gustke N, Mandelkow E (1993) Microtubule-associated protein tau, paired helical filaments, and phosphorylation. Ann N Y Acad Sci 695:209–216
Manukhina EB, Goryacheva AV, Barskov IV, Viktorov IV, Guseva AA, Pshennikova MG, Khomenko IP, Mashina SY, Pokidyshev DA, Malyshev IY (2010) Prevention of neurodegenerative damage to the brain in rats in experimental Alzheimer’s disease by adaptation to hypoxia. Neurosci Behav Physiol 40(7):737–743
Martins IJ, Hone E, Foster JK, Gnjec A, Fuller SJ, Nolan D, Gandy SE (2006) Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry 11(8):721–736
Masliah E, Sisk A, Mallory M, Games D (2001) Neurofibrillary pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. J Neuropathol Exp Neurol 60(4):357–368
Mazanetz MP, Fischer PM (2007) Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov 6(6):464–479
McManus T, Sadqrove M, Pringle AK, Chad JE, Sundstrom LE (2004) Intraischaemic hypothermia reduces free radical production and protects against ischaemic insults in cultured hippocampal slices. J Neurochem 91(2):327–336
Monje ML, Chatten-Brown J, Hye SE, Raley-Suman KM (2000) Free radicals are involved in the damage to protein synthesis after anoxia/aglycemia and NMDA exposure. Brain Res 857(1–2):172–182
Morais LH, Lima MMS, Martynhak BJ, Santiago R, Takahashi TT, Ariza D, Barbiero JK, Andreatini R, Vital MABF (2012) Characterization of motor, depressive-like and neurochemical alterations induced by a short-term rotenone administration. Pharmacol Rep 64:1081–1090
Newcomb-Fernandez JK, Zhao X, Pike BR, Wang KK, Kampfl A, Beer R, DeFord SM, Hayes RL (2001) Concurrent assessment of calpain and caspase-3 activation after oxygen-glucose deprivation in primary septo-hippocampal cultures. J Cereb Blood Flow Metab 21(11):1281–1294
Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH (1999) Conversion of p35 to p25 deregulates CDK5 activity and promotes neurodegeneration. Nature 402(6762):615–622
Picconi B, Tortiglione A, Barone I, Centonze D, Gardoni F, Gubellini P, Bonsi P, Pisani A, Bernardi G, Di Luca M, Calabresi P (2006) NR2B subunit exerts a critical role in postischemic synaptic plasticity. Stroke 37(7):1895–1901
Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, Siman R (1994) Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci 14(6):3934–3944
Sato S, Xu J, Okuyama S, Martinez LB, Walsh SM, Jacobsen MT, Swan RJ, Schlautman JD, Ciborowski P, Ikezu T (2008) Spatial learning impairment, enhanced CDK5/p35 activity, and downregulation of NMDA receptor expression in transgenic mice expressing tau-tubulin kinase 1. J Neurosci 28(53):14511–14521
Shewale PB, Patil RA, Hiray YA (2012) Antidepressant-like activity of anthocyanidins from Hibscus rosa-sinensis flowers in tail suspension test and forced swim test. Indian J Pharmacol 44(4):454–457
Skoog I, Gustafson D (2006) Update on hypertension and Alzheimer’s disease. Neurol Res 28(6):605–611
Toescu E, Verkhratsky A (2004) Ca2+ and mitochondria as substrates for deficits in synaptic plasticity in normal brain ageing. J Cell Mol Med 8(2):181–190
Wang X, Zheng W, Xei JW, Wang T, Wang SL, Teng WP, Wang ZY (2010) Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol Neurodegener 5:46
Zheng W, Xin N, Chi ZH, Zhao BL, Zhang J, Li JY, Wang ZY (2009) Divalent metal transporter 1 is involved in amyloid precursor protein processing and Abeta generation. FASEB J 23(12):4207–4217
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, L., Tian, S., Gao, H. et al. Hypoxia Increases Aβ-Induced Tau Phosphorylation by Calpain and Promotes Behavioral Consequences in AD Transgenic Mice. J Mol Neurosci 51, 138–147 (2013). https://doi.org/10.1007/s12031-013-9966-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-013-9966-y