Highlights
-
•
Physical exercise training favors large and positive changes in exerkines concentration in adults living with type 2 diabetes mellitus.
-
•
Changes in exerkines are related to improvements in clinical metabolic parameters (e.g., glycated hemoglobin, waist circumference, body mass).
-
•
We identified an optimal dosage for exercise prescription in this clinical population.
Keywords: Adipokines, Exercise training, Hepatokines, Myokines
Abstract
Background
This study investigates the effects of exercise training on exerkines in patients with type 2 diabetes mellitus to determine the optimal exercise prescription.
Methods
A systematic search for relevant studies was performed in 3 databases. Randomized controlled trials investigating the effects of exercise training on at least one of the following exerkines were included: adiponectin, apelin, brain-derived neurotrophic factor, fetuin-A, fibroblast growth factor-21, follistatin, ghrelin, interleukin (IL)-6, IL-8, IL-10, IL-15, IL-18, leptin, myostatin, omentin, resistin, retinol-binding protein 4, tumor necrosis factor-α, and visfatin.
Results
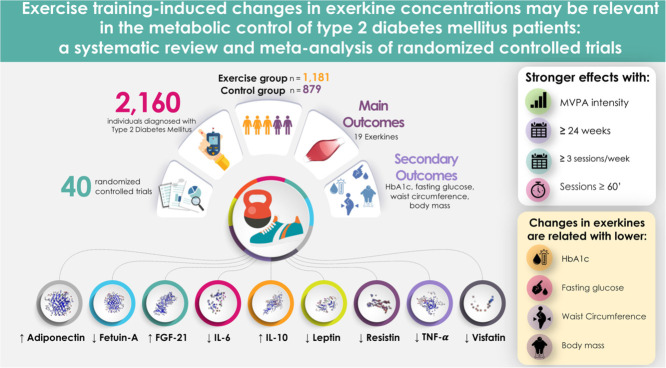
Forty randomized controlled trials were selected for data extraction (n = 2160). Exercise training induces changes in adiponectin, fetuin-A, fibroblast growth factor-21, IL-6, IL-10, leptin, resistin, and tumor necrosis factor-α levels but has no significant effects on apelin, IL-18, and ghrelin compared to controls. Physical exercise training favored large and positive changes in pooled exerkines (i.e., an overall effect size calculated from several exerkines) (Hedge's g = 1.02, 95% confidence interval (95%CI): 0.76−1.28), which in turn were related to changes in glycated hemoglobin (mean difference (MD) = −0.81%, 95%CI: −0.95% to −0.67%), fasting glucose (MD = −23.43 mg/dL, 95%CI: −30.07 mg/dL to −16.80 mg/dL), waist circumference (MD = −3.04 cm, 95%CI: −4.02 cm to −2.07 cm), and body mass (MD = −1.93 kg, 95%CI: −2.00 kg to −1.86 kg). Slightly stronger effects were observed with aerobic, resistance, or high-intensity interval protocols at moderate- to vigorous-intensity and with programs longer than 24 weeks that comprise at least 3 sessions per week and more than 60 min per session.
Conclusion
Exercise training represents an anti-inflammatory therapy and metabolism-improving strategy with minimal side effects for patients with type 2 diabetes mellitus.
Graphical abstract
1. Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by persistent hyperglycemia, and it is a leading cause of morbidity and mortality worldwide.1 T2DM is estimated to affect 463 million adults between the ages of 20 and 79 (data from 2019), and this is expected to rise to >578 million by 2030.2 A better understanding of the pathophysiology of T2DM and more effective methods of treating the disease are, therefore, urgently needed. According to the American Diabetes Association Professional Practice Committee, lifestyle management (e.g., weight management, physical activity) and psychosocial care are the cornerstones of T2DM management.3
Most adults with T2DM should engage in 150 min or more of moderate- to vigorous-intensity aerobic activity per week and 2–3 sessions/week of resistance exercise on nonconsecutive days.3 Physical exercise activates several signaling pathways, leading to the production of the bioactive molecules responsible for the beneficial effects of physical exercise. These molecules, which are potential pharmacological _targets/agents for the development of “exercise pills”, include circulating cytokines and humoral factors related to T2DM as well as “organokines”. They originate from skeletal muscle (myokines) as well as adipose tissue (adipokines) and the liver (hepatokines). Collectively, these are known as “exerkines”—a term coined by Safdar et al.4 to describe the circulating factors produced and secreted by any tissue or organ that are released in response to repeated bouts of exercise. Exerkines are increasingly recognized as critical intermediaries of exercise-related changes and health benefits, mainly due to their role in inter-organ and systemic communication and coordination.5
Most of the literature associates the benefits of exerkines with aerobic exercise;6 however, the effects of resistance or combined exercise (i.e., aerobic plus resistance training), intensity (i.e., light, moderate), length of program, frequency and/or duration of the session are less studied.7 Furthermore, the relationship between exerkines and response in individuals with T2DM remains unexplored. The aim of the present meta-analysis was 2-fold: (a) to examine major exerkines that may serve as biomarkers for monitoring the effects of exercise on T2DM and establishing the optimal exercise prescription; and (b) to determine the association of changes in exerkines with clinical parameters such as glycated hemoglobin (HbA1c), fasting glucose, waist circumference (WC), and body mass in an effort to explore the relationship between exercise and metabolism in T2DM patients.
2. Methods
The study was conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and recommendations.8 The protocol was registered in the international prospective register of systematic reviews (PROSPERO) (identification number: CRD42022283272). Two authors (AGH and RRV) independently performed the entire process, from literature selection to data extraction. Disagreements were resolved through consultation with a third researcher (MI).
2.1. Identification and selection criteria
To be eligible for inclusion in the present study, trials had to meet the following criteria (derived from the PICOS framework): (a) Participants: adults aged ≥18 years diagnosed with T2DM, excluding studies of patients with glucose intolerance, insulin resistance, or prediabetes; (b) Intervention: supervised exercise training program with a minimum duration of 2 weeks, excluding randomized controlled trials (RCTs) that used any additional diet or supplementation, such as green tea, Vitamin D, or drugs. Exercise training was defined as any body movement causing an increase in energy expenditure that involves planned or structured motion performed in a systematic manner in terms of frequency, intensity, duration, and progression and that was designed to maintain or enhance health-related outcomes according to the recommendations of the American Diabetes Association;9 (c) Comparison: control group receiving ordinary care, health education, and/or placebo, excluding healthy individuals (i.e., matched controls without T2DM); (d) Outcome: included studies measured at least one of the following exerkines, all of which have previously been linked to metabolic outcomes (Supplementary Table 1): adiponectin, apelin, brain-derived neurotrophic factor (BDNF), fetuin-A, fibroblast growth factor-21 (FGF-21), follistatin, ghrelin, interleukin-6 (IL-6), IL-8, IL-10, IL-15, IL-18, leptin, myostatin, omentin, resistin, retinol-binding protein 4 (RBP4), tumor necrosis factor-α (TNF-α), and visfatin; and (e) Study design: RCTs.
2.2. Search strategy
We performed an electronic search of PubMed, Embase, and Web of Science from inception to December 2, 2021. The search strategy was supplemented with manual searches of the existing literature and combined the following MeSH terms: “exercise” and “myokines” and “cytokines” and “adipokines” and “hepatokines” and “type 2 diabetes” (Supplementary Method 1). We also used the aforementioned “exerkines” as search terms. Two authors (AGH and RRV) independently assessed titles, abstracts, and full texts for eligibility for potential inclusion.
2.3. Data collection process
Two authors (AGH and RRV) independently extracted data from each RCT and collected the following information: (a) study design: authors’ names, year of publication, and country of the study; (b) participants: sample size, sex, age, and characteristics for all groups; (c) intervention: exercise modality, length (weeks), frequency (sessions/week), duration of training (minutes/session), and intensity of training; and (d) outcome data for each group regarding exerkines of interest and secondary outcomes (i.e., HbA1c, fasting glucose, WC, and body mass). When there was insufficient information, the authors of the included study were contacted. After extraction, a third reviewer (MI) examined the data for completeness and accuracy. Disagreements were resolved by review of the trial report and discussion.
2.4. Assessment of methodological quality and completeness of reporting
Methodological quality was appraised using Physiotherapy Evidence Database criteria,10 an 11-item scale designed for rating the methodological quality of RCTs. Two authors (AGH and RRV) independently scored each study, with disagreements resolved by consensus with a third investigator (MI). Physiotherapy Evidence Database scores of 0–3 are considered poor, 4–5 fair, 6–8 good, and 9–10 excellent.11
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used to evaluate the quality of the evidence of the overall effect.
2.5. Data synthesis and analysis
All analyses were carried out with STATA software using admetan and lfk modules (Version 17; StataCorp., College Station, TX, USA). We implemented the random-effects inverse-variance model with the Hartung–Knapp–Sidik–Jonkman adjustment. Changes in exerkines were calculated by subtracting change differences between the exercise and control groups, using the pooled standard deviation (SD) of change in both groups. If change score SDs were not available, they were calculated from pre-SD and post-SD values along with 95% confidence intervals (95%CIs) for either change outcome or exercise training effect differences.12 When the data were shown only by figures, Webplotdigitizer 4.3 software (Version 4.3; https://automeris.io/WebPlotDigitizer/) was used to extract them. Effect size was expressed as Hedge's g to correct for possible small sample bias.13 When a study included more than 2 arms in comparison with a control group, we halved the number of participants in the control group for each of the comparisons.
Meta-analyses were performed for each exerkine that was included in 3 or more RCTs. For this reason, we were unable to pool data for BDNF, follistatin, ghrelin, IL-15, myostatin, omentin, and RBP4.
We also determined the overall effect size (hereafter called pooled exerkines) by considering the following aspects: (a) when exercise typically favors a reduction in the exerkine (e.g., exercise has been shown to reduce IL-6 and TNF-α levels in patients with T2DM14), we changed the direction of effect size to ensure the same direction for all exerkines. This resulted in a change of direction for fetuin-A, IL-6, IL-18, leptin, myostatin, resistin, visfatin, and TNF-α in each study (i.e., negative effect sizes were changed to positive and vice versa); and (b) when a study included several exerkines (e.g., leptin, adiponectin), we used only the larger effect size to avoid duplication (i.e., the same group appearing on 2 or more occasions).
Using the pooled exerkines, subgroup analyses were performed according to type of exercise training (aerobic, high-intensity interval training, resistance, and both (concurrent)), intensity (using the categories of the American College of Sports Medicine: light, moderate, moderate to vigorous, and vigorous),7,15 length of program (<24 or ≥24 weeks), frequency (≤3 or >3 sessions per week) and duration of session (<60 and ≥60 min per session).
Meta-regression analyses were performed to evaluate the association between changes in exerkines and changes in HbA1c (%), fasting glucose (mg/dL), WC (cm), and body mass (kg). When possible, associations between changes in exerkines were analyzed. The following associations were possible: adiponectin/leptin, adiponectin/resistin, IL-6/TNF-α, and FGF-21/fetuin-A.
Heterogeneity across studies was calculated using the inconsistency index (I2), which is derived from the Cochran Q statistic:16 0%–40%, negligible heterogeneity; 30%–60%, moderate heterogeneity; 50%–90%, substantial heterogeneity; and 75%–100% considerable heterogeneity. Lastly, small-study effects and publication bias were examined using the Doi plot and the Luis Furuya–Kanamori index.17 Luis Furuya–Kanamori values beyond ± 1 are considered to be indicative of minor asymmetry; values ± 2 indicate major asymmetry and suggest the presence of publication bias.17
3. Results
3.1. Study selection
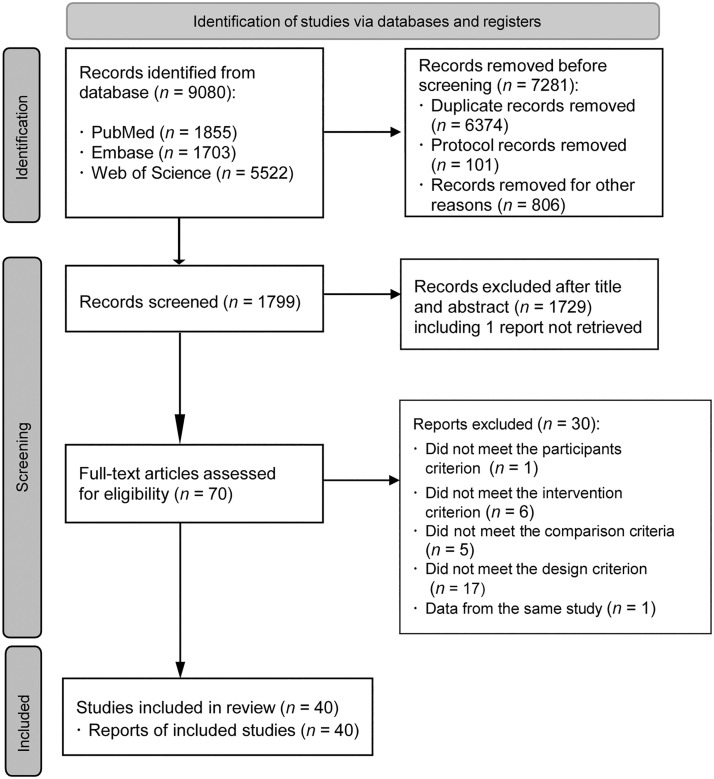
From the retrieved articles, a total of 40 RCTs18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 were included in the present study (Fig. 1). Four RCTs used the same control group50,51 or sample.21,23 Two others were included only in the systematic review due to lack of data needed to calculate an effect size.44,48 Reasons for exclusion are shown in Supplementary Method 2 in the Supplementary Materials.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
3.2. Characteristics of included trials
The study characteristics are summarized in Supplementary Table 2.
The study included a total of 2160 individuals with T2DM (1281 in the exercise groups and 879 in the control groups). Seven studies included only men19,24,27,38,40,42,45 and 9 included only women.21,23,29,39,49, 50, 51,54,56 Interventions included aerobic exercise (n = 639), high-intensity interval training (n = 95), resistance exercise (n = 255), concurrent training (n = 343), or several arms with the aforementioned protocols. Exercise training duration ranged from 8 weeks to 96 weeks,40 and training frequency ranged from 132 to 526,28,39,51,51 times weekly, with sessions from 15 min20,41 to 75 min43 in duration.
Of the 40 studies, 15 RCTs analyzed adiponectin, 19,22,24,25,28,31, 32, 33, 34,39,43,47,49,53,54 3 apelin,21,36,37 2 BDNF,44,48 4 fetuin-A,38,50,51,53 6 FGF-21,29,38,42,45,50,51 1 follistatin,42 12 IL-6,18,19,22,23,26,27,31,32,34,41,46,58 3 ghrelin,36,37,57 1 IL-8,18 3 IL-10,22,33,35 1 IL-15,23 3 IL-18,33,35,52 10 leptin,19,22,24,28,39, 40,43,47,49,53 2 myostatin,42,45 1 omentin,54 2 RBP4,19,39 4 resistin,22,31,34,53 12 TNF-α,18, 19, 20,22,27,30,31,33,34,41,57,58 and 3 visfatin31,37,56 (Supplementary Table 2).
Most of the RCTs did not relate information about side effects. Those studies that did report no major adverse effects or health problems attributable to the assessments or training sessions.19,22,53,23,32, 33, 34, 35, 36, 37,49 However, 3 studies reported post-exercise hypoglycemic events28,31 or minor injuries.41
3.3. Methodological quality within studies
The methodological quality assessment using the Physiotherapy Evidence Database scale showed a mean score of 4.8 out of a possible 10 points, with a range of 3 points46 to 7 points25,27 (Supplementary Table 3).
Overall, the quality of evidence (GRADE) for each pooled estimate comparing exercise protocols with the control group was low quality (Supplementary Table 4).
3.4. Effect estimates of exercise training on exerkines
Meta-analysis indicated that physical exercise training induces an increase in adiponectin (p = 0.008), FGF-21 (p = 0.022), and IL-10 (p = 0.041) levels and a decrease in fetuin-A (p = 0.001), IL-6 (p < 0.001), leptin (p = 0.001), resistin (p = 0.012), TNF-α (p < 0.001), and visfatin (p = 0.045). No significant changes were found in apelin (p = 0.170), IL-18 (p = 0.127), and ghrelin (p = 0.725) levels in comparison with the control groups (Table 1).
Table 1.
Effect of exercise programs on different exerkines in patients with type 2 diabetes mellitus.
| Exercise effect |
|||||
|---|---|---|---|---|---|
| Study (n) | Hedge's g | 95%CI | p | I2 | |
| Adiponectin | 13 | 0.43 | 0.11 to 0.76 | 0.008 | 74.4 |
| Apelin | 3 | 0.66 | −0.40 to 1.71 | 0.170 | 89.5 |
| Fetuin-A | 4 | −1.43 | −1.92 to −0.95 | 0.001 | 0 |
| Fibroblast growth factor-21 | 6 | 1.58 | 0.28 to 2.88 | 0.022 | 92.2 |
| Ghrelin | 3 | −0.21 | −1.69 to 1.26 | 0.725 | 89.3 |
| Interleukin-6 | 13 | −0.77 | −1.14 to −0.41 | <0.001 | 77.4 |
| Interleukin-10 | 3 | 1.00 | 0.08 to 1.91 | 0.041 | 65.4 |
| Interleukin-18 | 3 | −1.31 | −3.31 to 0.68 | 0.127 | 95.2 |
| Leptin | 10 | −0.48 | −0.72 to −0.23 | 0.001 | 24.1 |
| Resistin | 4 | −0.65 | −1.10 to −0.20 | 0.012 | 62.2 |
| Tumor necrosis factor-α | 11 | −1.04 | −1.45 to −0.64 | <0.001 | 81.0 |
| Visfatin | 3 | −0.46 | −0.92 to −0.01 | 0.045 | 71.0 |
Abbreviation: 95%CI = 95% confidence interval.
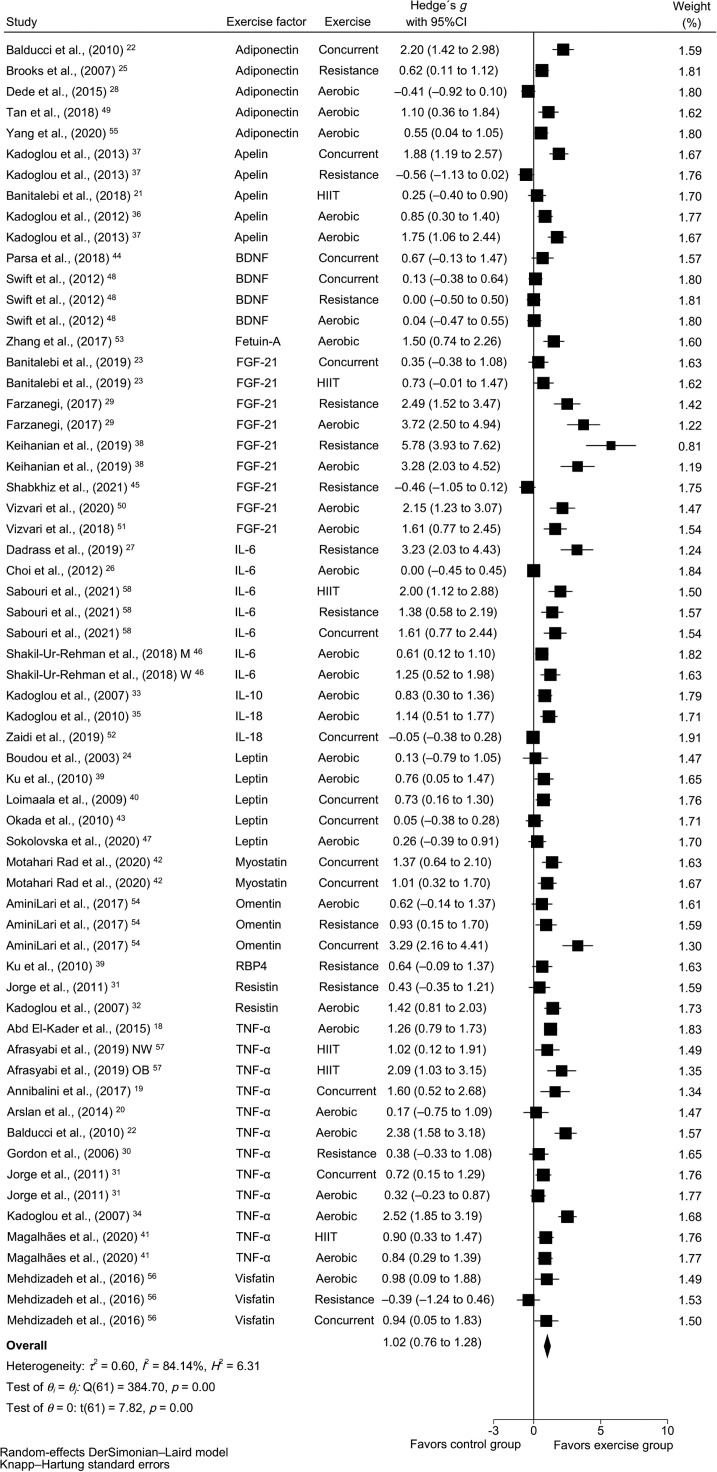
Of note, meta-analysis also demonstrated that physical exercise training favored a reduction in HbA1c (mean difference (MD) = −0.81%, 95%CI: −0.95% to −0.67%, p < 0.001, I2 = 93.3%) (Supplementary Fig. 1), fasting glucose (MD = −23.43 mg/dL, 95%CI: −30.07 mg/dL to −16.80 mg/dL, p < 0.001, I2 = 92.5%) (Supplementary Fig. 2), WC (MD = −3.04 cm, 95%CI: −4.02 cm to −2.07 cm, p < 0.001, I2 = 0%) (Supplementary Fig. 3), and body mass (MD = −1.93 kg, 95%CI: −2.00 kg to −1.86 kg, p < 0.001, I2 = 0%) (Supplementary Fig. 4), as well as large and positive changes in pooled exerkines (Hedge's g = 1.02, 95%CI: 0.76−1.28, p < 0.001, I2 = 84.14%) (Fig. 2).
Fig. 2.
Effects of exercise interventions on pooled exerkines in patients with type 2 diabetes mellitus. 95%CI = 95% confidence interval; BDNF = brain-derived neurotrophic factor; FGF-21 = fibroblast growth factor-21; HIIT = high intensity interval training; IL-6 = interleukin-6; M = men; NW = normal weight; OB = obese; RBP4 = retinol-binding protein 4; TNF-α= tumor necrosis factor-α; W = women.
No asymmetry suggestive of small-study effects was observed (Luis Furuya–Kanamori index = 1.90) (Supplementary Fig. 5).
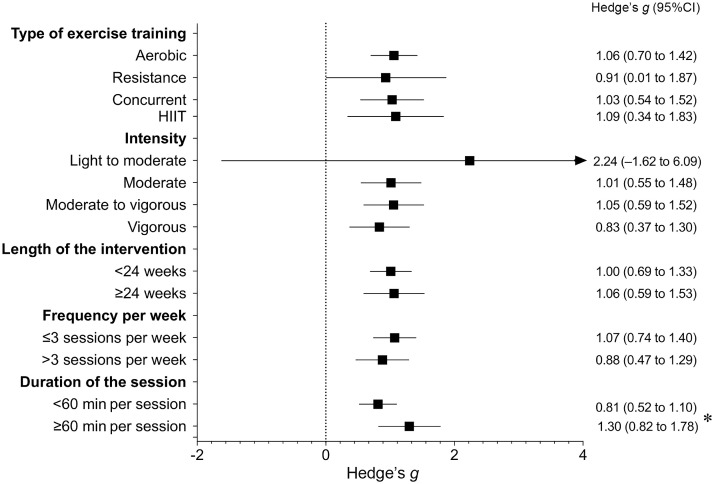
Fig. 3 depicts the effect of exercise on pooled exerkines in patients with T2DM according to different characteristics of the programs. Subgroup analysis showed similar effects according to type of exercise (aerobic training: Hedge's g = 1.06, 95%CI: 0.70−1.42, p < 0.001, I2 = 83.5%; high-intensity interval training: Hedge's g = 1.09, 95%CI: 0.34−1.83, p = 0.011, I2 = 66.1%; resistance training: Hedge's g = 0.91, 95%CI: 0.01−1.87, p = 0.039, I2 = 88.6%; concurrent training: Hedge's g = 1.03, 95%CI: 0.54−1.52, p < 0.001, I2 = 83.8%) (p difference between type of exercise 0.975), intensity (moderate: Hedge's g = 1.01, 95%CI: 0.55−1.48, p = 0.001, I2 = 85.0%; moderate to vigorous: Hedge's g = 1.05, 95%CI: 0.59−1.52, p < 0.001, I2 = 84.7%; vigorous: Hedge's g = 0.83, 95%CI: 0.37−1.30, p = 0.001, I2 = 80.2%) (p difference between intensity of the program 0.054), length of intervention (<24 weeks: Hedge's g = 1.00, 95%CI: 0.69 −1.33, p < 0.001, I2 = 83.6%; ≥24 weeks: Hedge's g = 1.06, 95%CI: 0.59−1.53, p < 0.001, I2 = 86.7%) (p difference between length of the programs 0.844), and frequency per week (≤3 sessions per week: Hedge's g = 1.07, 95%CI: 0.74−1.40, p < 0.001, I2 = 85.2%; >3 sessions per week: Hedge's g = 0.88, 95%CI: 0.47−1.29, p < 0.001, I2 = 81.0%) (p difference between frequency of exercise per week 0.398). Sessions equal to or longer than 60 min (Hedge's g = 1.30, 95%CI: 0.82−1.78, p < 0.001, I2 = 88.2%) showed a greater effect size than sessions less than 60 min (Hedge's g = 0.81, 95%CI: 0.52−1.10, p < 0.001, I2 = 76.7%) (p difference between duration of the session p = 0.033).
Fig. 3.
Overall effect of exercise on pooled exerkines in patients with type 2 diabetes mellitus according to different characteristics of the programs. *Difference between groups p = 0.033. 95%CI = 95% confidence interval; HIIT = high intensity interval training.
3.5. Changes in clinical parameters according to changes in exerkines
3.5.1. HbA1c
The meta-regression model showed positive (IL-6: β = 0.44, p = 0.012; leptin: β = 0.97, p = 0.009; resistin: β = 1.24, p = 0.001; TNF-α: β = 1.52, p < 0.001) and negative (adiponectin: β = −0.49, p = 0.014; apelin: β = −2.25, p < 0.001) associations between the decrease in each exercise factor and the decrease in percentage of HbA1c (Table 2).
Table 2.
Associations between changes in exerkines and changes in clinical outcomes (HbA1c, fasting glucose, waist circumference, and body mass).
| HbA1c (%) |
Fasting blood glucose (mg/dL) |
Waist circumference (cm) |
Body mass (kg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95%CI | p | β | 95%CI | p | β | 95%CI | p | β | 95%CI | p | |
| Adiponectin | −0.49 | −0.88 to −0.10 | 0.014 | 0.00 | −0.01 to 0.02 | 0.952 | −0.58 | −0.87 to −0.30 | <0.001 | −0.21 | −0.38 to 0.03 | 0.118 |
| Apelin | −2.25 | −3.23 to −1.28 | <0.001 | −0.03 | −0.02 to −0.05 | <0.001 | Insufficient data | Insufficient data | ||||
| Fetuin-A | −0.07 | −1.18 to 1.04 | 0.904 | 0.00 | −0.03 to 0.03 | 0.967 | Insufficient data | Insufficient data | ||||
| FGF-21 | 2.48 | −0.01 to 4.96 | 0.051 | −0.02 | −0.04 to −0.01 | 0.042 | Insufficient data | 0.11 | −0.15 to 0.37 | 0.410 | ||
| Ghrelin | 0.85 | −0.11 to 1.81 | 0.085 | −0.03 | −0.04 to −0.01 | 0.001 | Insufficient data | Insufficient data | ||||
| Interleukin-6 | 0.44 | 0.10 to 0.79 | 0.012 | 0.01 | −0.01 to 0.02 | 0.113 | 0.05 | −0.13 to 0.23 | 0.589 | 0.47 | 0.26 to 0.67 | <0.001 |
| Interleukin-10 | −3.72 | −8.05 to 0.61 | 0.092 | 0.03 | −0.19 to 0.25 | 0.791 | Insufficient data | Insufficient data | ||||
| Interleukin-18 | 1.23 | −2.58 to 5.05 | 0.525 | −0.19 | −0.31 to 0.07 | 0.101 | Insufficient data | Insufficient data | ||||
| Leptin | 0.97 | 0.24 to 1.69 | 0.009 | 0.01 | −0.01 to 0.03 | 0.117 | 0.31 | 0.07 to 0.54 | 0.010 | 0.11 | −0.05 to 0.27 | 0.185 |
| Resistin | 1.24 | 0.48 to 2.00 | 0.001 | 0.04 | 0.01 to 0.06 | 0.003 | 0.30 | 0.01 to 0.58 | 0.040 | 0.11 | −0.08 to 0.30 | 0.263 |
| TNF-α | 1.52 | 0.90 to 2.15 | <0.001 | 0.02 | 0.00 to 0.05 | 0.049 | 0.30 | 0.09 to 0.51 | 0.006 | 0.36 | 0.04 to 0.69 | 0.029 |
| Visfatin | −0.57 | −1.26 to 0.12 | 0.105 | 0.02 | 0.01 to 0.03 | 0.001 | Insufficient data | Insufficient data | ||||
Abbreviations: 95%CI = 95% confidence interval; FGF-21 = fibroblast growth factor-21; HbA1c = glycated hemoglobin; TNF-α = tumor necrosis factor-α.
3.5.2. Fasting glucose
The meta-regression model showed positive (resistin: β = 0.04, p = 0.003; TNF-α: β = 0.02, p = 0.049; visfatin: β = 0.02, p = 0.001) and negative (apelin: β = −0.03, p < 0.001; FGF-21: β = −0.02, p = 0.042) associations between the decrease in exerkines and changes in fasting glucose (Table 2).
3.5.3. WC
The meta-regression model showed positive (leptin: β = 0.31, p = 0.010; resistin: β = 0.30, p = 0.040; TNF-α: β = 0.30, p = 0.006) and negative (adiponectin: β = −0.58, p < 0.001) associations between changes in the aforementioned exerkines and changes in WC (Table 2).
3.5.4. Body mass
The meta-regression model showed positive associations between changes in IL-6 (β = 0.47, p < 0.001) and TNF-α (β = 0.36, p = 0.029) and changes in body mass (Table 2). Regarding pooled exerkines, the meta-regression models showed a negative association between the changes in exerkines and changes in HbA1c (β = −0.31, 95%CI: −0.51 to −0.11, p =0.003) (Supplementary Fig. 6), WC (β = −0.38, 95%CI: −0.58 to −0.18, p = 0.001) (Supplementary Fig. 7), and body mass (β = −0.11, 95%CI: −0.20 to −0.03, p = 0.006) (Supplementary Fig. 8), but not in fasting glucose (β = −0.01, 95%CI: −0.02 to 0.03, p = 0.723) (Supplementary Fig. 9).
3.6. Associations between changes in exerkines
The meta-regression model showed a positive association between the changes in adiponectin/leptin (β = −1.35, p < 0.001) (Supplementary Fig. 10) and IL-6/TNF-α (β = 0.34, p = 0.010) (Supplementary Fig. 11), respectively. The meta-regression model also showed that there is no association between the changes neither in adiponectin/resistin (β = −0.40, p = 0.085) (Supplementary Fig. 12), nor in FGF-21/fetuin-A (β = −1.04, p = 0.208) (Supplementary Fig. 13).
4. Discussion
In the last decade, an increasing number of clinical studies have demonstrated that the benefits of exercise training programs are at least partly the result of inter-tissue communication via exerkines (e.g., metabolites, hormones, myokines, microRNAs, and/or extracellular vesicles). Indeed, a single session of aerobic exercise was found to change the expression of ∼9800 molecular analytes in systemic circulation, including transcripts, proteins, metabolites, and lipids.59 Inter-organ signaling from skeletal muscle is found throughout the body, including in fat, liver, pancreas, bone, heart, immune, and brain cells.60 The notion that exerkines facilitate beneficial cross-talk among diverse organs and tissues has begun to be incorporated into treatment paradigms for T2DM.61
Our meta-analytic approach allowed us to generate novel evidence showing that any form of regular exercise changes the circulatory blood levels of several exerkines, including adiponectin, fetuin-A, FGF-21, IL-6, IL-10, leptin, resistin, and TNF-α, in patients with T2DM as compared to control groups. In this context, our results suggest that exerkines have utility as potential biomarkers and/or therapeutic _targets to treat T2DM and associated metabolic complications. This is in line with the strong body of evidence demonstrating that exercise is an effective strategy for the treatment of insulin resistance and T2DM.62
Most of the data in the present study were obtained for the effect of exercise on IL-6 levels.18,19,22,23,26,27,31,32,34,41,46 As a multifunctional cytokine, IL-6 has been implicated in the development of insulin resistance in T2DM63 through the generation of inflammation and control of cell differentiation, migration, proliferation, and apoptosis.64 Contrastingly, as a paradigmatic myokine, IL-6 is also thought to induce an anti-inflammatory cascade by triggering the release of anti-inflammatory cytokines.65 Similar to the results for IL-6, we found that chronic exercise reduced levels of IL-18 and TNF-α. On this basis we hypothesize that therapeutically _targeting inflammatory pathways may improve glycemic control and reduce the risk of complications in people with T2DM.66
Another novel finding from our meta-analysis was the positive effect exercise training had by decreasing the levels of fetuin-A, leptin, and resistin, which are broadly involved in obesity and related disorders, including T2DM, non-alcoholic fatty liver disease, and metabolic syndrome. Recently, Ramírez-Vélez et al.67 reported that exercise reduces fetuin-A levels in obesity, T2DM, and cardiovascular disease in adults and the elderly. The authors concluded, however, that these effects should be interpreted with caution because of the variety of exercise types investigated and the involvement of different obesity-related disorders. Our meta-analysis revealed that exercise training decreased leptin levels in adults with T2DM, which is in line with a previous meta-analysis done by Becic et al.,68 who reported that physical exercise reduces leptin levels in prediabetic and diabetic individuals. Resistin is an emerging cardiovascular risk factor implicated in T2DM.69 Contrary to other reports,70,71 we found that exercise induced a considerable decrease in resistin levels. Kadoglou et al.32 showed that a 16-week aerobic exercise training program of four 45–60-min sessions per week (50%–85% maximum oxygen consumption) might exert pleiotropic cardioprotective actions by modifying the expression of certain inflammatory cytokines, including resistin, in patients with T2DM. Following training, the reduction in resistin was associated with alterations in the level of high-sensitivity C-reactive protein, IL-18, and in maximum oxygen consumption. Interestingly, in our analysis, exercise-induced changes in resistin were positively related to changes in HbA1c and WC, suggesting an alternative explanation for the metabolic control in patients with T2DM.
It is well-recognized that hypoadiponectinemia (low levels of serum adiponectin) is associated with impaired glucose regulation, inflammation, obesity, atherosclerosis, and T2DM.72 Our meta-analysis revealed that overall physical exercise increased adiponectin levels, although significant study heterogeneity was evident. Notably, high heterogeneity for adiponectin levels was also found in previous meta-analyses.68,73 However, our meta-regression model also showed an association between the changes in adiponectin levels and changes in glycemic control based on HbA1c (and on WC). It is well-known that circulating adiponectin levels decrease with increasing levels of insulin resistance.74 This relationship was confirmed by Yamamoto et al.,75 who also showed that nearly 90% of patients who developed diabetes after a 3-year follow-up had prediabetes at baseline (fasting glucose ≥ 110 mg/dL and/or HbA1c ≥ 6.0%).
Evidence indicates that TNF-α is involved in the development and/or progression of T2DM.76 Overall, we found that physical exercise led to a significant decrease in serum TNF-α levels; however, significant study heterogeneity was present. This heterogeneity can likely be explained by the wide range of characteristics of both exercise programs and patients across studies. A study by Timper et al.77 indicated that IL-6 inhibits TNF-α production and may contribute to the stimulation of peripheral insulin sensitivity. Clinical studies have shown that FGF-21 analogs improve dyslipidemia in obese patients with T2DM.78 In addition to activating autophagy genes and improving inflammation, FGF-21 can also regulate glucose and lipid metabolism by controlling metabolism-related genes, such as adipose triglyceride lipase and acetyl-CoA carboxylase.79 FGF-21 is predominantly expressed in the liver, but it is also found in adipose tissue and, to a lesser extent, skeletal muscle in physiological conditions.80 Strikingly, our meta-analysis revealed that exercise decreased FGF-21 levels but, again, significant study heterogeneity was found. In a study of obese individuals and patients with T2DM, Sabaratnam et al.81 reported that a single 1-h bout of acute exercise increased the muscle mRNA and circulating levels of FGF-21 immediately after exercise, and both remained elevated 3 h into recovery without differences between the 2 groups. Other studies have reported an attenuated plasma FGF-21 response to acute exercise in obese individuals82 and in patients with T2DM.83 Thus, FGF-21 appears to be an important exercise factor that might play a key role in tissue crosstalk and promote the beneficial effects of exercise on whole-body metabolism; however, the mechanisms have yet to be unraveled.
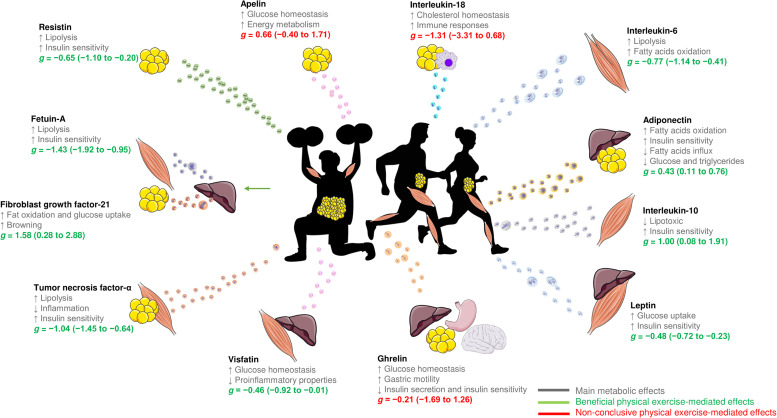
Overall, the results of this meta-analysis suggest that exercise training-induced changes in exerkines concentrations may be relevant in the metabolic control of T2DM due to their multisystemic benefits. Fig. 4 illustrates how exercise programs favor changes in exerkines concentration among patients with T2DM. However, much of the regulation by exercise of these exerkines remains unexplored, and there are still several challenges to their validation and translation into clinical practice. Therefore, it will be necessary for the treatment of T2DM patients to clarify these aspects and determine the molecular contribution of personalized patterns of physical exercise.
Fig. 4.
The effects of physical exercise on the regulation of exerkines. ↑ = increase; ↓ = decrease.
5. Strengths and limitations
To the best of our knowledge, this is the first meta-analysis to examine the efficacy of several types of exercise training protocols (and doses) on 19 exerkines in patients with T2DM. A strength of the present study is that we determined the therapeutic potential of exerkines and their association with clinical parameters, such as metabolic control measured by HbA1c, fasting glucose, WC, and body mass. These exerkines could emerge as biomarkers or as preventative or therapeutic _targets for T2DM. However, we recognize that there are some limitations to our findings. First, significant heterogeneity was found in the results for apelin, FGF-21, IL-6, IL-18, TNF-α, and pooled exerkines, which may be due to differences in methodology, participation criteria (e.g., age, years diagnosed with T2DM), and exercise training protocols; however, we did perform subgroup analyses to account for heterogeneity among RCTs. Second, not all RCTs included data on clinical outcomes (i.e., HbA1c, fasting glucose, WC, and body mass), thus our meta-regression analyses should be interpreted with caution. Also, substantial heterogeneity in the main results and in subgroup analyses generated uncertainty about the real change in magnitude that exerkines can produce in patients with T2DM. Finally, the quality of evidence in the overall analysis was determined to be low according to GRADE criteria.
In conclusion, this meta-analysis identified an optimal dosage for exercise prescription (any form of exercise at moderate and/or vigorous intensity with a program lasting longer than 24 weeks, with at least 3 sessions per week, ≥60 min per session) to induce significant improvements in exerkines in adults living with T2DM. In turn, these changes are associated with improvements in clinical metabolic parameters. Therefore, exerkines may emerge as biomarkers for monitoring the effects of exercise and as potential novel therapeutic _targets. While the quality of evidence in the overall analysis was determined to be low, clinicians could consider these findings when prescribing exercise for patients with T2DM in order to ensure optimal effectiveness.
Acknowledgments
Acknowledgment
AGH is a Miguel Servet Fellow (Instituto de Salud Carlos III – CP18/0150).
Authors' contributions
AGH and RRV were responsible for the conceptualization and design of the systematic review and meta-analysis, the screening process, data collection and extraction, risk of bias assessment, and data analysis, as well as the drafting of the original version of the manuscript; MI conceived the study, participated in its design, conducted the search and identification of studies, checked the information in the selected articles, and helped to draft the manuscript in its initial and post-review versions; JD and AG were responsible for drafting and revising the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.11.003.
Supplementary materials
References
- 1.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes–Global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. 5 Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Suppl. 1):S60–S82. doi: 10.2337/dc22-S005. [DOI] [PubMed] [Google Scholar]
- 4.Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12:504–517. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- 5.Chow LS, Gerszten RE, Taylor JM, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18:273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darragh IAJ, O'Driscoll L, Egan B. Exercise training and circulating small extracellular vesicles: Appraisal of methodological approaches and current knowledge. Front Physiol. 2021;12:738333. doi: 10.3389/fphys.2021.738333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanaley JA, Colberg SR, Corcoran MH, et al. Exercise/Physical activity in individuals with type 2 diabetes: A consensus statement from the American College of Sports Medicine. Med Sci Sport Exerc. 2022;54:353–368. doi: 10.1249/MSS.0000000000002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/Exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 11.Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) scale. J Physiother. 2020;66:59. doi: 10.1016/j.jphys.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.0.1. Available at: https://handbook-5-1.cochrane.org/. [accessed 09.08.2022].
- 14.Chen X, Sun X, Wang C, He H. Effects of exercise on inflammatory cytokines in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Oxid Med Cell Longev. 2020;2020:6660557. doi: 10.1155/2020/6660557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riebe D, Ehrman JK, Liguori G, Magal M. 10th ed. Wolters Kluwer; Philadelphia, PA: 2018. ACSM's guidelines for exercise testing and prescription. [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16:195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 18.Abd El-Kader SM, Al-Jiffri OH, Al-Shreef FM. Aerobic exercises alleviate symptoms of fatigue related to inflammatory cytokines in obese patients with type 2 diabetes. Afr Health Sci. 2015;15:1142–1148. doi: 10.4314/ahs.v15i4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annibalini G, Lucertini F, Agostini D, et al. Concurrent aerobic and resistance training has anti-inflammatory effects and increases both plasma and leukocyte levels of IGF-1 in late middle-aged type 2 diabetic patients. Oxid Med Cell Longev. 2017;2017:3937842. doi: 10.1155/2017/3937842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arslan M, Ipekci SH, Kebapcilar L, et al. Effect of aerobic exercise training on MDA and TNF-α levels in patients with type 2 diabetes mellitus. Int Sch Res Not. 2014;2014:820387. doi: 10.1155/2014/820387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banitalebi E, Faramarzi M, Nasiri S. High-intensity interval training versus moderate intensity combined training (resistance and aerobic) for improving insulin-related adipokines in type 2 diabetic women. Zahedan J Res Med Sci. 2018;20:e68793. doi: 10.5812/zjrms.68793. [DOI] [Google Scholar]
- 22.Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608–617. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Banitalebi E, Kazemi AR, Faramarzi M, Nasiri S, Haghighi MM. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. 2019;217:101–109. doi: 10.1016/j.lfs.2018.11.062. [DOI] [PubMed] [Google Scholar]
- 24.Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JF. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol. 2003;149:421–424. doi: 10.1530/eje.0.1490421. [DOI] [PubMed] [Google Scholar]
- 25.Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4:19–27. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi KM, Han KA, Ahn HJ, et al. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: A randomized controlled trial. J Clin Endocrinol Metab. 2012;97:3751–3758. doi: 10.1210/jc.2012-1951. [DOI] [PubMed] [Google Scholar]
- 27.Dadrass A, Mohamadzadeh Salamat K, Hamidi K, Azizbeigi K. Anti-inflammatory effects of vitamin D and resistance training in men with type 2 diabetes mellitus and vitamin D deficiency: A randomized, double-blinded, placebo-controlled clinical trial. J Diabetes Metab Disord. 2019;18:323–331. doi: 10.1007/s40200-019-00416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dede ND, İpekci SH, Kebapcılar L, et al. Influence of exercise on leptin, adiponectin and quality of life in type 2 diabetics. Turkish J Endocrinol Metab. 2015;19:7–13. [Google Scholar]
- 29.Farzanegi P. Aerobic and resistance exercises modulate fibroblast Growth factor-21 level in menopause women with type II diabetic. West Indian Med J. 2022;69:471–477. [Google Scholar]
- 30.Gordon PL, Vannier E, Hamada K, et al. Resistance training alters cytokine gene expression in skeletal muscle of adults with type 2 diabetes. Int J Immunopathol Pharmacol. 2006;19:739–749. doi: 10.1177/039463200601900404. [DOI] [PubMed] [Google Scholar]
- 31.Jorge MLMP, de Oliveira VN, Resende NM, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60:1244–1252. doi: 10.1016/j.metabol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Kadoglou NPE, Perrea D, Iliadis F, Angelopoulou N, Liapis C, Alevizos M. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care. 2007;30:719–721. doi: 10.2337/dc06-1149. [DOI] [PubMed] [Google Scholar]
- 33.Kadoglou NPE, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14:837–843. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 34.Kadoglou NPE, Iliadis F, Liapis CD, Perrea D, Angelopoulou N, Alevizos M. Beneficial effects of combined treatment with rosiglitazone and exercise on cardiovascular risk factors in patients with type 2 diabetes. Diabetes Care. 2007;30:2242–2244. doi: 10.2337/dc07-0341. [DOI] [PubMed] [Google Scholar]
- 35.Kadoglou NPE, Iliadis F, Sailer N, et al. Exercise training ameliorates the effects of rosiglitazone on traditional and novel cardiovascular risk factors in patients with type 2 diabetes mellitus. Metabolism. 2010;59:599–607. doi: 10.1016/j.metabol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Kadoglou NPE, Vrabas IS, Kapelouzou A, et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monit. 2012;18:CR290–CR295. doi: 10.12659/MSM.882734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadoglou NPE, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet Med. 2013;30:e41–e50. doi: 10.1111/dme.12055. [DOI] [PubMed] [Google Scholar]
- 38.Keihanian A, Arazi H, Kargarfard M. Effects of aerobic versus resistance training on serum fetuin-A, fetuin-B, and fibroblast growth factor-21 levels in male diabetic patients. Physiol Int. 2019;106:70–80. doi: 10.1556/2060.106.2019.01. [DOI] [PubMed] [Google Scholar]
- 39.Ku YH, Han KA, Ahn H, et al. Resistance exercise did not alter intramuscular adipose tissue but reduced retinol-binding protein-4 concentration in individuals with type 2 diabetes mellitus. J Int Med Res. 2010;38:782–791. doi: 10.1177/147323001003800305. [DOI] [PubMed] [Google Scholar]
- 40.Loimaala A, Groundstroem K, Rinne M, et al. Effect of long-term endurance and strength training on metabolic control and arterial elasticity in patients with type 2 diabetes mellitus. Am J Cardiol. 2009;103:972–977. doi: 10.1016/j.amjcard.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Magalhães JP, Santos DA, Correia IR, et al. Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: A secondary analysis from a 1-year randomized controlled trial. Cardiovasc Diabetol. 2020;19:169. doi: 10.1186/s12933-020-01136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motahari Rad M, Bijeh N, Attarzadeh Hosseini SR, Raouf Saeb A. The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch Physiol Biochem. 2020 doi: 10.1080/13813455.2020.1829649. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Okada S, Hiuge A, Makino H, et al. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb. 2010;17:828–833. doi: 10.5551/jat.3798. [DOI] [PubMed] [Google Scholar]
- 44.Parsa TA, Attarzadeh Hosseini SR, Bije N, Hamedi Nia MR. The study of the effect of a 16-week program of resistance-aerobic training on BDNF, Hba1c, pain, and Michigan Neuropathy Score among type 2 diabetic patients with peripheral neuropathy. J Diabetes Metab. 2018;9:11. doi: 10.4172/2155-6156.1000811. [DOI] [Google Scholar]
- 45.Shabkhiz F, Khalafi M, Rosenkranz S, Karimi P, Moghadami K. Resistance training attenuates circulating FGF-21 and myostatin and improves insulin resistance in elderly men with and without type 2 diabetes mellitus: A randomised controlled clinical trial. Eur J Sport Sci. 2021;21:636–645. doi: 10.1080/17461391.2020.1762755. [DOI] [PubMed] [Google Scholar]
- 46.Shakil-Ur-Rehman S, Karimi H, Gillani SA, Amjad I, Ahmad S, Yaseen A. Response to a supervised structured aerobic exercise training program in patients with type 2 diabetes mellitus – Does gender make a difference? A randomized controlled clinical trial. J Natl Med Assoc. 2018;110:431–439. doi: 10.1016/j.jnma.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Sokolovska J, Ostrovska K, Pahirko L, et al. Impact of interval walking training managed through smart mobile devices on albuminuria and leptin/adiponectin ratio in patients with type 2 diabetes. Physiol Rep. 2020;8:e14506. doi: 10.14814/phy2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swift DL, Johannsen NM, Myers VH, et al. The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes. PLoS One. 2012;7:e42785. doi: 10.1371/journal.pone.0042785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan S, Du P, Zhao W, Pang J, Wang J. Exercise training at maximal fat oxidation intensity for older women with type 2 diabetes. Int J Sports Med. 2018;39:374–381. doi: 10.1055/a-0573-1509. [DOI] [PubMed] [Google Scholar]
- 50.Vizvari E, Farzanegi P, Abbas Zade H. Effect of Moderate aerobic exercise on serum levels of FGF21 and fetuin A in women with type 2 diabetes. Med Lab J. 2020;14:17–22. [Google Scholar]
- 51.Vizvari E, Farzanegi P, Abbas Zade Sourati H. Effect of vigorous aerobic exercise on serum levels of SIRT1, FGF21, and Fetuin A in women with type II diabetes. Med Lab J. 2018;12:1–6. [Google Scholar]
- 52.Zaidi H, Byrkjeland R, Njerve IU, et al. Effects of exercise training on inflammasome-related mediators and their associations to glucometabolic variables in patients with combined coronary artery disease and type 2 diabetes mellitus: Sub-study of a randomized control trial. Diabetes Vasc Dis Res. 2019;16:360–368. doi: 10.1177/1479164119836922. [DOI] [PubMed] [Google Scholar]
- 53.Zhang LY, Liu T, Teng YQ, et al. Effect of a 12-week aerobic exercise training on serum Fetuin-A and adipocytokine levels in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2017;126:487–492. doi: 10.1055/s-0043-115904. [DOI] [PubMed] [Google Scholar]
- 54.AminiLari Z, Fararouei M, Amanat S, et al. The effect of 12 weeks aerobic, resistance, and combined exercises on Omentin-1 levels and insulin resistance among type 2 diabetic middle-aged women. Diabetes Metab J. 2017;41:205–212. doi: 10.4093/dmj.2017.41.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang D, Li Y, Fan X, Liang H, Han R. The impact of exercise on serum irisin, osteocalcin, and adiponectin levels and on glycolipid metabolism in patients with type 2 diabetes. Int J Clin Exp Med. 2020;13:7816–7824. [Google Scholar]
- 56.Mehdizadeh A, Hamzezadeh S, Tofighi A. Investigation of plasma visfatin changes in women with type 2 diabetes followed by endurance, resistance, and combined exercise: The role of lipid profile. J Diabetes Metab. 2016;7:703. doi: 10.4172/2155-6156.1000703. [DOI] [Google Scholar]
- 57.Afrasyabi S, Marandi SM, Kargarfard M. The effects of high intensity interval training on appetite management in individuals with type 2 diabetes: Influenced by participants weight. J Diabetes Metab Disord. 2019;18:107–117. doi: 10.1007/s40200-019-00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabouri M, Hatami E, Pournemati P, Shabkhiz F. Inflammatory, antioxidant and glycemic status to different mode of high-intensity training in type 2 diabetes mellitus. Mol Biol Rep. 2021;48:5291–5304. doi: 10.1007/s11033-021-06539-y. [DOI] [PubMed] [Google Scholar]
- 59.Contrepois K, Wu S, Moneghetti KJ, et al. Molecular choreography of acute exercise. Cell. 2020;181:1112–1130. doi: 10.1016/j.cell.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magliulo L, Bondi D, Pini N, Marramiero L, Di Filippo ES. The wonder exerkines—Novel insights: A critical state-of-the-art review. Mol Cell Biochem. 2021;477:105–113. doi: 10.1007/s11010-021-04264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang D, Yang Y, Li Y, Han R. Physical exercise as therapy for type 2 diabetes mellitus: From mechanism to orientation. Ann Nutr Metab. 2019;74:313–321. doi: 10.1159/000500110. [DOI] [PubMed] [Google Scholar]
- 62.Sabaratnam R, Wojtaszewski JFP, Højlund K. Factors mediating exercise-induced organ crosstalk. Acta Physiol (Oxf) 2022;234:e13766. doi: 10.1111/apha.13766. [DOI] [PubMed] [Google Scholar]
- 63.Bowker N, Shah RL, Sharp SJ, et al. Meta-analysis investigating the role of interleukin-6 mediated inflammation in type 2 diabetes. EBioMedicine. 2020;61:103062. doi: 10.1016/j.ebiom.2020.103062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, Rasul A. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2017;27:229–236. doi: 10.1615/CritRevEukaryotGeneExpr.2017019712. [DOI] [PubMed] [Google Scholar]
- 65.Welc SS, Clanton TL. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp Physiol. 2013;98:359–371. doi: 10.1113/expphysiol.2012.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cruz NG, Sousa LP, Sousa MO, Pietrani NT, Fernandes AP, Gomes KB. The linkage between inflammation and type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013;99:85–92. doi: 10.1016/j.diabres.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Ramírez-Vélez R, García-Hermoso A, Hackney AC, Izquierdo M. Effects of exercise training on Fetuin-a in obese, type 2 diabetes and cardiovascular disease in adults and elderly: A systematic review and meta-analysis. Lipids Health Dis. 2019;18:23. doi: 10.1186/s12944-019-0962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Becic T, Studenik C, Hoffmann G. Exercise increases adiponectin and reduces leptin levels in prediabetic and diabetic individuals: Systematic review and meta-analysis of randomized controlled trials. Med Sci (Basel) 2018;6:97. doi: 10.3390/medsci6040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 70.Monzillo LU, Hamdy O, Horton ES, et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res. 2003;11:1048–1054. doi: 10.1038/oby.2003.144. [DOI] [PubMed] [Google Scholar]
- 71.Giannopoulou I, Fernhall B, Carhart R, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 72.Kishida K, Funahashi T, Shimomura I. Molecular mechanisms of diabetes and atherosclerosis: Role of adiponectin. Endocr Metab Immune Disord Drug _targets. 2012;12:118–131. doi: 10.2174/187153012800493468. [DOI] [PubMed] [Google Scholar]
- 73.Hayashino Y, Jackson JL, Hirata T, et al. Effects of exercise on C-reactive protein, inflammatory cytokine, and adipokine in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Metabolism. 2014;63:431–440. doi: 10.1016/j.metabol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 74.Hivert MF, Sullivan LM, Shrader P, et al. Insulin resistance influences the association of adiponectin levels with diabetes incidence in two population-based cohorts: The Cooperative Health Research in the Region of Augsburg (KORA) S4/F4 study and the Framingham Offspring Study. Diabetologia. 2011;54:1019–1024. doi: 10.1007/s00125-011-2067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamoto S, Matsushita Y, Nakagawa T, Hayashi T, Noda M, Mizoue T. Circulating adiponectin levels and risk of type 2 diabetes in the Japanese. Nutr Diabetes. 2014;4:e130. doi: 10.1038/nutd.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 77.Timper K, Denson JL, Steculorum SM, et al. IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Rep. 2017;19:267–280. doi: 10.1016/j.celrep.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 78.Yan J, Nie Y, Cao J, et al. The roles and pharmacological effects of FGF21 in preventing aging-associated metabolic diseases. Front Cardiovasc Med. 2021;8:655575. doi: 10.3389/fcvm.2021.655575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat Rev Endocrinol. 2020;16:654–667. doi: 10.1038/s41574-020-0386-0. [DOI] [PubMed] [Google Scholar]
- 80.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sabaratnam R, Pedersen AJT, Kristensen JM, Handberg A, Wojtaszewski JFP, Højlund K. Intact regulation of muscle expression and circulating levels of myokines in response to exercise in patients with type 2 diabetes. Physiol Rep. 2018;6:e13723. doi: 10.14814/phy2.13723. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slusher AL, Whitehurst M, Zoeller RF, Mock JT, Maharaj M, Huang CJ. Attenuated fibroblast growth factor 21 response to acute aerobic exercise in obese individuals. Nutr Metab Cardiovasc Dis. 2015;25:839–845. doi: 10.1016/j.numecd.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92:157–191. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.