Abstract
Revlimid® (Lenalidomide, CC-5013) and CC-4047 are IMiDs® immunomodulatory drugs that have been described as having immunomodulatory properties and anti-tumor activity. Here we report proapoptotic effects of CC-5013 and CC-4047 on tumor cells in a co-culture model of PBMC and tumor cells. CC-5013 and CC-4047 enhanced PBMC activity leading to tumor cell apoptosis in K562/PBMC co-culture model. We also demonstrate that the natural killer (NK) cell population of PBMC was essential in inducing K562 apoptosis. Increases of NK and natural killer T (NKT) cell populations by CC-5013 and CC-4047 was observed along with modulation of NK cell CD56 adhesion marker. In addition, our data indicate that NK activation by CC-4047 was dependent on other cell types of PBMC. We expanded the application of K562/PBMC co-culture model to other hematological and solid tumors. In Raji/PBMC co-culture model, CC-5013 and CC-4047 dose-dependently augmented tumor cell apoptosis. Pre-treatment of Raji cells with Rituximab further enhanced apoptosis induced by CC-5013 or CC-4047-treated PBMC. Moreover, CC-5013 and CC-4047 significantly increased PC-3 prostate cancer cell apoptosis in PC-3/PBMC co-culture, either as single agent or in combination with Docetaxel. Together, the results reveal that co-culture models are suitable cellular systems to assess anti-tumor activities of these compounds. Our findings support clinical evaluation of CC-5013 and CC-4047 in relapsed NHL with Rituximab and in prostate cancer with Docetaxel.
Keywords: Tumor cell apoptosis, Co-culture model, IMiDs® immunomodulatory drugs
Introduction
Many IMiDs® immunomodulatory drugs were developed for improved anti-cancer and anti-inflammatory properties and decreased side effects [2, 4]. Several members of this class of drugs have shown activities in hematological cancers and solid malignancies [1, 20, 23], as well as having profound effects on the bone marrow microenvironment [20, 23, 30]. These compounds exert functions in part by altering the production of cytokines and growth factors that in turn enhance immune responses against tumor cells [8, 33] and inhibit tumor angiogenesis [10, 11]. Importantly many of these drugs are potent co-stimulators for T-cell activation, including increasing production of T cell cytokines and activation of CD8+ T cells and NK cells [28]. Because of these indirect effects of selected IMiDs® immunomodulatory drugs on tumor cells, there are only special cases where some of them have demonstrated anti-proliferative activity in simple tumor cell proliferation assays [16, 37]. Therefore, there is an urgent need for an in vitro assay model capable of revealing the anti-tumor activity of many IMiDs® immunomodulatory drugs in both hematological and solid tumor cell lines. Such model would also be useful for the screening of new compounds with immunomodulatory properties.
Here we adapted a co-culture assay of peripheral blood mononuclear cells (PBMC) and tumor cells [12] as an in vitro model of tumor–host immune system interaction, to further explore the anti-tumor potential of these drugs. This assay is non-radioactive and flow cytometry based. In this co-culture assay, anti-tumor activities of CC-5013 and CC-4047 against both hematopoietic and solid tumors cell lines were detected. In addition, our results demonstrate that these activities were mediated by NK cells. More significantly, we show here, for the first time, that CC-4047 and CC-5013 are capable of enhancing the anti-tumor activity of Docetaxel in a prostate cancer PC-3/PBMC co-culture model.
Materials and methods
Reagents
CC-4047 and CC-5013 (Celgene Corporation, Summit, NJ, USA) were freshly dissolved in dimethyl sulfoxide (DMSO). Human recombinant Interleukin-2 protein (IL-2) was purchased from BD Biosciences (BD Biosciences, San Jose, CA, USA).
Cells
Tumor-derived cell lines K562, Raji, and PC-3 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), and maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, San Diego, CA, USA), 50 unit/ml penicillin, and 50 μg/ml streptomycin (Invitrogen). Peripheral blood was obtained from healthy donors (San Diego Blood Bank, San Diego, CA, USA). PBMC were isolated by Ficoll-Paque PLUS (GE Healthcare Bioscience, Piscataway, NJ, USA) gradient centrifugation and maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 unit/ml of penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin (Cascade Biologics, Portland, OR, USA).
NK cell depletion and isolation
PBMC were isolated as described above and cultured in tissue culture plates for 2 h to deplete monocytes. For NK cell depletion, non-adherent PBMC were first incubated with CD56 MicroBeads (Miltenyi Biotec, Auburn, CA, USA). Then CD56-positive NK cells were depleted through magnetic separation according to the manufacturer’s protocol. For NK cell isolation, non-adherent PBMC were used to isolate NK cells using NK cell Isolation Kit II (Miltenyi Biotec).
BrdU incorporation assay
Raji cells were plated at 10,000 cells/well in 96-well plates in triplicate. Cells were then treated with CC-4047, CC-5013 and Rituximab in the presence of various doses of complements. Plates were incubated in a 37°C humidified CO2 incubator for 72 h. Six hours before the end of the incubation period, Bromodeoxyuridine (BrdU) (Roche, Indianapolis, IN, USA) was added to the cells. Cells were then dried at 60°C for 1 h in an oven and anti-BrdU antibody was added followed by substrate and chemiluminescence intensity of the substrate was read with Wallac Victor 2 plate reader (Perkin Elmer, Atlanta, GA, USA) according to the manufacturer’s instructions.
Pre-label of tumor cells with PKH26
Tumor cells were labeled with fluorescence membrane labeling dye, PKH26, by adapting a protocol described previously [12]. Briefly, K562, Raji or PC-3 cells were washed twice with serum-free RPMI 1640 medium, and then re-suspended in 1 ml Diluent C of Red Fluorescent Cell Linker Kit (Sigma, St Louis, MO, USA). PKH26 dye stock (1 mM) was diluted to 4 μM with Diluent C and added into the cell suspension. The cell suspension was shaken gently at room temperature for 40 min. The reaction was stopped by adding one volume of heat inactivated FBS and incubating for 1 min at room temperature. One volume of complete medium was added and cells were washed three times with complete medium.
Treatment of PBMC, NK-depleted PBMC, and NK cells
PBMC, NK-depleted PBMC, or NK cells were treated either in flasks or in 96-well plates for different applications. In some studies, each group of effector cells was divided into two groups. Twenty units/ml of human recombinant IL-2 were added to one group, while the other group served as no IL-2 control. Both groups were incubated in a 37°C humidified CO2 incubator for 72 h. PBMC were then spun down and resuspended in fresh RPMI complete medium to 2 × 106 cells/ml. Each of two groups (+IL-2 and −IL-2) was further divided into several subgroups to be treated with vehicle control (DMSO) or various doses of CC-4047 and CC-5013 for 72 h prior to co-culture with tumor cells. The final concentration of DMSO in all experiments was 0.2%.
Co-culture model and FACS analysis
Co-culture models were set up in two formats to accommodate analysis by FACSAria flow cytometer (BD Biosciences) or by FACSArray bioanalyzer (BD Biosciences). For FACSAria analysis, PBMC treated in flasks were counted, mixed with PKH26 labeled tumor cells at various ratios in 1.5 ml microcentrifuge tubes, and incubated at 37°C for 3–4 h for K562 and Raji or 18 h for PC-3. The cells were subsequently labeled with Annexin V-FITC (BD Biosciences) and subject to FACSAria scan. For FACSArray bioanalyzer analysis, PBMC were treated with CC-4047 or CC-5013 in 96-well plate, then transferred to another 96-well plate where pre-labeled tumor cells were seeded. The PBMC and tumor cell ratios and incubation time for the mixture were the same as stated in previous format. After incubation, cells were labeled with Annexin V-APC (BD Biosciences) and subject to FACSArray scan. Non-labeled tumor cells, PKH26 labeled tumor cells and Annexin V-FITC/APC labeled apoptotic tumor cells (pretreated by cytotoxic agent) were included in each assay as compensation controls. Spontaneous apoptosis of tumor cells in the absence of PBMC served as background and was subtracted from all treated samples. Co-culture of tumor cells with NK-depleted PBMC or NK cells was set up following the same procedures as stated above.
Combination studies with chemodrugs using the co-culture model
PKH26-labeled Raji cells were resuspended to 1 × 106 cells/ml in fresh complete medium containing 10 μg/ml Rituximab (Genentech, South San Francisco, CA, USA) and incubated for 20 min at room temperature. Cells were then washed once with complete medium and proceeded to co-culture. For combination studies with solid tumor cells, PKH26-labeled PC-3 cells were plated at 20,000 cells/well in low adherence 96-well plates (Costar, Lowell, MA, USA) and treated with Docetaxel (Save-Mart Pharmaceuticals, San Diego, CA, USA) for 6 h prior to co-culture with PBMC.
Data analysis
To determine whether the combination treatment with CC-4047 or CC-5013 and other chemodrugs showed additive, synergistic or antagonistic effects, we used the fractional product method. In the fractional product method, the effect of two independently acting agents is defined as the product of the unaffected fractions after treatment with either drug alone: fu(1, 2) = fu(1) × fu(2). This formula allows calculation of the predicted effect of co-treatment, based on the assumption that two agents do not interact or cooperate in inducing their effects. If the observed unaffected fraction, that is, the remaining live cells, is below, equal or above the calculated product fu(1, 2) after co-treatment, the two agents show synergy, additivity or antagonism, respectively [7].
Results
CC-4047 enhances PBMC-mediated apoptosis of human leukemia cell K562 in co-culture model
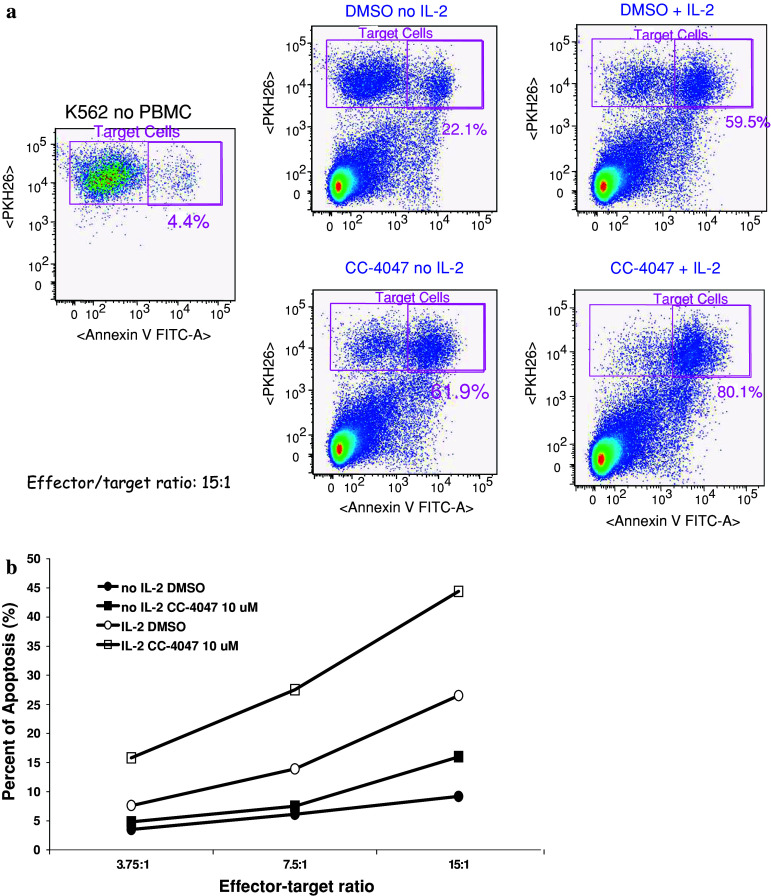
We initially evaluated the direct effects of CC-4047 on human leukemia cell K562 apoptosis in a simple mono-cell culture model. No apoptosis of K562 cells was induced by CC-4047 at concentrations up to 100 μM (data not shown). These data suggest that CC-4047 may have minimal direct effects on leukemia cells or that one of the drug metabolites is the active form. Considering the potent properties of certain IMiDs® immunomodulatory drugs shown in various immune cell models [8], a co-culture model using PBMC and K562 cells was used to study anti-cancer activities of those drugs. In this model, freshly isolated human PBMC were treated with or without IL-2 for 72 h followed by CC-4047 for 72 h and then co-cultured with K562 cells for 3–4 h. K562 tumor cells were prelabeled with a lipophilic membrane dye PKH26, using an optimal protocol that minimizes the impact of staining on tumor cell viability [12]. After co-culture, the mixture of K562 cells and PBMC was labeled with Annexin V-FITC and analyzed by FACSAria flow cytometer. Prelabeling of K562 cells with PKH26 allowed us to distinguish them from PBMC. Annexin V-FITC and PKH26 double positive cells represent tumor cells in early apoptotic state. Percentage of apoptotic K562 cells was calculated for every experiment. First, we detected that in this co-culture model, PBMC increased apoptosis of K562 cells from 4.4% basal level (no PBMC) to 22.1% (DMSO no IL-2) (Fig. 1a). PBMC pretreated with IL-2 further increased K562 apoptosis to 59.5% (DMSO + IL-2) (Fig. 1a). Next, we determined the effect of CC-4047 in this co-culture model in the presence or absence of IL-2. Importantly, treatment of PBMC with CC-4047 further induced K562 apoptosis from 22.1 to 61.9% in the no IL-2 group, and from 59.5 to 80.1% in the IL-2 group (Fig. 1a). Therefore, we conclude that this co-culture model is able to detect anti-tumor properties of CC-4047, which were not observed in the mono-cell culture model. In addition, these data show that the addition of IL-2 is not required to reveal the proapoptotic effects of CC-4047. We also analyzed the effect of CC-4047 on PBMC-mediated K562 apoptosis at three different effector-to-_target cell ratios. CC-4047 was able to enhance K562 apoptosis at all three ratios tested with or without IL-2 co-treatment (Fig. 1b). The percentage of apoptotic K562 cells correlated well with effector-to-_target cell ratio (Fig. 1b). This result suggests that the apoptotic effects observed are likely directly dependent on the amount of effector cells present.
Fig. 1.
CC-4047 enhances PBMC activity in inducing K562 apoptosis with or without IL-2 pretreatment. a PBMC were treated with or without 20 units/ml of IL-2 for 72 h followed by 10 μM CC-4047 for 72 h. K562 cells were labeled with PKH26 dye prior to co-culture with PBMC at 15:1 effector-to-_target ratio. After co-culture, the mixture of K562 cells and PBMC was labeled with Annexin V-FITC. This experiment was performed with PBMC from three different donors. Representative density plots of K562/PBMC co-culture with or without CC-4047 treatment are shown here. K562 cells can be distinguished from PBMC by gating PKH26 positive population (y-axis). Double positive cell population represents apoptotic K562 cells as shown in the upper right-hand box of each plot. CC-4047 increased apoptosis of K562 cells to 61.9% (no IL-2) or 80.1% (with IL-2) compared to DMSO-treated groups (22.1 or 59.5%, respectively). b CC-4047 enhances PBMC-mediated apoptosis of K562 cells at various effector-to-_target ratios with or without IL-2 treatment. PBMC pretreated with CC-4047 were mixed with K562 cells at the indicated effector-to-_target ratios for 3 h. Cells were then analyzed as described in Materials and methods. Results shown are representative of three independent experiments with different PBMC donors
CC-4047 and CC-5013 enhance PBMC-induced K562 apoptosis in the absence of IL-2 co-stimulation
Since CC-4047 showed profound activity in inducing K562 apoptosis, even in the absence of IL-2, we next assessed CC-5013 in this co-culture model without IL-2 co-stimulation. PBMC pretreated with various concentrations of CC-4047 and CC-5013 for 72 h were incubated with K562 at a 15:1 effector-to-_target ratio for 3 h. When compared to control PBMC cultures in DMSO, twofold increase in K562 apoptosis were detected in CC-4047 treated K562/PBMC co-culture (Fig. 2). CC-5013 appeared to enhance PBMC-mediated K562 apoptosis to a similar degree as CC-4047 (Fig. 2). Together with the minimal impact of either CC-4047 or CC-5013 on K562 apoptosis observed in mono-cell culture model, these data indicate that the K562/PBMC co-culture model provides a suitable in vitro model to reveal anti-tumor activity of CC-4047 and CC-5013.
Fig. 2.
CC-4047 and CC-5013 increase PBMC-mediated apoptosis of K562 cells in the absence of IL-2. Various doses of CC-4047 and CC-5013 were evaluated in the co-culture model without IL-2 pretreatment of PBMC. Fold increase in K562 apoptosis in CC-4047 and CC-5013 treated group versus DMSO treated group was calculated. Results shown are mean fold increase of apoptosis (±SD) from three independent experiments using different PBMC donors
NK cells are required but not sufficient for CC-4047 to enhance PBMC cytotoxicity against K562
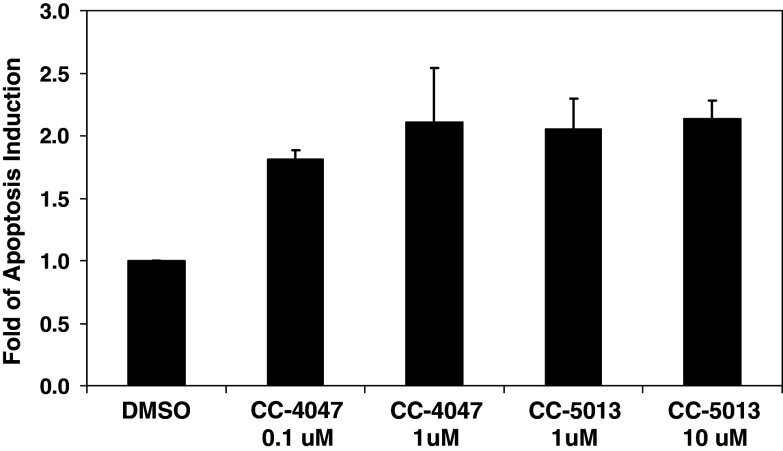
Cell-mediated cytotoxicity is a major effector pathway of immune protection against intracellular pathogens or tumors, and a fundamental mechanism to maintain homeostasis of the immune system. It has been demonstrated previously that selected IMiDs® immunomodulatory drugs seem to augment the cytotoxicity of natural killer (NK) cells against multiple myeloma cells [15]. We first evaluated the effects of CC-4047 and CC-5013 on NK cell surface markers. Treatment of PBMC with CC-4047 and CC-5013 caused a marked upregulation of the NK cell marker and adhesion molecule CD56 (NCAM1) in the CD3–CD56+ NK cell population (Fig. 3a). We also observed a twofold increase in the numbers of NKT (CD3+CD56+) cells in the samples treated with CC-4047 and CC-5013 (Fig. 3a).
Fig. 3.
NK cells play important roles in inducing K562 apoptosis enhanced by CC-4047. a After three days in culture with CC-4047 or CC-5013, increased CD56bright NK cells and NKT cells (CD56+CD3+ cells) numbers were detected in PBMC from six tested donors. Plots show representative results. b Histograms of a representative experiment comparing regular PBMC and NK-depleted PBMC in the co-culture model. NK cells were depleted from PBMC and tested in the co-culture model with K562 cells as described in Materials and methods. Non-depleted regular PBMC from the same donor are shown on the left panel and NK-depleted PBMC on the right. For each double-peaked sample, the right peak indicates the apoptotic population of K562. The level of apoptosis is listed above each histogram. This experiment was repeated three times using PBMC from different donors. c Comparison of CC-4047 effects on regular non-depleted PBMC (PBMC), NK-depleted PBMC (PBMC-NK), and purified NK cells (NK). NK cells were isolated from PBMC and treated with IL-2 followed by DMSO or 1 μM CC-4047 as described in Materials and methods. As a control, regular PBMC and NK-depleted PBMC from the same donor were treated under the same conditions. Data show representative results of four independent experiments with PBMC from different donors. d Effect of 1 μM CC-4047 on purified NK-mediated K562 apoptosis at different NK-to-_target ratios. Different effector-to-_target ratios were tested for isolated NK-mediated K562 apoptosis. Ratio of 1:1 matches the NK cell-to-K562 ratio in non-depleted K562/PBMC co-culture. Data in c and d show representative results of four independent experiments with PBMC from different donors
Next, we determined whether NK cells are required in mediating CC-4047 activity in this K562/PBMC co-culture model. NK cells were depleted from PBMC as described in Materials and methods. Typically, 94–97% NK depletion was achieved in those experiments. NK-depleted PBMC and non-depleted, regular PBMC from the same donor were treated with IL-2 followed by incubation with CC-4047. These PBMC were tested in the co-culture model with K562 cells. While non-depleted PBMC induced K562 cell apoptosis at the similar level as shown previously, NK-depleted PBMC lost this ability (Fig. 3b). Only basal level of K562 apoptosis was detected in the NK-depleted PBMC group (Fig. 3b). Furthermore, CC-4047 was not able to rescue or augment the ability of NK-depleted PBMC to induce K562 apoptosis (Fig. 3b). These data demonstrate that the NK cell subpopulation of PBMC is required for CC-4047 activity in K562/PBMC co-culture model.
To further investigate the role of NK cells in CC-4047 function, NK cells were purified from PBMC and treated with IL-2 followed by CC-4047 as described under Materials and methods. As controls, non-depleted regular PBMC and NK-depleted PBMC from the same donor were also treated with IL-2 and CC-4047. In this co-culture assay using isolated NK cells with 88% purity, the total NK cell number was adjusted to match the total NK cell number of non-depleted PBMC samples. One micromolar of CC-4047 increased non-depleted PBMC activity against K562 more than twofold over DMSO treated PBMC (Fig. 3c). DMSO-treated purified NK cells were able to induce K562 apoptosis to a similar degree as non-depleted PBMC (Fig. 3c). CC-4047 marginally enhanced purified NK cell activity against K562 (Fig. 3c), even though CC-4047 treatment did not stimulate proliferation or apoptosis of purified NK cells (data not shown). Additionally, in all three effector-to-_target cell ratios tested, only a slight increase of NK-mediated K562 apoptosis was induced by CC-4047 treatment (Fig. 3d). Together, these data indicate that CC-4047 enhancement of NK cell cytotoxicity requires the presence of other cell population(s) within PBMC.
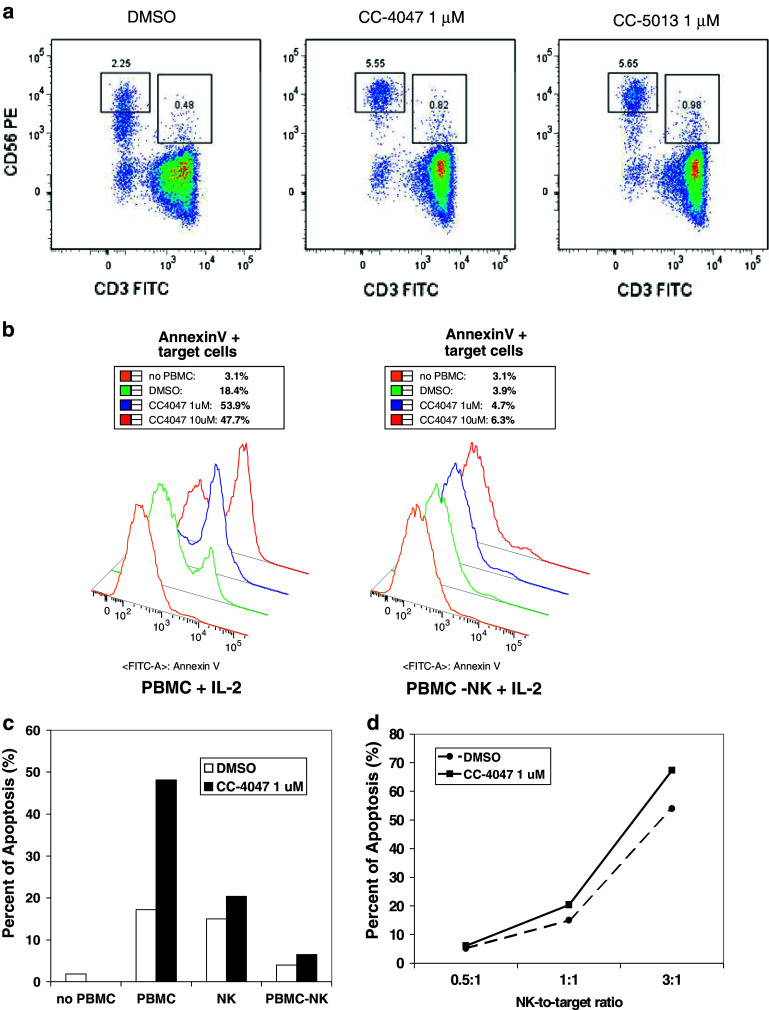
CC-4047 and CC-5013 show modest effect on Raji cell proliferation and no additive effect with Rituximab in Raji mono-cell culture model
CC-5013 has been shown to have anti-tumor activities in multiple hematological tumors, including NHL [16, 30, 37]. Here, we first tested the direct anti-proliferative activities of CC-4047 and CC-5013 on NHL cells by treating Raji cells with a standard of care for NHL, Rituximab, in the absence or presence of CC-4047 and CC-5013. As single agent, Rituximab significantly inhibited Raji cell proliferation in the presence of complement (Fig. 4a). CC-4047 and CC-5013 alone had a modest effect on Raji cell proliferation (Fig. 4a). In combination with Rituximab and complement, neither CC-4047 nor CC-5013 showed additive effect in inhibiting Raji cell proliferation in the mono-cell culture model (Fig. 4a).
Fig. 4.
CC-4047 and CC-5013 show no additive effect in combination with Rituximab and complement in Raji mono-cell culture but enhance Raji apoptosis in co-culture model as single agent or in combination with Rituximab. a Raji cells were cultured with Rituximab (10 μg/ml) and increasing concentrations of complement in the absence or presence of CC-4047 and CC-5013. Rituximab plus complement inhibited cell proliferation in a dose dependent manner. CC-4047 and CC-5013 had a very modest effect on Raji cell proliferation and showed no additive effect with Rituximab plus complement in this mono-cell culture assay. b Enhancement of PBMC-mediated Raji apoptosis by CC-4047 and CC-5013 is dose-dependent. PBMC were treated with CC-4047 or CC-5013 for 72 h prior to co-culture. These PBMC were then co-cultured with pre-labeled Raji cells at 30:1 or 15:1 ratio for 3 h, followed by labeling with AnnexinV-APC and analysis with FACSArray Bioanalyzer. Each data point represents the average of triplicate results. Shown is the result from 30:1 ratio. Similar trend of apoptosis induction was obtained with 15:1 ratio (data not shown). c Combination studies of Rituximab and CC-4047 and CC-5013 in Raji/PBMC co-culture model. Raji cells were treated with or without various doses of Rituximab for 20 min. PBMC were treated with CC-4047 (10 μM) and CC-5013 (10 μM) for 72 h prior to the co-culture with Raji cells. Each data point represents the average of triplicate results (* Synergistic and ♣ additive as determined by fractional product method.)
CC-4047 and CC-5013 enhance Raji cell apoptosis mediated by PBMC, as single agent or in combination with Rituximab
Since no additive effect between CC-4047 or CC-5013 and Rituximab was observed in the Raji mono-cell culture model, we next evaluated the co-culture model as a more appropriate cellular model to assess CC-4047 and CC-5013 anti-tumor activities in NHL. PBMC and prelabeled Raji cells were co-cultured, and apoptotic cells were labeled by Annexin V and quantified by FACS analysis. We validated a 96-well plate format FACS analysis using the BD FACSArray Bioanalyzer to perform dose response studies of CC-4047 and CC-5013. CC-4047 or CC-5013 alone did not induce Raji apoptosis in the mono-cell culture model. In the co-culture model, both CC-4047 and CC-5013 enhanced human PBMC activity in inducing Raji cell apoptosis in a dose dependent manner (Fig. 4b). We further evaluated CC-4047 and CC-5013 in combination with Rituximab. Raji cells were incubated with Rituximab for 30 min prior to co-culture for 4 h with PBMC that had been treated with CC-4047 and CC-5013. As shown in Fig. 4c, Rituximab was able to trigger PBMC-mediated Raji apoptosis in the absence of CC-4047 or CC-5013. Very importantly, synergistic effects between Rituximab and CC-4047 or CC-5013 to induce Raji cell apoptosis were detected in the Raji/PBMC co-culture model (Fig. 4c). These data provide the preclinical basis for clinical application of CC-4047 and CC-5013 in combination with Rituximab to improve the outcome of NHL patients.
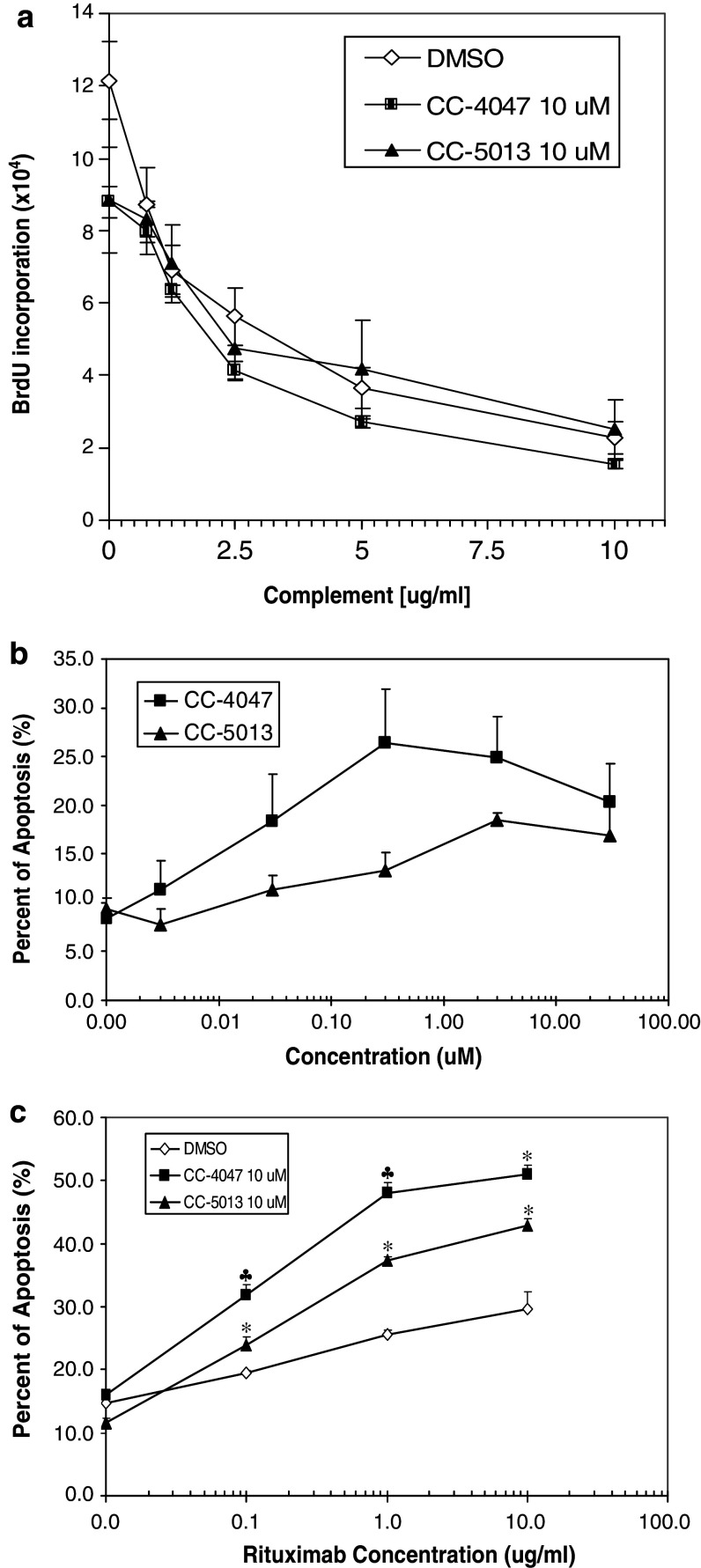
CC-4047 and CC-5013 enhance PBMC-induced apoptosis of PC-3 with or without Docetaxel pretreatment
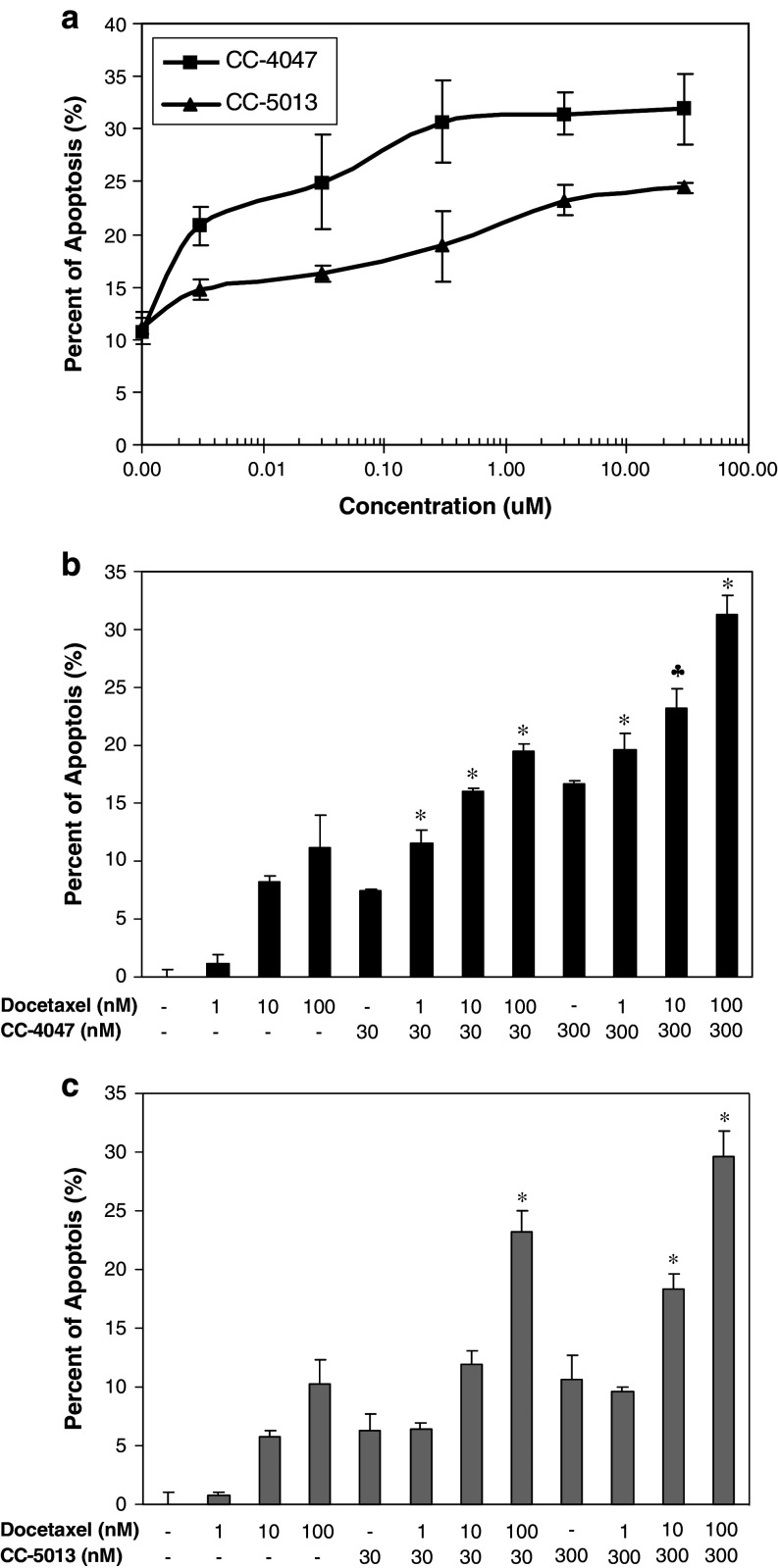
There have been numerous reports on certain IMiDs® immunomodulatory drugs anti-tumor activities against hematological tumors [1, 37]. However, very few studies have been done to investigate the effect of these drugs on solid tumor cells. Minimum impact of CC-4047 and CC-5013 on PC-3 proliferation or apoptosis was detected in mono-cell culture model (data not shown). Thus, we expanded the utility of our co-culture model into prostate cancer. In this model, PBMC and PKH26 labeled PC-3 cells were co-cultured in low-adherence 96-well plates for 18 h, and apoptotic cells were labeled by Annexin V-APC and quantified by FACS analysis using BD FACSArray Bioanalyzer. CC-4047 and CC-5013 dramatically enhanced human PBMC activity in inducing PC-3 apoptosis, in a dose dependent manner (Fig. 5a). Using this co-culture model, we also evaluated CC-4047 and CC-5013 anti-tumor activities in combination with Docetaxel, the standard of care for prostate cancer. PC-3 cells were exposed to the indicated suboptimal concentrations of Docetaxel for 6 h prior to co-culture with PBMC that had been treated with CC-4047 and CC-5013. Various doses of Docetaxel alone exerted minimum level of PC-3 apoptosis (10% at 100 nM), as shown in Fig. 5b. In the absence of PBMC, the combination of various doses of Docetaxel and CC-4047 or CC-5013 induced the same low level of PC-3 apoptosis as Docetaxel alone. In contrast, the combination of Docetaxel with CC-4047 or CC-5013-treated PBMC showed a dramatic increase in PC-3 cell apoptosis (Fig. 5b, c). In co-culture model, the combination treatment with Docetaxel and all doses of CC-4047 resulted in additive or synergistic apoptosis as determined by the fractional product method of drug interactions as described in Materials and methods (Fig. 5b). Co-administration of 10 or 100 nM Docetaxel with either 30 or 300 nM CC-5013-treated PBMC also resulted in a significant increase of apoptosis in PC-3 cells (Fig. 5c). Collectively these findings suggest, for the first time, that these two compounds, particularly CC-4047, may provide potential clinical benefits to prostate cancer patients as single agent or in combination with standard chemotherapy.
Fig. 5.
CC-4047 and CC-5013 enhance PC-3 cell apoptosis in co-culture model, in the presence or absence of Docetaxel pretreatment. a Enhancement of PBMC-mediated apoptosis by CC-4047 and CC-5013 is dose-dependent. PBMC were treated with CC-4047 or CC-5013 for 72 h. These PBMC were then co-cultured with pre-labeled PC-3 cells at 10:1 or 5:1 ratio for 18 h, followed by labeling with AnnexinV-APC and analysis with FACSArray Bioanalyzer. Each data point represents the average of triplicate results. Shown above is the result from 10:1 ratio. A similar dose response was obtained with the 5:1 ratio. Combination studies of Docetaxel and CC-4047 (b) or CC-5013 (c) in PC-3/PBMC co-culture model. PKH26 pre-labeled PC-3 cells were treated with or without various doses of Docetaxel for 6 h. PBMC were treated with CC-4047 or CC-5013 at various doses for 72 h prior to co-culture with PC-3 cells. Each data point represents the average of triplicate results (* Synergistic and ♣ additive as determined by fractional product method.)
Discussion
IMiDs® immunomodulatory drugs are a novel class of compounds with numerous effects on the human immune system [8, 21] and the tumor microenvironment [10]. In general, these compounds do not show strong cytotoxic effect on tumor cell lines or primary tumor cells, with a few exceptions as published previously [17, 19, 24, 29, 37]. Consistently, we observed no or modest direct effect of CC-4047 and CC-5013 on proliferation of the three cancer cell lines used in this study. One of these cell lines represents a leukemia tumor for which anti-tumor properties of CC-5013 has been observed in patients [18, 22]. We therefore adapted a more physiologically relevant in vitro model where PBMC are co-cultured with tumor cells. Our data demonstrate that in vitro anti-tumor activity of CC-4047 and CC-5013 is only fully revealed when immune cells are present in co-culture.
It has been previously reported that certain IMiDs® immunomodulatory drugs may mediate their anti-tumor effects, at least in part, by modulating NK cell number and function in cancer patients [9, 15]. Consistently, the induction of tumor cell apoptosis in our co-culture model is attributed to NK cell activation by CC-4047 and CC-5013. Both compounds upregulated the expression of CD56 adhesion molecule in NK cells, to the same extent as the upregulation reported for IL-2 and NK cell stimulatory factor (NKSF) [31, 32]. NK cells have been categorized into two major groups based on the level of CD56 expression. In general, CD56bright NK cells are known as the major cytokine producing subset, whereas CD56dim NK cells as the subset exhibiting greater cytotoxic activity [6]. However, upon IL-2 or IL-12 stimulation, CD56bright cells have also been shown to exhibit enhanced cytotoxicity against NK _targets, comparable to CD56dim cells [27, 32, 36].
As shown previously, CC-4047 and CC-5013 can stimulate the production of cytokines by anti-CD3-stimulated T cells [8]. Increased IL-2 production by these compounds was detected in non-stimulated PBMC as reported by Hayashi et al. [15]. We also observed elevated levels of IL-2, TNF-α and IFN-γ in the supernatants of PBMC treated with CC-4047 or CC-5013 for 24 h (data not shown). The increased IL-2 level might have been responsible for upregulation of CD56 and the enhanced cytotoxic activity of NK cells in our model. Hayashi et al. showed that blockage of IL-2R abrogated several IMiDs® immunomodulatory drugs-induced enhancement of NK cytotoxicity [15], supporting this hypothesis.
Our data also demonstrate that the presence of other cell population(s) within PBMC is required for the enhanced NK cell cytotoxicity. In co-culture assays performed in a transwell system where PBMC and _target cells were separated by a membrane, PBMC activity in inducing K562 apoptosis was completely abolished [13]. Therefore, cell–cell interaction is also critical for NK cell cytotoxicity in this co-culture model. Together, these data indicate that CC-4047 and CC-5013 might enhance cytotoxicity by stimulating cytokine production and activating NK cells.
Additionally, by applying the co-culture in a combination setting we demonstrated that CC-4047 and CC-5013 strongly enhance the ability of Rituximab to induce antibody-dependent cell-mediated cytotoxicity (ADCC) of NHL cells. It has been shown that CC-5013 augments NK cell cytotoxicity in a mouse lymphoma model in combination with Rituximab and this augmentation was NK cell dependent [16]. Positive preliminary results of a phase II trial to assess CC-5013 efficacy in NHL as monotherapy have been reported recently [38]. Trials with Rituximab in combination with CC-5013 are also ongoing for both NHL and CLL. In multiple myeloma, CC-5013 has been shown to augment NK-mediated ADCC induced by antibodies to different antigens [34, 35]. It is reasonable to hypothesize that CC-4047 and CC-5013 might synergize with other therapeutic antibodies under clinical development for hematological cancers. We are actively investigating this potential application of selected IMiDs® immunomodulatory drugs.
Furthermore, we provide the first in vitro evidence that CC-4047 and CC-5013 enhance the killing of a prostate tumor cell line, alone or in combination with Docetaxel. Increases in NK cell numbers by CC-5013 were previously observed in patients with another solid tumor, metastatic melanoma [3]. These observations together with our results suggest that CC-4047 and CC-5013 could be efficacious in prostate cancer via enhancing NK-mediated cytotoxicity. A phase I clinical trial to evaluate escalating doses of CC-5013 and Docetaxel in androgen independent prostate cancer is ongoing. Preliminary results are promising in terms of PSA declines, disease response and pain reduction [26].
In solid tumors, localization of activated NK cells to the tumor site may provide more effective tumor destruction. One potential strategy to improve the antitumor efficacy of activated NK cells is addition of tumor-reactive monoclonal antibody (mAb) to facilitate ADCC. In vitro and in vivo studies have also demonstrated that ADCC of tumor cells is increased when effector cells are initially activated with IL-2. Certain IMiDs® immunomodulatory drugs, via stimulating IL-2 production and activating effector cells in vivo, might be able to improve clinical outcome of mAb treatment in cancer patients. It has been shown that CC-4047 acts in synergy with Herceptin in inducing apoptosis of a breast cancer cell line [5]. A number of mAb against tumor antigens are being evaluated in prostate cancer patients, including mAb J591 which was shown to induce ADCC [14, 25]. Therefore, combination therapy of selected IMiDs® immunomodulatory drugs with antibodies to tumor-specific antigens has the potential to provide clinical benefits to patients with solid tumors.
In summary, this study describes the application of an in vitro co-culture model that is amenable to drug discovery of anti-cancer agents with immunomodulatory properties. Our results provide strong rationale for clinical evaluation of CC-5013 and CC-4047 in NHL and prostate cancer either as single therapy or in combination with Rituximab or Docetaxel. Combination therapy of certain IMiDs® immunomodulatory drugs with cytotoxic agents in solid tumors is beginning to gain momentum and early data are encouraging.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- BrdU
Bromodeoxyuridine
- CD56
NCAM1, Neural cell adhesion molecule 1
- DMSO
Dimethyl sulfoxide
- FBS
Fetal bovine serum
- IL-2
Interleukin-2 protein
- IL-2R
Interleukin-2 receptor
- LLT1
Lectin-like transcript 1
- mAb
Monoclonal antibody
- NK
Natural killer cell
- NKSF
NK cell stimulatory factor
- NKT
Natural killer T cell
- PBMC
Peripheral blood mononuclear cells
- SD
Standard deviation
Footnotes
D. Zhu and L. G. Corral contributed equally to this study.
References
- 1.Bamias A, Dimopoulos MA. Thalidomide and immunomodulatory drugs in the treatment of cancer. Expert Opin Investig Drugs. 2005;14:45–55. doi: 10.1517/13543784.14.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JB, Michael A, Clarke IA, Dredge K, Nicholson S, Kristeleit H, Polychronis A, Pandha H, Muller GW, Stirling DI, Zeldis J, Dalgleish AG. Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. Br J Cancer. 2004;90:955–961. doi: 10.1038/sj.bjc.6601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett JB, Tozer A, Stirling D, Zeldis JB. Recent clinical studies of the immunomodulatory drug (IMiD) lenalidomide. Br J Cancer. 2005;93:613–619. doi: 10.1038/sj.bjc.6602774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett JB, Wu L, Adams M, Schafer P, Muller G, Stirling D (2007) lenalidomide and pomalidomide strongly enhance tumor cell killing in vitro during antibody-dependent cellular cytotoxicity (ADCC) mediated by trastuzumab, cetuximab and rituximab. Paper presented at 2007 ASCO annual meeting abstract 3023
- 6.Chidrawar SM, Khan N, Chan YL, Nayak L, Moss PA. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun Ageing. 2006;3:10. doi: 10.1186/1742-4933-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 8.Corral LG, Kaplan G. Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis. 1999;58(Suppl 1):I107–I113. doi: 10.1136/ard.58.2008.i107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, Treon SP, Richardson PG, Schlossman RL, Morgan GJ, Muller GW, Stirling DI, Anderson KC. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.V98.1.210. [DOI] [PubMed] [Google Scholar]
- 10.Dredge K, Marriott JB, Macdonald CD, Man HW, Chen R, Muller GW, Stirling D, Dalgleish AG. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87:1166–1172. doi: 10.1038/sj.bjc.6600607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, Dalgleish AG, Bartlett JB. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69:56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Fischer K, Mackensen A. The flow cytometric PKH-26 assay for the determination of T-cell mediated cytotoxic activity. Methods. 2003;31:135–142. doi: 10.1016/S1046-2023(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhu D, Corral L, Hong W, Stein B. IMiDs enhance tumor cell apoptosis in tumor/PBMC co-cultures. Proc Am Assoc Cancer Res. 2005;46:190–191. [Google Scholar]
- 14.Gu Z, Yamashiro J, Kono E, Reiter RE. Anti-prostate stem cell antigen monoclonal antibody 1G8 induces cell death in vitro and inhibits tumor growth in vivo via a Fc-independent mechanism. Cancer Res. 2005;65:9495–9500. doi: 10.1158/0008-5472.CAN-05-2086. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, Kumar S, Chauhan D, Treon SP, Richardson P, Anderson KC. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005;11:5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- 17.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, Muller G, Stirling DI, Anderson KC. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 18.Kastritis E, Dimopoulos MA. The evolving role of lenalidomide in the treatment of hematologic malignancies. Expert Opin Pharmacother. 2007;8:497–509. doi: 10.1517/14656566.8.4.497. [DOI] [PubMed] [Google Scholar]
- 19.Lentzsch S, LeBlanc R, Podar K, Davies F, Lin B, Hideshima T, Catley L, Stirling DI, Anderson KC. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia. 2003;17:41–44. doi: 10.1038/sj.leu.2402745. [DOI] [PubMed] [Google Scholar]
- 20.List AF. Emerging data on IMiDs in the treatment of myelodysplastic syndromes (MDS) Semin Oncol. 2005;32:S31–S35. doi: 10.1053/j.seminoncol.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Marriott JB, Dredge K, Dalgleish AG. Thalidomide derived immunomodulatory drugs (IMiDs) as potential therapeutic agents. Curr Drug _targets Immune Endocr Metabol Disord. 2003;3:181–186. doi: 10.2174/1568008033340207. [DOI] [PubMed] [Google Scholar]
- 22.Mesa RA, Tefferi A, Li CY, Steensma DP. Hematologic and cytogenetic response to lenalidomide monotherapy in acute myeloid leukemia arising from JAK2(V617F) positive, del(5)(q13q33) myelodysplastic syndrome. Leukemia. 2006;20:2063–2064. doi: 10.1038/sj.leu.2404398. [DOI] [PubMed] [Google Scholar]
- 23.Miller AA, Case D, Harmon M, Savage P, Lesser G, Hurd D, Melin SA. Phase I study of lenalidomide in solid tumors. J Thorac Oncol. 2007;2:445–449. doi: 10.1097/01.JTO.0000268679.33238.67. [DOI] [PubMed] [Google Scholar]
- 24.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.V99.12.4525. [DOI] [PubMed] [Google Scholar]
- 25.Morris MJ, Pandit-Taskar N, Divgi CR, Bender S, O’Donoghue JA, Nacca A, Smith-Jones P, Schwartz L, Slovin S, Finn R, Larson S, Scher HI. Phase I evaluation of J591 as a vascular _targeting agent in progressive solid tumors. Clin Cancer Res. 2007;13:2707–2713. doi: 10.1158/1078-0432.CCR-06-2935. [DOI] [PubMed] [Google Scholar]
- 26.Moss RA, Mohile SG, Shelton G, Melia J, Petrylak DP (2007) A phase I open-label study using lenalidomide and docetaxel in androgen independent prostate cancer (AIPC). Paper presented at 2007 prostate cancer symposium abstract 89
- 27.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–3191. [PubMed] [Google Scholar]
- 28.Payvandi F, Wu L, Naziruddin SD, Haley M, Parton A, Schafer PH, Chen RS, Muller GW, Hughes CC, Stirling DI. Immunomodulatory drugs (IMiDs) increase the production of IL-2 from stimulated T cells by increasing PKC-theta activation and enhancing the DNA-binding activity of AP-1 but not NF-kappaB, OCT-1, or NF-AT. J Interferon Cytokine Res. 2005;25:604–616. doi: 10.1089/jir.2005.25.604. [DOI] [PubMed] [Google Scholar]
- 29.Raje N, Kumar S, Hideshima T, Ishitsuka K, Chauhan D, Mitsiades C, Podar K, Le Gouill S, Richardson P, Munshi NC, Stirling DI, Antin JH, Anderson KC. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004;104:4188–4193. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- 30.Richardson PG, Mitsiades CS, Hideshima T, Anderson KC. Novel biological therapies for the treatment of multiple myeloma. Best Pract Res Clin Haematol. 2005;18:619–634. doi: 10.1016/j.beha.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Robertson MJ, Caligiuri MA, Manley TJ, Levine H, Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990;145:3194–3201. [PubMed] [Google Scholar]
- 32.Robertson MJ, Soiffer RJ, Wolf SF, Manley TJ, Donahue C, Young D, Herrmann SH, Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man HW, Schnetkamp PP, Wolbring G, Govinda S, Corral LG, Payvandi F, Muller GW, Stirling DI. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther. 2003;305:1222–1232. doi: 10.1124/jpet.102.048496. [DOI] [PubMed] [Google Scholar]
- 34.Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, Song W, Podar K, Hideshima T, Chauhan D, Schlossman R, Richardson P, Treon SP, Grewal IS, Munshi NC, Anderson KC. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res. 2005;65:11712–11720. doi: 10.1158/0008-5472.CAN-05-1657. [DOI] [PubMed] [Google Scholar]
- 35.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weler E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DE, Anderson KC (2007) Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood (prepublished online Oct 9, 2007) [DOI] [PMC free article] [PubMed]
- 36.Takahashi E, Kuranaga N, Satoh K, Habu Y, Shinomiya N, Asano T, Seki S, Hayakawa M. Induction of CD16+CD56bright NK cells with antitumour cytotoxicity not only from CD16-CD56bright NK cells but also from CD16-CD56dim NK cells. Scand J Immunol. 2007;65:126–138. doi: 10.1111/j.1365-3083.2006.01883.x. [DOI] [PubMed] [Google Scholar]
- 37.Verhelle D, Corral LG, Wong K, Mueller JH, Moutouh-de Parseval L, Jensen-Pergakes K, Schafer PH, Chen R, Glezer E, Ferguson GD, Lopez-Girona A, Muller GW, Brady HA, Chan KW. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67:746–755. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- 38.Wiernik P, Lossos IS, Tuscano J, Justice G, Vose JM, Pietronigro D, Takeshita K, Ervin-Haynes A, Zeldis J, Habermann T (2007) Preliminary results from a phase II study of lenalidomide oral monotherapy in relapsed/refractory aggressive non-Hodgkin lymphoma. Paper presented at 2007 ASCO annual meeting abstract 8052