Abstract
The efficacy of chemotherapy is mostly restricted by the drug resistance developed during the course of cancer treatment. Mitophagy, as a pro-survival mechanism, crucially maintains mitochondrial homeostasis and it is one of the mechanisms that cancer cells adopt for their progression. On the other hand, mitochondrial apoptosis, a precisely regulated form of cell death, acts as a tumor-suppressive mechanism by _targeting cancer cells. Mitochondrial lipids, such as cardiolipin, ceramide, and sphingosine-1-phosphate, act as a mitophageal signal for the clearance of damaged mitochondria by interacting with mitophagic machinery as well as activate mitochondrial apoptosis via the release of cytochrome c into the cytoplasm. In the recent time, the lipid-mediated lethal mitophagy has also been used as an alternative approach to abolish the survival role of lipid in cancer. Therefore, by _targeting mitochondrial lipids in cancer cells, the detailed mechanism linked to drug resistance can be unraveled. In this review, we precisely discuss the current knowledge about the multifaceted role of mitochondrial lipid in regulating mitophagy and mitochondrial apoptosis and its application in effective cancer therapy.
Keywords: Cardiolipin, Ceramide, Sphingosine-1-phosphate, Mitophagy, Mitochondrial apoptosis, Cancer therapy
Introduction
Mitochondria are essential organelles for cell proliferation and metabolic processes, and act as a central player in the mitophagy and mitochondrial apoptosis. A defect in mitochondrial functions leads to severe health disorders such as cancer, neuro-degeneration diseases, and other age-related disorders [1, 2]. Mitochondrial membranes are mainly composed of phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), cardiolipin (CL), and phosphatidylserine (PS) (given in the decreasing order of their abundance) [3–5]. Among these, CL and PE are mainly synthesized by the mitochondria, whereas for other phospholipids, mitochondria depend on endoplasmic reticulum (ER) [5]. Since several decades, lipids have been believed to be only a part of membrane structure and an alternative energy source. However, current findings have revealed their involvement (e.g., phospholipids, sterols, and sphingolipids) in several cellular signaling cascades leading to the achievement of desired cellular outcomes [6].
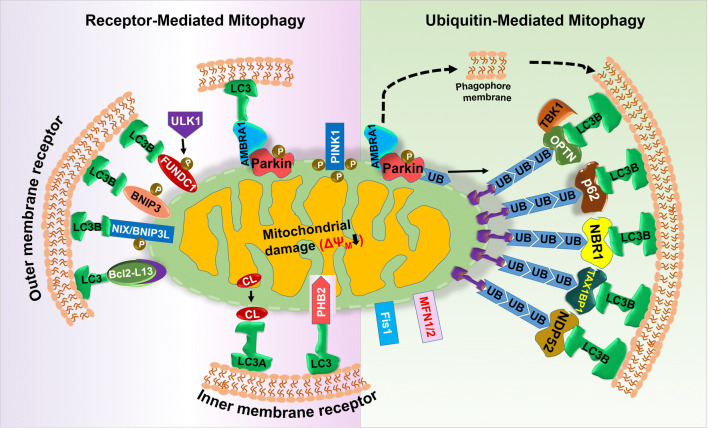
In mitophagy, which is a selective form of autophagy, the entire dysfunctional mitochondria with inefficient oxidative phosphorylation are enclosed in a double-membranous vesicle called mitophagosome and are _targeted for lysosomal degradation [7, 8]. In 2005 for the first time, John Lemasters used the term ‘mitophagy’, since then the majority of research is focusing on identifying its different components. In 2008, Richard Youle and his groups reported that the elimination of damaged mitochondria is mediated through the complex interplay between the E3 ubiquitin ligase Parkin and the Ser/Thr kinase PINK1 (PTEN-induced putative kinase 1) [9]. In healthy mitochondria, PINK1 is imported to mitochondria, followed by its proteasomal degradation via mitochondria intermembrane space protease called as PARL, which cleaves its mitochondrial _targeting sequence [10–12]. A mitochondrial damage causes PINK1 accumulation in the MOM, with subsequent phosphorylation of Parkin and ubiquitin at Ser65 [13, 14]. Being selective autophagy, mitophagy requires all autophagic proteins necessary for autophagosome formation [15], mitochondrial fission (such as Drp1, fis1, and mfn), ubiquitin adaptors (such as NDP52, p62/SQSTRM1, NBR1, TAX1BP1, and Optineurin) [16] and several mitophagy receptors (such as NIX/BNIP3L [17], BNIP3 [18], FUNDC1 [19], AMBRA1 [20], and Bcl2-L13 [21]). These proteins directly and/or indirectly interacts with LC3 via its LIR motif Y (18) xxL promoting mitophagy (see Fig. 1). When mitophagy degrades mitochondria before activating caspase-dependent apoptosis, mitophagy behaves as a cell survival mechanism. Conversely, excessive mitophagy leads to the release of lysosomal enzymes (i.e., cathepsins) to stimulate caspase-dependent apoptosis [22].
Fig. 1.
Mechanistic detail of mitophagy. Under different stress, proteins present on the MOM such as Bcl2-L13, NIX, BNIP3, FUNDC1, and AMBRA1 act as mitophagic receptor and interact directly with LC3 to remove superfluous mitochondria, a process categorized as receptor-mediated mitophagy. On the contrary, NDP52, TAX1BP1, NBR1, p62/SQSTM, and OPTN act as mitophagic adaptor and interact simultaneously with ubiquitin and LC3-promoting ubiquitin-mediated mitophagy
Mitochondrial apoptosis is a precisely regulated form of cell death, where cells undergo drastic changes (i.e., shrinkage, chromatin condensation, and nuclear fragmentation) with subsequent removal in the form of apoptotic bodies by phagocytosis [23]. During this process, the proteins [e.g., cytochrome c (cyt c) and other pro-apoptotic factors] that act normally in the intermembrane space of mitochondria pass into the cytosol depending on mitochondrial damage and subsequent permeabilization of mitochondrial outer membrane (MOM) [24]. Once in the cytosol, cyt c binds to the adaptor molecule APAF-1, which undergoes extensive conformational changes and oligomerizes to form apoptosome (a heptameric structure) followed by recruitment and activation of a cascade of caspases (cysteine aspartyl-specific proteases) (such as caspase-9, caspase-3, -6, -7); this eventually leads to the cell death [25, 26]. In another way, if caspases are not activated, the mitochondrial outer membrane permeabilization (MOMP) also leads to cell death [27]. As mitochondria maintain the balance between tumorigenesis and cell death, the major focus of cancer research is _targeting mitochondria (more specifically mitochondrial lipid) for an effective anti-cancer therapy. In this review, we focus on the status of our current understanding on the involvement of mitochondrial lipids in mitochondrial function, homeostasis with a specific focus on mitophagy and mitochondrial apoptosis, and its therapeutic applications in cancer.
Multifaceted role of mitochondrial lipids in regulating mitophagy
The subcellular distribution of individual lipids has a significant impact on the lipid–protein interactions, modulating the cellular functionality and activity of _target proteins or by directing proteins to different regions of the cell for organelle-specific activities.
Cardiolipin: role in clearing dysfunctional mitochondria
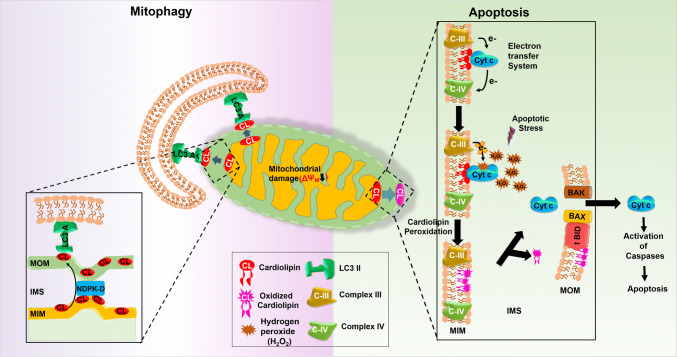
In eukaryotes, CL is a specialized non-bilayer forming phospholipid synthesized by the action of CL synthase (CRD1), which condenses PG and CDP-diacylglycerol (CDP-DAG) [28, 29]. In healthy mitochondria, CL is involved in lipid–protein interactions, necessary for mitochondrial function including, mitochondrial cristae formation, better assembly, and functioning of electron transport chain complexes as well as membrane fusion [30–33]. Any damage to mitochondria or depolarization of its membrane results in the translocation of CL to MOM, which is an initiator signal for mitophagy [34–36]. In 2013, Chu et al. [34] established the functional relationship between CL translocation from mitochondrial inner membrane (MIM) to MOM and mitophagy. Through direct liposome-binding assays, site-directed mutagenesis and computational modeling, microtubule-associated-protein-1 light-chain-3 (LC3) was found to bind with CL. Inhibiting this molecular interaction prevents rotenone-induced mitochondrial delivery to autophagic machinery and reduces mitochondrial loss [37]. LC3 has a putative CL binding site at its N-terminal α-helices necessary for the direct interaction between MOM and mitophagosome culminating into mitophagy but not important for non-selective form of autophagy [34, 37]. When cells were exposed to carbonyl cyanide m-chlorophenyl hydrazine (CCCP) (membrane depolarizer) and rotenone (a complex I inhibitor and mitophagy inducer), a high percentage of CL was observed on the MOM [34]. This translocation of CL is tightly regulated by phospholipid scramblase-3 (PLS3) [34] or nucleoside diphosphate kinase D (NDPK-D) [35]. PLS3 maintains the balanced distribution of CL between outer and inner membrane leaflets, [34] whereas NDPK-D a hexameric intermembrane space protein acts as a CL-translocating machinery, which assists in CL redistribution to the MOM [35]. Additionally, in situ proximity ligation assay (PLA) confirmed the CL-transfer activity of NDPK-D, in close association with OPA1, a dynamin-like GTPase-linking mitochondrial fission–fusion cycle with mitophagy [35]. Therefore, CL redistribution acts as an “eat-me” signal for the removal of dysfunctional mitochondria (see Fig. 2).
Fig. 2.
Multifunctional role of cardiolipin in regulating mitophagy and mitochondrial apoptosis. During mitophagy, cardiolipin translocates from MIM to MOM by the action of NDPK-D, followed by interaction with LC3A for the clearance of dysfunctional mitochondria. Whereas, in mitochondrial apoptosis cardiolipin oxidized by the peroxidase activity, cyt c results in its translocation to the MOM where tBID–CL interaction induces the Bax/Bak oligomerization initiating the mitochondrial disruption and release of cyt c to initiates apoptosis
The changes in CL species, specifically a decline in tetralinoleoyl CL (the most abundant CL in highly active metabolic tissues), interrupt mitophagy [34, 38], causing the accumulation of fragmented and/or dysfunctional mitochondria. Hsu et al. [38] reported the functional involvement of Tafazzin (TAZ)—a known CL remodeling transacylase—in regulating mitochondrial function and mitophagy without affecting autophagosome biogenesis. The molecular interaction between LC3B and CL is more specific than other di-anionic lipids [e.g., phosphatidylinositol-4-phosphate (PtdIns4P)] with positive cooperativity. Moreover, it depends on both electrostatic forces and CL-specific changes in the membrane properties where the C terminal end of LC3B remains open toward the hydrophilic environment after binding with CL-enriched membranes [39]. Shen et al. [40] have reported that CL controls the mitophageal degradation via protein kinase C pathway. Recently, Shimasaki et al. [41] observed that CL plays a major role in the translocation of p210 BCR-ABL [most common variant causing chronic myeloid leukemia (CML)] from cytosol to mitochondria to manage mitochondrial damage. The Pleckstrin homology (PH) domain of p210 BCR-ABL efficiently recognizes CL, via its Arg726 residue present in the ligand-binding region essential for this lipid–protein interaction [41]. This study unraveled the importance of CL, PH domain, and p210 BCR-ABL in CML pathogenesis. By looking at these studies, the importance of CL translocation, an indicator of mitochondrial dysfunction in mitophagy and cancer progression, could be ascertained.
Ceramide: a critical regulator of mitophagy
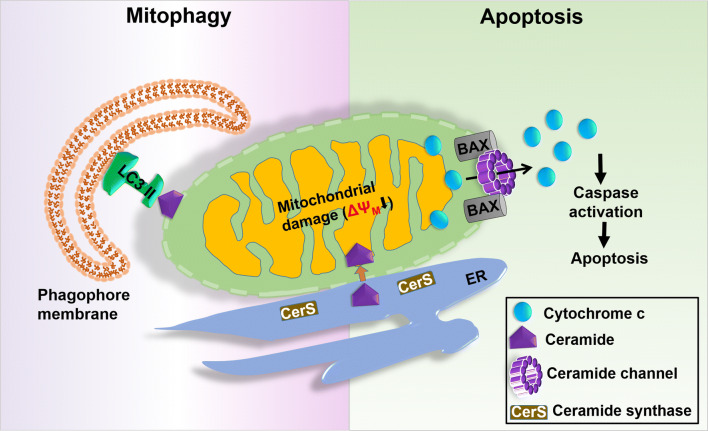
Numerous studies in the recent times suggest that ceramide could act locally in mitochondria and get involved in the regulation of mitophagy (see Fig. 3). When cells are exposed to various cell stressors, the activity of ceramide synthase (CerS) inside the cells goes high with subsequent accumulation of ceramide in the cell that leads to cell death [42, 43]. Obeid and his group reported a novel pathway involved in the production of ceramide in the intact liver mitochondria. Two mitochondrial enzymes, viz., mitochondrial thioesterase and neutral ceramidase (NCDase) regulate this pathway. First, thioesterase hydrolyzes palmitoyl-CoA to palmitate and CoA, and then NCDase forms ceramide by condensing palmitate with sphingosine in a reverse ceramidase reaction inside the mitochondria [44]. In another study, Novgorodov et al. identified two isoforms of CerS (i.e., CerS6 and CerS2) in isolated rat brain mitochondria. CerS6 is present in MIM in association with adenine nucleotide translocase and CerS2 is abundant in MOM along with Tom20 [45]. In 2012, Sentelle et al. [46] reported a mechanistic connection between ceramide signaling and mitophagy while exploring the autophagy inducing potential of C18-ceramide. In this study, they found that C18-ceramide selectively _targets mitochondria to LC3B-II containing autophagolysosomes. After C-18 treatment, LC3B-phosphatidylethanolamine gets lipidated to form LC3B-II, which then binds to ceramide on the MOM suggesting that ceramide–LC3B-II interaction is the key factor leading to lethal mitophagy [46].
Fig. 3.
Role of ceramide in controlling mitophagy and mitochondrial apoptosis. During mitophagy, ceramide translocates to MOM where it interacts with ceramide-binding domain of LC3II for the removal of damaged mitochondria. On the other hand, in mitochondrial apoptosis ceramides form stable pores known as ceramide channel in the phospholipid bilayer. Along with activated BAX, it makes the release of cytochrome c from the mitochondria to activate apoptosis
In 2016, Ogretmen and his group reported that CerS1/C18-ceramide selectively induces lethal mitophagy in FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD)-mediated acute myeloid leukemia (AML) [47]. The inhibition of FLT3-ITD signaling (both at molecular and pharmacological level) activates Drp1 and generates CerS1/C18-ceramide by the mitochondrial CerS1. Further, the mitochondrial C18-ceramide interacts with LC3B-II via I35/F52 residues present in its ceramide-binding domain, in order to recruit autophagosomes to mitochondria to initiate mitophagy-dependent cell death [47]. Recently, we reported that PUMA, a BH3-only pro-apoptotic Bcl2 family protein, is responsible for inducing lethal mitophagy in glioblastoma cells [48]. Upon ceramide stress, ceramide synthase-1 activation leads to ER stress and the accumulation of ROS, which triggers mitochondrial damage. In this condition, PUMA interacts with LC3 via its C-terminally located LC3 interacting region (LIR) and leads to lethal mitophagy [48].
Sphingosine-1-phosphate and mitophagy
Sphingosine-1-phosphate (S1P) is one of the most widely studied bioactive sphingolipids [49] produced intracellularly by two closely related sphingosine kinases: SphK1 and SphK2. Mitochondrial S1P is mostly generated from sphingosine by the action of SphK2 [50]. SphK2 enhances autophagy level by directly interacting with Bcl-2 via a putative BH3 domain to displace Beclin-1 independent of its catalytic activity [51]. S1P is directly involved in the LC3 lipidation after a cleavage mediated by the action of Sphingosine-1-phosphate lyase 1 (SGPL1) to form hexadecenal and ethanolamine phosphates, which can be directed to the synthesis of PE. S1P directly interacts with prohibitin2 (PHB2), which is a highly conserved chaperone that regulates mitochondrial assembly and function [50] and a receptor protein for mitophagy [52]. Mitroi et al. [53] reported that the depletion of SGPL1 reduces PE levels and impairs LC3 lipidation causing an accumulation of phagophore-like structures and autophagic substrates, including p62 and aggregate-prone proteins. The addition of PE to SGPL1-deficient cells restores LC3-II levels and autophagic flux suggesting that S1P regulates PE availability for LC3 lipidation and the elongation of phagophores [53].
Mitochondrial lipid: the dynamic regulator of mitochondrial apoptosis
Mitochondrial lipids act as a stress sensor in cancer cells, which not only regulate the shape, structure, and function of mitochondria but also are involved in the regulation and propagation of mitochondrial apoptosis. In this section, we describe the functional role of different mitochondrial lipids with respect to mitochondrial apoptosis.
Cardiolipin (CL): mitochondrial localization, molecular interaction under apoptotic stimuli
In healthy cells, CL interacts with all the proteins present in the MIM (such as the electron transport chain complexes I, III, IV, V and cyt c) and because of the presence of a small head group with four fatty acyl chains, it serves as a proton sink in the proximity of energy transducing macromolecules [54]. Free and loosely bound cyt c (about 85% of the total cyt c) contributes to the transfer of electrons, inhibiting ROS formation in preventing oxidative stress. Conversely, the tightly bound cyt c (15%) confers peroxidase activity, a crucial event for initiating apoptosis [55]. Under ‘normal’ conditions when cyt c carrying electrons, the functional positions in its haeme iron are occupied and it undergoes conformational change when bound to CL and it becomes partially unfolded [56]. During mitochondrial apoptosis, cyt c drives the oxidation of protein and lipid substrates (preferably CL) leading to the accumulation of CL-hydroperoxides and translocation of both cyt c and CL-hydroperoxide species to the MOM [56]. The formation of highly oxidized heme in the CL/cyt c complex in the presence of hydrogen peroxide (H2O2) implies the subtraction of one electron from unsaturated acyl chains of CL and formation of a lipid hydroperoxide. In the absence of H2O2, this lipid hydroperoxide acts as an alternative substrate for the peroxidase activity of cyt c, which is required for lipid peroxidation [57].
CL is mostly located at the contact region between MOM and MIM and acts as the _target site for tBID, a protein appointed for the apoptotic message transmission [58]. tBID first alters the structure of the mitochondrion by binding to CL via its alphaH6 helix; successively, in cooperation with CL, it induces the Bax/Bak oligomerization (a process requiring the BH3 domain of tBID), which initiates the mitochondrial disruption [59]. The tBID–CL interaction also plays a critical role in the cristae-remodeling essential for the release of cyt c into the cytosol and for the formation of fission sites in the mitochondrion [60]. The release of cyt c is attributed to the formation of the mitochondrial apoptosis-induced channel (MAC, formed by Bak and/or Bax) in the outer mitochondrial membrane followed by activation of caspase 9, via cyt c to initiates apoptosis [61]. In a recent study, Lai et al. [62] have reported that BAX undergoes dimerization to form active oligomers (lethal oligomer) in the presence of CL. The active oligomers form a stable pore in the membrane that leads to MOMP [62] (see Fig. 2).
Ceramide: a bona fide transducer in mitochondrial apoptosis
Mitochondrial ceramide is produced inside cells in response to various stress (pro-apoptotic) stimuli, such as IR, CD95/Fas, and TNF-α cell via sphingomyelin hydrolysis or de novo synthesis [63, 64]. Birbes et al. [65] reported that MCF-7 cells possess a set of mitochondria-specific ceramide (not present in any other organelle), responsible for the induction of apoptosis. The addition of exogenous ceramides to mitochondria or to cultured cells induces the release of cytochrome c from mitochondria, which is the initiator signal for mitochondrial apoptosis [65]. Ceramide activates serine/threonine protein phosphatases (e.g., PP1 and PP2A), which are the important intracellular effector molecules of apoptosis [42]. PP1 is responsible for the dephosphorylation of pRB (retinoblastoma susceptibility gene) that leads to cell cycle arrest at the G1 phase in the presence of ceramide [66].
Ceramide and activated BAX together are responsible to form stable pores known as ceramide channel in the phospholipid bilayer that makes the release of cytochrome c from the mitochondria to activate apoptosis [67]. The formation and stability of ceramide channel are mostly affected by the ceramide concentration, frequency of their synthesis, and breakdown [68], and their transport for the mitochondrial _targeting of the ceramide transfer protein (CERT), which is involved in their import into mitochondria [69]. Bcl-2 overexpression leads to the accumulation of ceramide due to the reduction of nSmase1 activation followed by subsequent apoptosis in glioma cells [70]. Yabu et al. [71] showed that when neutral sphingomyelinase1 (nSmase1) or bacterial sphingomyelinase was _targeted to mitochondria, apoptosis was induced by promoting the hydrolysis of sphingomyelin that was finally converted into ceramide. The addition of Bcl-xL to rat mitochondria prevented ceramide-induced MOMP by disassembling ceramide channels in the phospholipid membranes that inhibited the release of cytochrome c [72].
The ceramide-enriched membrane is the primary requirement for BAX oligomerization [73] and its translocation is facilitated by activating p38 MAPK or downregulating AKT [74]. Ceramide arbitrates MOMP by activating glycogen synthase kinase 3β (GSK3β) through PP2A and activates cathepsin D, which then activates a series of caspases including caspase-2 and caspase-8, and finally leads to the cleavage of a BID to form tBID [66]. Activation of BAK is essential and enough to drive the activity of ceramide synthase (CerS) via a feed-forward mechanism that leads to the accumulation of ceramide inside the cell following inhibition of BCL2-like proteins leading to the formation of the ceramide channel [75] (see Fig. 3).
Sphingosine-1-phosphate (S1P) and its role in mitochondrial apoptosis
In addition to ceramide, sphingosine also regulates mitochondrial apoptosis in a ceramide-independent pathway. When human neutrophils were exposed to TNF-α, a higher production of sphingosine and ceramide inside the cells leads to apoptosis. However, when sphingosine was supplemented alone exogenously, the effect of TNF-α was recapitulated suggesting that the sphingosine-deacetylated product of ceramide controls induction of TNF-α [76]. Sphingosine-induced apoptosis was found to be caspase dependent and started earlier than ceramide-mediated apoptosis, which signifies that apoptosis induction is solely because of sphingosine and not because of its conversion to ceramide [77].
Under different stress, S1P is degraded by S1P lyase to form hexadecenal, which then binds to the apoptosis regulator BAX, promoting its oligomerization followed by the release of cytochrome c [78]. The BH3 domain of SphK2 favors its binding with pro-apoptotic BCL-XL, which abolishes its anti-apoptotic effect [79]. Chipuk et al. [78] have shown, using purified mitochondria, that sphingolipid metabolism works together with BAK and BAX activation leading to the release of cytochrome c in coordination with BH3-only proteins and a specific lipid environment followed by MOMP sensitization promoting the mitochondrial apoptosis. The overexpression of SphK/S1P signaling develops resistance to chemotherapy, radiation therapy, and hormonal therapy in various types of cancers, including breast, prostate, and pancreatic cancers [49, 80]. Whereas, knocking down SphK2 with siRNA or inhibiting its activity with the selective pharmacological drugs reduces cancer cell growth, migration, and invasion [81, 82].
Mitochondrial lipid: a potential _targets for anti-cancer therapy
Even though chemotherapy is one of the most widely adopted approaches for effective cancer treatment, its efficacy is restricted because of drug resistance developed due to drug _target alterations, pro-survival pathway activation, and ineffective cell death induction [83]. Currently, many attempts are being made to develop drugs, which specifically inactivate the pro-survival pathway or stimulate cell death pathway (mitochondrial apoptosis) so that the tumor cells are removed. This could be an ideal strategy for anti-cancer therapy.
How can the survival role of mitochondrial lipid be _targeted for cancer cell death?
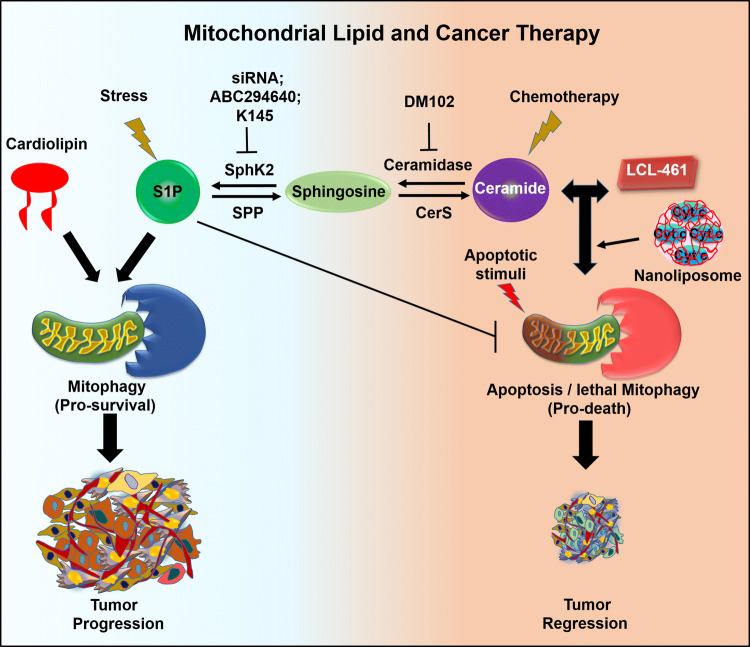
Mitochondrial lipid-induced mitophagy is context dependent and acts either as a cell survival or as a death mechanism in cancer cells. For cell survival, it eliminates the mitochondria which are getting ready to initiate caspase-dependent apoptosis [84]. However, when excessive removal of mitochondria without proper biogenesis or metabolic balance occurs for longer duration, it leads to lethal mitophagy and cell death [85]. For example, C18-ceramide was accumulated in the MOM to interact with LC3B-II recruiting autophagic machinery to trigger lethal mitophagy and tumor suppression in head and neck squamous cell carcinoma (HNSCC) in a non-apoptotic manner, independent of caspase and Bax/Bak activity. Mitochondrial depolarization, decreased ATP generation, and DRP-1 oligomerization accompanied this process [46, 85]. Dany et al. [47] reported that LCL-461 which is an analog drug for mitochondria-_targeted C18-ceramide is effective against crenolanib resistance by inducing lethal mitophagy in FLT3 mutated AML cells, in NSG mice with crenolanib-resistant AML xenografts, and in human FLT3-ITD1 AML blasts, whereas the known inhibitors of FLT3-ITD, such as quizartinib (AC220), sorafenib, and crenolanib, showed a high therapeutic efficacy in preclinical models but failed to produce desirable results in clinical trials due to the resistance developed against the drugs [86]. In another study, Thomas et al. [87] reported that triggering ceramide signaling in human papillomavirus (HPV)-associated HNSCC leads to lethal mitophagy in cancer cells resulting in tumor suppression. Moreover, using different pharmacological, and genetic approaches, they showed that HPV early protein 7 (E7) increases the level of ceramide-mediated lethal mitophagy by selectively inhibiting retinoblastoma protein (RB), which releases E2F5. E2F5 then associates with DRP1, providing a supportive platform for the activation of Drp1 and its mitochondrial translocation, resulting in mitochondrial fission mediating high level of lethal mitophagy-driven tumor suppression [87]. Thus, _targeting mitophagy might affect the equilibrium between cancer progression and cell death (see Fig. 4).
Fig. 4.
Mitochondrial lipid in _targeting cancer cells for better anti-cancer therapy. Mitochondrial lipids could be useful for _targeted removal of cancer cells by either inhibiting various enzymes necessary for S1P and cardiolipin-mediated protective mitophagy or activating ceramide production or exogenous supplying of LCL-461 to induce lethal mitophagy and apoptosis in cancer cells
In another mechanism, cancer cells develop therapeutic resistance by upregulating SphK/S1P signaling, which leads to a higher production of S1P, a tumor-promoting lipid. In the recent time, multiple lines of experiments show that the inhibition of SphK2 at both pharmaceuticals as well as at the genetic level can rule out MDR-associated chemo-resistance in different cancer types [88, 89]. A breast cancer cell line, MCF-7, shows enhanced sensitivity toward doxorubicin by the loss of function of SphK2 [90]. Sorafenib (BAY 43-9006), one of the clinically approved drugs against renal cell carcinoma [91], is also used against melanoma and breast cancer in combination with nanoliposomal ceramide for effective treatment [92]. The selective inhibition of SphK2 by the pharmacological inhibitors such as ABC294640 and K145 has shown anti-cancer effects [89]. Furthermore, a phase I clinical study on ABC294640 in patients with advanced solid tumors reported a partial response in a patient with cholangiocarcinoma with various solid tumors, suggesting that SphK2 is an attractive therapeutic _target [93]. In addition, a combination of sorafenib and an inhibitor of either SphK1/2 or SphK2 can inhibit the growth of kidney carcinoma and human pancreatic adenocarcinoma cells under in vitro as well as in vivo condition [94]. However, more studies are needed to be performed _targeting various mitochondrial lipids or specific enzymes required for their biosynthesis to abolish the survival role of mitochondrial lipid that would be beneficial for effective cancer treatments.
Which switch should be on to activate cancer cell death?
Mitochondrial lipids are widely believed to be a master regulator of apoptosis but how these molecules specifically _target cancer cells and commit to death is not well known. Ceramide as a tumor-suppressive lipid acts as a crucial factor in modulating cancer progression. Ceramide-induced apoptosis can be enhanced by _targeting enzymes involved in ceramide biosynthesis pathway (see Fig. 4). Gain of function of CerS1 increased the sensitivity of HEK293T cells toward different anti-cancer drugs including carboplatin, doxorubicin, vincristine, and cisplatin, while CerS5 overexpression augmented its sensitivity toward vincristine and doxorubicin [95]. In prostate cancer cells, the addition of DM102 (an acid ceramidase inhibitor) enhances cytotoxic effects of fenretinide [96], whereas, in case of breast cancer cell lines, exogenous addition of C6 ceramide showed similar effects [97]. Tamoxifen increases the cytotoxicity of C6 ceramide in breast cancer cells, by arresting the cell cycle and causing high MOMP that leads to caspase-dependent apoptosis [98]. Downregulation of Protein Tyrosine Phosphatase localized to the Mitochondrion 1 (PTPMT1), an MIM protein with a phosphatase domain exposed to the matrix, sensitizes cancer cells towards currently available chemotherapeutics through induction of apoptosis [99].
During the course of treatment, cancer cells develop resistance towards drugs by overexpressing anti-apoptotic proteins (Bcl-2 and Bcl-xL), which prevent the permeabilization of MOM and thus the release of cyt c into the cytoplasm [100]. Therefore, intracellular delivery of cyt c from outside of the cell is one way to initiate apoptosis in cancer cells by evading the anti-apoptotic factors preventing the release of the “own” cyt c from the cell mitochondria. However, cyt c is impermeable to the cellular membrane. In the recent time, several different nano-sized cyt c delivery systems have been developed to address these issues [101–103]. In 2012, Kim et al. developed a lipoprotein-based delivery platform using refined lipid bilayers of DOTAP/DOPE lipids and apo-lipoprotein for transporting MPS-conjugated cyt c. This nanocarrier-mediated platform specifically delivers cyt c into cancer cells (both into non-small cell lung carcinoma cells and tumor tissue) to induce apoptosis and tumor growth retardation in vivo [101]. In another study, Mendez et al. [102] designed a mesoporous silica nanoparticle system for the _targeted delivery of immobilized chemically glycosylated cyt c into HeLa cells to induce apoptosis. The major drawback of these systems is that all cyt c carriers are formulated using synthetic compounds (i.e., mimicking a natural event through non-naturally occurring compounds). Then, in 2017, Vladimirov et al. [103] designed a biomaterial-based anti-cancer nano-formulation having cyt c and CL, which directly acts upon the cell membrane and/or mitochondrial membranes leading to lipid peroxidation. In this study, they examined the cytotoxic and pro-apoptotic effect of this cyt-CL complex in both drug-sensitive and -resistant cancer cells. Moreover, they studied the mechanism by which free radicals are formed by the cyt-CL complex using tetraoleoyl CL (cardiolipin resistant toward lipid peroxidation) to mediate pro-apoptotic effects [103]. It can be inferred through these findings that a combination of mitophagy inhibitors and classical chemotherapeutics can improve the effectiveness of chemotherapy.
Conclusion and future prospective
Mitochondria, as a central player, maintain the balance between the cancer cell survival and mitochondria-mediated cancer cell death. Therefore, in this review, we have made an effort to summarize the present understanding about various mitochondrial lipids and their dynamic roles in both mitophagy and mitochondrial apoptosis in connection with more effective cancer therapy. Even though many developments have been made about mitochondrial lipids, several concerns remain such as how mitochondrial dynamics regulate the distribution and trafficking of individual lipids. Do mitochondrial lipids translocate to any specific organelle to carry selective autophagy? Is there any condition where an alteration in CL biosynthesis can lead to cancer development? What are the factors that decide whether sphingolipid-induced mitophagy is cytoprotective or lethal? Is it the length of fatty acyl chain or its intracellular location that decide the nature of mitophagy? Cancer cells mostly alter lipid metabolism to develop therapeutic resistance against different chemotherapeutic drugs. Therefore, by _targeting enzymes of lipid metabolism using specific inhibitors or a combination of different drugs along with inhibitors could offer a new promising approach for cancer therapy (Fig. 4). In addition, inhibiting the pro-survival pathway adopted by cancer cells and activating pro-death signaling cascade using specific pharmacological inhibitors or inducers would be an ideal strategy for cancer treatment. Gathering information about how cancer cells skip apoptosis via mitochondrial lipid-mediated signaling cascades and discovering more potent and safe inhibitors of mitochondrial lipids will provide opportunities for more practical and effective cancer therapy.
Acknowledgements
Research support was partly provided by Department of Biotechnology (Grant number BT/PR7791/BRB/10/1187/2013); Science and Technology Department, Government of Odisha; the Board of Research in Nuclear Sciences (BRNS) (number 37(1)/14/38/2016-BRNS/37276), Department of Atomic Energy (DAE); Science and Engineering Research Board (SERB) (number EMR/2016/001246), Department of Science and Technology.
Compliance with ethical standards
Conflict of interest
The authors disclose no conflict of interest.
References
- 1.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang D, Hamasaki N. Alterations of mitochondrial DNA in common diseases and disease states: aging, neurodegeneration, heart failure, diabetes, and cancer. Curr Med Chem. 2005;12:429–441. doi: 10.2174/0929867053363081. [DOI] [PubMed] [Google Scholar]
- 3.Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 4.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic (Cph, Den) 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 6.Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, Spener F, van Meer G, Wakelam MJ, Dennis EA. Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res. 2009;50(Suppl):S9–S14. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campello S, Strappazzon F, Cecconi F. Mitochondrial dismissal in mammals, from protein degradation to mitophagy. Biochem Biophys Acta. 2014;1837:451–460. doi: 10.1016/j.bbabio.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meissner C, Lorenz H, Hehn B, Lemberg MK. Intramembrane protease PARL defines a negative regulator of PINK1- and PARK2/Parkin-dependent mitophagy. Autophagy. 2015;11:1484–1498. doi: 10.1080/15548627.2015.1063763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamano K, Youle RJ. PINK1 is degraded through the N-end rule pathway. Autophagy. 2013;9:1758–1769. doi: 10.4161/auto.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205:143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 15.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akematsu T, Akporiaye ET, Al-Rubeai M, Albaiceta GM, Albanese C, Albani D, Albert ML, Aldudo J, Algul H, Alirezaei M, Alloza I, Almasan A, Almonte-Beceril M, Alnemri ES, Alonso C, Altan-Bonnet N, Altieri DC, Alvarez S, Alvarez-Erviti L, Alves S, Amadoro G, Amano A, Amantini C, Ambrosio S, Amelio I, Amer AO, Amessou M, Amon A, An Z, Anania FA, Andersen SU, Andley UP, Andreadi CK, Andrieu-Abadie N, Anel A, Ann DK, Anoopkumar-Dukie S, Antonioli M, Aoki H, Apostolova N, Aquila S, Aquilano K, Araki K, Arama E, Aranda A, Araya J, Arcaro A, Arias E, Arimoto H, Ariosa AR, Armstrong JL, Arnould T, Arsov I, Asanuma K, Askanas V, Asselin E, Atarashi R, Atherton SS, Atkin JD, Attardi LD, Auberger P, Auburger G, Aurelian L, Autelli R, Avagliano L, Avantaggiati ML, Avrahami L, Awale S, Azad N, Bachetti T, Backer JM, Bae DH, Bae JS, Bae ON, Bae SH, Baehrecke EH, Baek SH, Baghdiguian S, Bagniewska-Zadworna A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birgisdottir AB, Lamark T, Johansen T. The LIR motif—crucial for selective autophagy. J Cell Sci. 2013;126:3237–3247. doi: 10.1242/jcs.126128. [DOI] [PubMed] [Google Scholar]
- 17.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, Rogov V, Lohr F, Popovic D, Occhipinti A, Reichert AS, Terzic J, Dotsch V, Ney PA, Dikic I. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinsay MN, Thomas RL, Lee Y, Gustafsson AB. Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy. 2010;6:855–862. doi: 10.4161/auto.6.7.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, Huang L, Xue P, Li B, Wang X, Jin H, Wang J, Yang F, Liu P, Zhu Y, Sui S, Chen Q. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 20.Strappazzon F, Nazio F, Corrado M, Cianfanelli V, Romagnoli A, Fimia GM, Campello S, Nardacci R, Piacentini M, Campanella M, Cecconi F. AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ. 2015;22:419–432. doi: 10.1038/cdd.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, Yasui H, Ueda H, Akazawa Y, Nakayama H, Taneike M, Misaka T, Omiya S, Shah AM, Yamamoto A, Nishida K, Ohsumi Y, Okamoto K, Sakata Y, Otsu K. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6:7527. doi: 10.1038/ncomms8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, De Dawson VL, Laurenzi V De, Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M Di, Daniele N Di, Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott M, Zhivotovsky B, Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- 25.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 26.Zaman S, Wang R, Gandhi V. _targeting the apoptosis pathway in hematologic malignancies. Leuk Lymphoma. 2014;55:1980–1992. doi: 10.3109/10428194.2013.855307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 2015;112:957–962. doi: 10.1038/bjc.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlattner U, Tokarska-Schlattner M, Rousseau D, Boissan M, Mannella C, Epand R, Lacombe ML. Mitochondrial cardiolipin/phospholipid trafficking: the role of membrane contact site complexes and lipid transfer proteins. Chem Phys Lipid. 2014;179:32–41. doi: 10.1016/j.chemphyslip.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Houtkooper RH, Akbari H, van Lenthe H, Kulik W, Wanders RJ, Frentzen M, Vaz FM. Identification and characterization of human cardiolipin synthase. FEBS Lett. 2006;580:3059–3064. doi: 10.1016/j.febslet.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 30.Arnarez C, Marrink SJ, Periole X. Identification of cardiolipin binding sites on cytochrome c oxidase at the entrance of proton channels. Sci Rep. 2013;3:1263. doi: 10.1038/srep01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharpley MS, Shannon RJ, Draghi F, Hirst J. Interactions between phospholipids and NADH:ubiquinone oxidoreductase (complex I) from bovine mitochondria. Biochemistry. 2006;45:241–248. doi: 10.1021/bi051809x. [DOI] [PubMed] [Google Scholar]
- 32.Sinibaldi F, Howes BD, Droghetti E, Polticelli F, Piro MC, Di Pierro D, Fiorucci L, Coletta M, Smulevich G, Santucci R. Role of lysines in cytochrome c-cardiolipin interaction. Biochemistry. 2013;52:4578–4588. doi: 10.1021/bi400324c. [DOI] [PubMed] [Google Scholar]
- 33.Khalifat N, Fournier JB, Angelova MI, Puff N. Lipid packing variations induced by pH in cardiolipin-containing bilayers: the driving force for the cristae-like shape instability. Biochem Biophys Acta. 2011;1808:2724–2733. doi: 10.1016/j.bbamem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagan VE, Jiang J, Huang Z, Tyurina YY, Desbourdes C, Cottet-Rousselle C, Dar HH, Verma M, Tyurin VA, Kapralov AA, Cheikhi A, Mao G, Stolz D, St Croix CM, Watkins S, Shen Z, Li Y, Greenberg ML, Tokarska-Schlattner M, Boissan M, Lacombe ML, Epand RM, Chu CT, Mallampalli RK, Bayir H, Schlattner U. NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy. Cell Death Differ. 2016;23:1140–1151. doi: 10.1038/cdd.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren M, Phoon CK, Schlame M. Metabolism and function of mitochondrial cardiolipin. Prog Lipid Res. 2014;55:1–16. doi: 10.1016/j.plipres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Chu CT, Bayir H, Kagan VE. LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: implications for Parkinson disease. Autophagy. 2014;10:376–378. doi: 10.4161/auto.27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu P, Liu X, Zhang J, Wang HG, Ye JM, Shi Y. Cardiolipin remodeling by TAZ/tafazzin is selectively required for the initiation of mitophagy. Autophagy. 2015;11:643–652. doi: 10.1080/15548627.2015.1023984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anton Z, Landajuela A, Hervas JH, Montes LR, Hernandez-Tiedra S, Velasco G, Goni FM, Alonso A. Human Atg8-cardiolipin interactions in mitophagy: specific properties of LC3B, GABARAPL2 and GABARAP. Autophagy. 2016;12:2386–2403. doi: 10.1080/15548627.2016.1240856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Z, Li Y, Gasparski AN, Abeliovich H, Greenberg ML. Cardiolipin regulates mitophagy through the protein kinase C pathway. J Biol Chem. 2017;292:2916–2923. doi: 10.1074/jbc.M116.753574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimasaki K, Watanabe-Takahashi M, Umeda M, Funamoto S, Saito Y, Noguchi N, Kumagai K, Hanada K, Tsukahara F, Maru Y, Shibata N, Naito M, Nishikawa K. Pleckstrin homology domain of p210 BCR-ABL interacts with cardiolipin to regulate its mitochondrial translocation and subsequent mitophagy. Genes Cells Devoted Mol Cell Mech. 2018;23:22–34. doi: 10.1111/gtc.12544. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez-Corbacho MJ, Canals D, Adada MM, Liu M, Senkal CE, Yi JK, Mao C, Luberto C, Hannun YA, Obeid LM. Tumor necrosis factor-alpha (TNFalpha)-induced ceramide generation via ceramide synthases regulates loss of focal adhesion kinase (FAK) and programmed cell death. J Biol Chem. 2015;290:25356–25373. doi: 10.1074/jbc.M115.658658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J. 2012;441:789–802. doi: 10.1042/BJ20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J Biol Chem. 2011;286:25352–25362. doi: 10.1074/jbc.M110.214866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novgorodov SA, Chudakova DA, Wheeler BW, Bielawski J, Kindy MS, Obeid LM, Gudz TI. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J Biol Chem. 2011;286:4644–4658. doi: 10.1074/jbc.M110.164392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ, Szulc ZM, Bielawski J, Ogretmen B. Ceramide _targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dany M, Gencer S, Nganga R, Thomas RJ, Oleinik N, Baron KD, Szulc ZM, Ruvolo P, Kornblau S, Andreeff M, Ogretmen B. _targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood. 2016;128:1944–1958. doi: 10.1182/blood-2016-04-708750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panda PK, Naik PP, Meher BR, Das DN, Mukhopadhyay S, Praharaj PP, Maiti TK, Bhutia SK. PUMA dependent mitophagy by Abrus agglutinin contributes to apoptosis through ceramide generation. Biochem Biophys Acta. 2018;1865:480–495. doi: 10.1016/j.bbamcr.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song DD, Zhang TT, Chen JL, Xia YF, Qin ZH, Waeber C, Sheng R. Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain. Cell Death Dis. 2017;8:e2912. doi: 10.1038/cddis.2017.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei Y, Chiang WC, Sumpter R, Jr, Mishra P, Levine B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell. 2017;168:224–238.e10. doi: 10.1016/j.cell.2016.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitroi DN, Karunakaran I, Graler M, Saba JD, Ehninger D, Ledesma MD, van Echten-Deckert G. SGPL1 (sphingosine phosphate lyase 1) modulates neuronal autophagy via phosphatidylethanolamine production. Autophagy. 2017;13:885–899. doi: 10.1080/15548627.2017.1291471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haines TH, Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett. 2002;528:35–39. doi: 10.1016/S0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- 55.Huttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, Doan JW, Lee I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion. 2011;11:369–381. doi: 10.1016/j.mito.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Min L, Jian-xing X. Detoxifying function of cytochrome c against oxygen toxicity. Mitochondrion. 2007;7:13–16. doi: 10.1016/j.mito.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Zhang T, Saghatelian A. Emerging roles of lipids in BCL-2 family-regulated apoptosis. Biochem Biophys Acta. 2013;1831:1542–1554. doi: 10.1016/j.bbalip.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Raemy E, Martinou JC. Involvement of cardiolipin in tBID-induced activation of BAX during apoptosis. Chem Phys Lipid. 2014;179:70–74. doi: 10.1016/j.chemphyslip.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Shamas-Din A, Bindner S, Zhu W, Zaltsman Y, Campbell C, Gross A, Leber B, Andrews DW, Fradin C. tBid undergoes multiple conformational changes at the membrane required for Bax activation. J Biol Chem. 2013;288:22111–22127. doi: 10.1074/jbc.M113.482109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caroppi P, Sinibaldi F, Fiorucci L, Santucci R. Apoptosis and human diseases: mitochondrion damage and lethal role of released cytochrome C as proapoptotic protein. Curr Med Chem. 2009;16:4058–4065. doi: 10.2174/092986709789378206. [DOI] [PubMed] [Google Scholar]
- 62.Lai YC, Li CC, Sung TC, Chang CW, Lan YJ, Chiang YW (2019) The role of cardiolipin in promoting the membrane pore-forming activity of BAX oligomers. Biochim Biophys Acta Biomembr 1861:268–280 [DOI] [PubMed]
- 63.Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science (New York, NY) 2008;322:110–115. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 66.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 67.Ganesan V, Perera MN, Colombini D, Datskovskiy D, Chadha K, Colombini M. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis Int J Programmed Cell Death. 2010;15:553–562. doi: 10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- 68.Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J Lipid Res. 2008;49:625–634. doi: 10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Jain A, Beutel O, Ebell K, Korneev S, Holthuis JC. Diverting CERT-mediated ceramide transport to mitochondria triggers Bax-dependent apoptosis. J Cell Sci. 2017;130:360–371. doi: 10.1242/jcs.194191. [DOI] [PubMed] [Google Scholar]
- 70.Sawada M, Nakashima S, Banno Y, Yamakawa H, Takenaka K, Shinoda J, Nishimura Y, Sakai N, Nozawa Y. Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene. 2000;19:3508–3520. doi: 10.1038/sj.onc.1203699. [DOI] [PubMed] [Google Scholar]
- 71.Yabu T, Shiba H, Shibasaki Y, Nakanishi T, Imamura S, Touhata K, Yamashita M. Stress-induced ceramide generation and apoptosis via the phosphorylation and activation of nSMase1 by JNK signaling. Cell Death Differ. 2015;22:258–273. doi: 10.1038/cdd.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siskind LJ, Feinstein L, Yu T, Davis JS, Jones D, Choi J, Zuckerman JE, Tan W, Hill RB, Hardwick JM, Colombini M. Anti-apoptotic Bcl-2 family proteins disassemble ceramide channels. J Biol Chem. 2008;283:6622–6630. doi: 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee H, Rotolo JA, Mesicek J, Penate-Medina T, Rimner A, Liao WC, Yin X, Ragupathi G, Ehleiter D, Gulbins E, Zhai D, Reed JC, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim HJ, Oh JE, Kim SW, Chun YJ, Kim MY. Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer Lett. 2008;260:88–95. doi: 10.1016/j.canlet.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 75.Beverly LJ, Howell LA, Hernandez-Corbacho M, Casson L, Chipuk JE, Siskind LJ. BAK activation is necessary and sufficient to drive ceramide synthase-dependent ceramide accumulation following inhibition of BCL2-like proteins. Biochem J. 2013;452:111–119. doi: 10.1042/BJ20130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohta H, Yatomi Y, Sweeney EA, Hakomori S, Igarashi Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 1994;355:267–270. doi: 10.1016/0014-5793(94)01218-0. [DOI] [PubMed] [Google Scholar]
- 77.Cuvillier O, Edsall L, Spiegel S. Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J Biol Chem. 2000;275:15691–15700. doi: 10.1074/jbc.M000280200. [DOI] [PubMed] [Google Scholar]
- 78.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 80.Nagahashi M, Tsuchida J, Moro K, Hasegawa M, Tatsuda K, Woelfel IA, Takabe K, Wakai T. High levels of sphingolipids in human breast cancer. J Surg Res. 2016;204:435–444. doi: 10.1016/j.jss.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang L, Liu X, Zuo Z, Hao C, Ma Y. Sphingosine kinase 2 promotes colorectal cancer cell proliferation and invasion by enhancing MYC expression. Tumour Biol J Int Soc Oncodev Biol Med. 2016;37:8455–8460. doi: 10.1007/s13277-015-4700-8. [DOI] [PubMed] [Google Scholar]
- 82.Venant H, Rahmaniyan M, Jones EE, Lu P, Lilly MB, Garrett-Mayer E, Drake RR, Kraveka JM, Smith CD, Voelkel-Johnson C. The sphingosine kinase 2 inhibitor ABC294640 reduces the growth of prostate cancer cells and results in accumulation of dihydroceramides in vitro and in vivo. Mol Cancer Ther. 2015;14:2744–2752. doi: 10.1158/1535-7163.MCT-15-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 84.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dany M, Ogretmen B. Ceramide induced mitophagy and tumor suppression. Biochem Biophys Acta. 2015;1853:2834–2845. doi: 10.1016/j.bbamcr.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stein EM, Tallman MS. Emerging therapeutic drugs for AML. Blood. 2016;127:71–78. doi: 10.1182/blood-2015-07-604538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomas RJ, Oleinik N, Panneer Selvam S, Vaena SG, Dany M, Nganga RN, Depalma R, Baron KD, Kim J, Szulc ZM, Ogretmen B. HPV/E7 induces chemotherapy-mediated tumor suppression by ceramide-dependent mitophagy. EMBO Mol Med. 2017;9:1030–1051. doi: 10.15252/emmm.201607088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, Delisle MB, Cuvillier O, Susini C, Bousquet C. _targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol Cancer Ther. 2009;8:809–820. doi: 10.1158/1535-7163.MCT-08-1096. [DOI] [PubMed] [Google Scholar]
- 89.Liu K, Guo TL, Hait NC, Allegood J, Parikh HI, Xu W, Kellogg GE, Grant S, Spiegel S, Zhang S. Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2,4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent. PLoS One. 2013;8:e56471. doi: 10.1371/journal.pone.0056471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, Dent P, Milstien S, Spiegel S. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 91.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, Sridhara R, Garvey P, Justice R, Pazdur R. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 92.Tran MA, Smith CD, Kester M, Robertson GP. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:3571–3581. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- 93.Britten CD, Garrett-Mayer E, Chin SH, Shirai K, Ogretmen B, Bentz TA, Brisendine A, Anderton K, Cusack SL, Maines LW, Zhuang Y, Smith CD, Thomas MB. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23:4642–4650. doi: 10.1158/1078-0432.CCR-16-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beljanski V, Knaak C, Zhuang Y, Smith CD. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Investig New Drugs. 2011;29:1132–1142. doi: 10.1007/s10637-010-9452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, Alexander S. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res. 2007;5:801–812. doi: 10.1158/1541-7786.MCR-07-0100. [DOI] [PubMed] [Google Scholar]
- 96.Gouaze-Andersson V, Flowers M, Karimi R, Fabrias G, Delgado A, Casas J, Cabot MC. Inhibition of acid ceramidase by a 2-substituted aminoethanol amide synergistically sensitizes prostate cancer cells to N-(4-hydroxyphenyl) retinamide. Prostate. 2011;71:1064–1073. doi: 10.1002/pros.21321. [DOI] [PubMed] [Google Scholar]
- 97.Flowers M, Fabrias G, Delgado A, Casas J, Abad JL, Cabot MC. C6-ceramide and _targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast Cancer Res Treat. 2012;133:447–458. doi: 10.1007/s10549-011-1768-8. [DOI] [PubMed] [Google Scholar]
- 98.Morad SA, Levin JC, Shanmugavelandy SS, Kester M, Fabrias G, Bedia C, Cabot MC. Ceramide–antiestrogen nanoliposomal combinations–novel impact of hormonal therapy in hormone-insensitive breast cancer. Mol Cancer Ther. 2012;11:2352–2361. doi: 10.1158/1535-7163.MCT-12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niemi NM, Lanning NJ, Westrate LM, MacKeigan JP. Downregulation of the mitochondrial phosphatase PTPMT1 is sufficient to promote cancer cell death. PLoS One. 2013;8:e53803. doi: 10.1371/journal.pone.0053803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 101.Kim SK, Foote MB, Huang L. The _targeted intracellular delivery of cytochrome C protein to tumors using lipid-apolipoprotein nanoparticles. Biomaterials. 2012;33:3959–3966. doi: 10.1016/j.biomaterials.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mendez J, Morales Cruz M, Delgado Y, Figueroa CM, Orellano EA, Morales M, Monteagudo A, Griebenow K. Delivery of chemically glycosylated cytochrome c immobilized in mesoporous silica nanoparticles induces apoptosis in HeLa cancer cells. Mol Pharm. 2014;11:102–111. doi: 10.1021/mp400400j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vladimirov YA, Sarisozen C, Vladimirov GK, Filipczak N, Polimova AM, Torchilin VP. The cytotoxic action of cytochrome C/cardiolipin nanocomplex (Cyt-CL) on cancer cells in culture. Pharm Res. 2017;34:1264–1275. doi: 10.1007/s11095-017-2143-1. [DOI] [PubMed] [Google Scholar]