Abstract
Mycobacterium tuberculosis (Mtb) has adapted its metabolism for persistence in the human macrophage. The adaptations are likely to involve Mtb's core intermediary metabolism, whose enzymes have been little studied. The tricarboxylic acid cycle is expected to yield precursors for energy, lipids, amino acids, and heme. The genome sequence of Mtb H37Rv predicts the presence of a complete tricarboxylic acid cycle, but we recently found that α-ketoglutarate dehydrogenase (KDH) activity is lacking in Mtb lysates. Here we showed that citrate synthase, aconitase, isocitrate dehydrogenase, fumarase, malate dehydrogenase, and succinate dehydrogenase, but not KDH, are present, raising the possibility of separate oxidative and reductive half-cycles. As a potential link between the half-cycles, we found that Rv1248c, annotated as encoding SucA, the putative E1 component of KDH, instead encodes α-ketoglutarate decarboxylase (Kgd) and produces succinic semialdehyde. Succinic semialdehyde dehydrogenase activity was detected in Mtb lysates and recapitulated with recombinant proteins GabD1 (encoded by Rv0234c) and GabD2 (encoded by Rv1731). Kgd and GabD1 or GabD2 form an alternative pathway from α-ketoglutarate to succinate. Rv1248c, which is essential or required for normal growth of Mtb [Sassetti, C., Boyd, D. H. & Rubin, E. J. (2003) Mol. Microbiol 48, 77-84] is the first gene shown to encode a Kgd. Kgd is lacking in humans and may represent a potential _target for chemotherapy of tuberculosis.
Keywords: succinic semialdhehyde, succinic semialdhehyde dehydrogenase
Mycobacterium tuberculosis (Mtb), the leading cause of death from a single bacterial species, severely impacts global health and threatens further havoc with the spread of drug resistance (1). Chemotherapy directed against new classes of _targets is an urgent need (2). Unfortunately, knowledge is scant regarding the metabolism of Mtb during its residence in host cells and organs. Transcriptional adaptations of the bacillus in those sites imply that some of the environments faced by Mtb in the host are hypoxic, carbon-poor, oxidative, and nitrosative (3-6).
Part of the difficulty in delineating Mtb's metabolic profile during infection is the meagerness of biochemical knowledge pertaining to its core metabolic processes, much of which has been tentatively inferred from bioinformatics. Several metabolic enzymes are linked with persistence and antioxidant defense of Mtb. Isocitrate lyase (Icl) and glyoxylate dehydrogenase, two enzymes of the glyoxylate shunt, are activated during adaptation to microaerophilic conditions (7), and icl expression is enhanced during infection of macrophages (8). Mtbs deficient in icl were attenuated for survival in activated macrophages in vitro and in lungs of mice (9). Two constituents of pyruvate dehydrogenase (PDH) serve also as components of one of the antioxidant and antinitrosative systems in Mtb; these are lipoamide dehydrogenase (Lpd; Rv0462) and dihydrolipoamide acyltransferase (DlaT; Rv2215; formerly SucB). Lpd and DlaT interact with two other proteins to constitute a four-protein, NADH-dependent peroxidase and peroxynitrite reductase. The two additional proteins are alkylhydroperoxide reductase subunit C (AhpC) and the thioredoxin-like protein coexpressed with it, AhpD (10). This close intersection between core intermediary metabolism and resistance to host-imposed biochemical stress in Mtb directed our attention to Mtb's tricarboxylic acid (TCA) cycle.
The TCA cycle plays essential roles in cell metabolism, providing reducing equivalents for energy generation and biosynthetic reactions, along with precursors for lipids, amino acids and heme. Many bacteria operate variant TCA cycles, reflecting a diversity of metabolic niches and needs (11). The classic cycle favors generation of energy as well as biosynthetic precursors, whereas variants with oxidative and reductive branches, as in many phototropes, lithotrophs and methylotrophs, are more focused on production of biosynthetic precursors (12). The distinguishing feature of the branched variants is their lack of α-ketoglutarate dehydrogenase (KDH). The oxidative branch terminates in production of α-ketoglutarate, whereas the reductive branch leads to succinate. Some bacteria, such as Escherichia coli, operate a complete TCA cycle aerobically but can switch under anaerobic conditions to a branched pathway lacking KDH. Helicobacter pylori, which thrives under microaerophilic conditions, operates a branched TCA pathway, but both branches can be connected by α-ketoglutarate ferredoxin oxidoreductase (13).
The Mtb genome is annotated to encode a complete TCA cycle as well as α-ketoglutarate ferredoxin oxidoreductase (14). However, the annotations are not based on biochemical evidence from Mtb itself. In fact, the only systematic study of TCA cycle enzymes in Mtb dates back to 1962 (15). In that work, PDH and KDH activities were both found to be NADP+-dependent, and KDH was not CoA-dependent. Such unusual patterns of utilization of cofactors call those identifications into question.
Our previous studies (16) suggest that DlaT and Lpd do not conform to their annotations (http://genolist.pasteur.fr/TubercuList) as components of KDH. In fact, Mtb appears to lack KDH, and DlaT and Lpd serve as components of PDH instead. Mtb's PDH activities depend on NAD+, not NADP+, as measured both in cell extracts and in vitro with recombinant proteins. In the present study, we explored the implications of the absence of KDH activity in Mtb. We surveyed the remaining enzymes of the classic TCA cycle and sought additional enzymes that might bridge the oxidative and reductive half-cycles that are potentially left when KDH is absent.
As a starting point, we focused on Rv1248c, whose protein product was annotated as SucA (http://genolist.pasteur.fr/TubercuList) in the expectation that it served as the E1 component of KDH. Because the Rv1248c protein does not perform such a function in vitro (16), we considered additional actions that it might catalyze with α-ketoglutarate.
Materials and Methods
Bacterial Lysates. Mtb lysates were prepared as described (16). E. coli, Mycobacterium smegmatis, and Mycobacterium bovis bacillus Calmette-Guérin were grown in LB or 7H9 medium at 37°C. Log phase and stationary phase cells were harvested and washed three times in PBS. Cell pellets were resuspended in PBS/1 mM PMSF and broken by beating with glass beads. Lysates were centrifuged twice at 17,900 × g for 10 min at 4°C, except that for succinyl CoA synthetase and succinate dehydrogenase assays, lysates were centrifuged once at 2,700 × g. Supernatants were used as cell extracts.
Recombinant Proteins. The Rv1248c protein was purified from recombinant E. coli as described (16). MtbH37Rv gabD1 (Rv0234c) and gabD2 (Rv1731) genes were amplified from genomic DNA by PCR with Pfu DNA polymerase using primers containing 5′ NdeI and 3′ NheI sites. PCR products were digested with NdeI and NheI, and ligated with T4 ligase into pET28b vectors digested with the same enzymes. E. coli expression strain BL21 (DE3) cells were transformed with pET28b encoding His6-tagged GabD1 and GabD2 and cultured in 1-liter batches in LB media with 50 μg/ml kanamycin. Protein expression was induced with 1 mM IPTG at 25°C for 4 h. Cells were harvested by centrifugation at 4°C at 5,500 × g, resuspended in lysis buffer (1 mM PMSF/30 mM imidazole/25 mM Tris, pH 8.0/500 mM NaCl) and broken in a French press. The soluble fraction of the lysates was loaded on a 5-ml Ni+ column (equilibration buffer: 30 mM imidazole/25 mM Tris, pH 8.0; washing buffer: 30 mM imidazole/300 mM NaCl/25 mM Tris, pH 8.0; and elution buffer: 250 mM imidazole/300 mM NaCl/25 mM Tris, pH 8.0). Fractions containing recombinant proteins were identified by SDS/PAGE. Protein concentrations were measured by Bradford assay, and proteins were stored at -20°C.
Enzyme Assays. All reactions were performed at room temperature. KDH. Reaction mixtures (0.5 ml) contained 50 mM potassium phosphate (KPi) (pH 7.0), 0.2 mM thiamine pyrophosphate (TPP), 1 mM MgCl2, 2 mM NAD+ or NADP+, and 50 μg MtbH37Rv lysates. Reactions were initiated by adding 0.165 mM CoA and 1 mM α-ketoglutarate and monitored by following the production of NADH or NADPH at 340 nm. An extinction coefficient of 6,223 M-1·cm-1 was used to calculate the rates of NADH or NADPH formation.
Citrate synthase. Reaction mixtures (0.5 ml) contained 50 mM Hepes (pH 8.0), 2 mM EDTA, 100 mM NaCl, 100 μM 5,5′-dithiobis-2-nitrobenzoic acid, and 25 μg of MtbH37Rv lysates. Reactions were initiated by addition of 0.2 mM oxaloacetate and 0.14 mM acetyl-CoA, and thionitrobenzene formation was followed. An extinction coefficient of 13,600 M-1·cm-1 was used to calculate the rates. Aconitase. Reaction mixtures (0.25 ml) contained 25 mM Hepes (pH 8.0), 100 mM NaCl, and 50 μg MtbH37Rv lysates. Reactions were initiated by adding 0.1 mM cis-aconitate and monitored by following the disappearance of cis-aconitate at 240 nm. An extinction coefficient of 3,500 M-1·cm-1 was used to calculate the rates. Isocitrate dehydrogenase. Reaction mixtures (0.5 ml) contained 50 mM Hepes (pH 8.0), 0.25 mM NAD+ or NADP+, 10 mM MgCl2, and 50 μg of MtbH37Rv lysates. Reactions were initiated by adding 1 mM isocitrate and monitored by following the production of NADH or NADPH at 340 nm. An extinction coefficient of 6,223 M-1·cm-1 was used to calculate the rates.
Succinyl-CoA synthetase. Reaction mixtures (0.5 ml) contained 50 mM KPi (pH 7.0), 100 μM 5,5′-dithiobis-2-nitrobenzoic acid, 250 μM ADP, and 195 μg of MtbH37Rv lysates. Reactions were initiated by adding 150 μM succinyl-CoA and monitored by following the formation of thionitrobenzene at 412 nm. Extinction coefficient 13,600 M-1·cm-1 was used to calculate the rates.
Succinate dehydrogenase. Reaction mixtures (0.5 ml) contained 50 mM Tris·HCl (pH 7.4), 0.1 mM EDTA, 0.18 mM benzyl viologen, and 195 μg of MtbH37Rv cell lysates. Reactions were initiated by adding 20 mM fumarate and monitored by following the oxidation of benzyl viologen at 550 nm. An extinction coefficient of 7,800 M-1·cm-1 was used to calculate the rates.
Fumarase. Reaction mixtures (0.25 ml) contained 50 mM KPi (pH 7.0) and 50 μg of MtbH37Rv lysates. Reactions were initiated by adding 25 mM l-malate and monitored by following the production of fumarate at 250 nm. An extinction coefficient of 1,479 M-1·cm-1 was used to calculate the rates.
Malate dehydrogenase. Reaction mixtures (0.5 ml) contained 50 mM Hepes (pH 8.0), 1 mM NADH, and 50 μg of MtbH37Rv lysates. Reactions were initiated by adding 400 μM oxaloacetate and monitored by following the consumption of NADH at 340 nm. An extinction coefficient of 6,223 M-1·cm-1 was used to calculate the rates.
Succinic semialdehyde dehydrogenase (SSADH). Reaction mixtures (0.5 ml) contained 50 mM Hepes (pH 8.0), 1 mM 2-mercaptoethanol, 2 mM NAD+ or NADP+, and 50 μg of MtbH37Rv lysates. Reactions were initiated by adding 0.1 mM succinic semialdehyde (SSA) and monitored by following the production of NADH or NADPH at 340 nm. An extinction coefficient of 6,223 M-1·cm-1 was used to calculate the rates.
Ferricyanide reductase. Ferricyanide reductase was assayed as described (16).
Linked assay of Rv1248c protein and GabD1 or GabD2. Recombinant Rv1248c protein was preincubated with reaction mix A containing 50 mM KPi (pH 7.0), 0.3 mM TPP, 1 mM MgCl2, and 1 mM α-ketoglutarate for 1 h at room temperature. Recombinant GabD1or GabD2 was added to reaction mix B containing 100 mM KPi (pH 7.0), 0.5 mM NADP+, and 1 mM DTT. SSADH activity was followed by measuring the production of NADPH at A340 after combining the above two reactions.
Identification of Reaction Product of Rv1248c Protein with α-Ketoglutarate. Recombinant Rv1248c protein was preincubated with reaction mix A (see above) for 1 h. Reaction products were derivatized with 2,4-dinitrophenylhydrazine as described (17) with modifications: 2.5 ml reaction mixture contained 10 mM Tris·HCl (pH 6.5), 0.3 mM TPP, 1 mM MgCl2, and 1 mM α-ketoglutarate. Reaction was initiated by adding 2 μM Rv1248c protein, and 0.5-ml aliquots were removed at time 0, 15, 30, and 60 min. A total of 84 μl of 6.3 mM 2,4-dinitrophenylhydrazine in 3 M HCl was added to each aliquot and the samples incubated for 45 min at 50°C. The derivatized products were extracted three times with 0.5 ml of ethyl acetate, and the combined extracts were evaporated to dryness. The residue was redissolved in 10 μl of ethyl acetate, 5 μl of which was spotted onto a TLC plate (Silica Gel 60/Kieselguhr F254). The plate was developed with 1-butanol saturated with 3% NH4OH. α-ketoglutarate and SSA (Sigma) were derivatized in the same way and used as standards. For NMR identification, a 0.65-ml reaction mixture contained 50 mM KPi (pH 6.5), 0.3 mM TPP, 1 mM MgCl2, and 5 mM α-ketoglutarate. The reaction was initiated by adding 2 μM Rv1248c protein. Deuterium oxide (D2O) was added immediately before NMR data collection to a final concentration of 10%. The one-dimensional proton spectra were acquired at 25°C with presaturation of the water resonance. Chemical shifts are quoted relative to external sodium 4, 4-dimethyl-4-silapentane-1-sulfonate at 0 ppm.
Western Blots. MtbH37Rv lysates (25 μg) were run on SDS/10% PAGE, transferred to nitrocellulose, and subjected to Western blotting with antiserum (1:10,000) raised by injecting rabbits with purified α-ketoglutarate decarboxylase (Kgd) in incomplete Freund's adjuvant.
Determinations of TCA Cycle Metabolites Concentrations in Mtb Lysates. Protein concentrations of log phase and stationary phase lysates were 4 mg/ml and 7 mg/ml, respectively. Methanol (100 ml) was added to lysates (25 ml), and the mixture was centrifuged (17,000 × g) at 4°C for 10 min. The methanol supernatant was diluted 2-fold with distilled water, and a 5-μl aliquot was injected into an Agilent 1100 LC system with a YMC Hydrosphere C18 column connected to an Applied Biosystems/MDS Sciex API 3000 mass spectrometer. Five-milliliter mixtures of calibration standards prepared at concentrations of 1, 10, and 100 mM in distilled water were also injected. Quantification was performed by the absolute calibration method using peak area. Recovery rates were also estimated (Table 3, which is published as supporting information on the PNAS web site).
Estimation of Intracellular Glutamate Concentration. Volume of a bacterial cell was taken as 2 × 10-15 liters (18) or 2.5 μl/mg dry weight (19) assuming that the weight of one cell is 9.5 × 10-13 g and the dry weight of one cell is 2.8 × 10-13 g (20). Alternatively, the volume of a tubercle bacillus was calculated assuming that one cell represents a cylinder with width ranging from 0.3 to 0.6 μm and length from 1 to 4 μm and that lipid represents ≈60% of wet weight (21). Total cell numbers were calculated by using 25-ml cultures at 0.6 OD (logarithmic phase) or 3.0 OD (stationary phase) with the observed relationship that 1 OD unit corresponds to 5 × 108 colony forming units/ml and the assumption that each bacillus is a colony-forming unit. Nonpeptidyl glutamate was measured in lysates with volume 0.7 ml (4.5 mg per ml of protein) from a logarithmic phase culture and 1 ml (7.5 mg per ml of protein) from a stationary phase culture.
Results
TCA Cycle Enzyme Activities in Mtb Lysates. All classical TCA cycle activities other than KDH were detected in Mtb lysates (Table 1). Nor was KDH activity detected in M. smegmatis and M. bovis bacillus Calmette-Guérin grown on glycerol or glutamate. Specific activities of the other TCA cycle enzymes were comparable to those reported for Mtb in 1962 (15). Thus, it appears that Mtb's TCA cycle is interrupted by a lack of KDH.
Table 1.
TCA cycle and related enzyme activities in Mtb H37Rv, M. smegmatis, M. bovis bacillus Calmette-Guérin, and E. coli lysates, compared to those published for Mtb (15)
| Enzyme | Mtb H37Rv Vmax, nmol·min−1·mg−1 | M. smegmatis Vmax, nmol·min−1·mg−1 | M. bovis BCG Vmax, nmol·min−1·mg−1 | Mtb H37Rv Vmax,* nmol·min−1·mg−1 | E. coli Vmax, nmol·min−1·mg−1 |
|---|---|---|---|---|---|
| PDH | 64 | 33.4 | 4.4 | 3 | 206 |
| Citrate synthase | 405 | 182 | 99.2 | NM | 570 |
| Aconitase | 195 | 770 | 244.4 | 154 | ND |
| Isocitrate dehydrogenase | 64 | 233 | 22.2 | 87 | 92 |
| KDH | ND | ND | ND | 3 | 224 |
| Succinyl-CoA synthetase | 7.5 | 25 | 20.5 | NM | 20 |
| Succinate dehydrogenase | 6.4 | 47.5 | 11.5 | 7† | 46.1 |
| Fumarase | 548 | 1,222 | 1,042 | 633 | 279 |
| Malate dehydrogenase | 1,149 | ND | 35.8 | 2,981 | 2,229 |
ND, not detected; NM, not measured.
Published TCA cycle and related enzyme activities in Mtb H37Rv (15)
Measured by reduction of 2,6-dichlorophenol-indophenol.
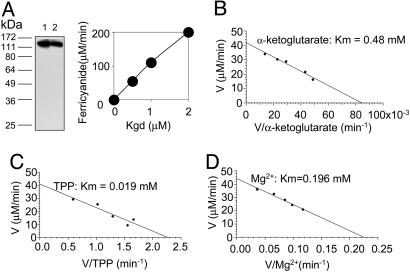
Rv1248c Encodes Kgd. We next sought an alternative pathway from α-ketoglutarate to succinate. We began by testing Rv1248c for reactions with α-ketoglutarate other than its dehydrogenation. Recombinant Rv1248c protein was purified to homogeneity as described (16). Immunoblot with antiserum raised against the pure protein documented expression of Kgd in Mtb during both logarithmic growth and stationary phase (Fig. 1A Left). Although the Rv1248c protein generated no KDH activity together with DlaT and Lpd (16), it did react with α-ketoglutarate in the presence of TPP and Mg2+ as monitored by using ferricyanide as an electron acceptor (Fig. 1A Right). The ferricyanide reductase activity of Rv1248c protein (0.8 μmol/min/mg; 109 min-1) was strictly α-ketoglutarate-, TPP-, and Mg2+-dependent, but did not require CoA, NAD+, or NADP+ (data not shown). The enzyme was specific for α-ketoglutarate, showing no activity with pyruvate. The Km values for α-ketoglutarate, TPP, and Mg2+ were 480 μM, 19 μM, and 196 μM, respectively (Fig. 1 B-D), similar to those of the Euglena gracilis mitochondrial Kgd (22). Based on the results to be described next, the Rv1248c protein was renamed Kgd.
Fig. 1.
Expression of native Kgd and activity of recombinant Kgd. (A) (Left) Immunoblot of lysates (25 μg) of Mtb grown in logarithmic (lane 1) and stationary phase (lane 2) after separation by SDS/10% PAGE, using rabbit antiserum raised against pure recombinant Kgd, which has a calculated Mr of 136 kDa. (Right) Ferricyanide reductase activity of recombinant Kgd. (B-D) Determination of Kgd Km for α-ketoglutarate (B), TPP (C), and Mg2+ (D).
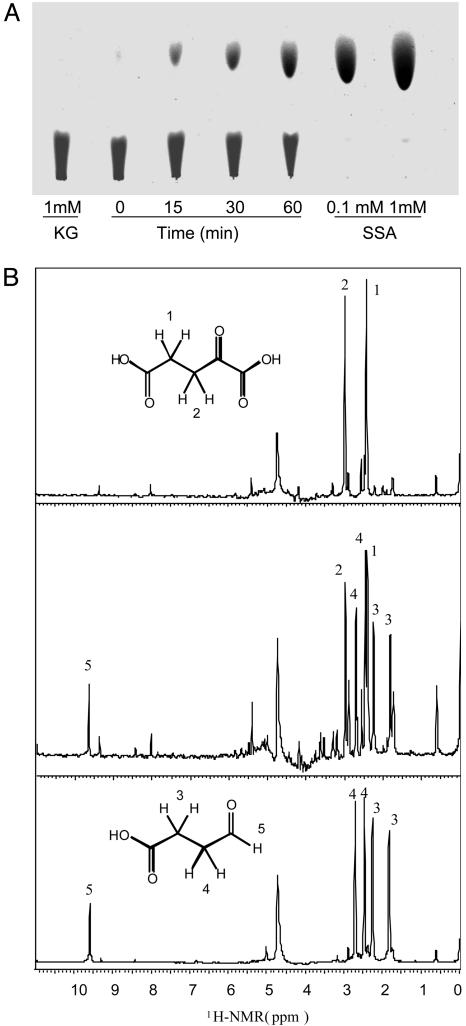
Given that the foregoing activity of Kgd did not depend on CoA, NAD+, or NADP+, we hypothesized that Kgd catalyzes an oxidative decarboxylation from five-carbon α-ketoglutarate to four-carbon SSA (molecular mass, 102 Da). TLC demonstrated that the derivatized reaction product formed by incubating Kgd with α-ketoglutarate comigrated with derivatized, authentic SSA (Fig. 2A). Formation of SSA was confirmed by one-dimensional proton [1H] NMR spectra (Fig. 2B). Two major peaks between 1.5 ppm and 3 ppm represented α-ketoglutarate, and four major peaks were generated from authentic SSA. The reaction mixture of α-ketoglutarate and Kgd generated all six peaks, corresponding to α-ketoglutarate and SSA. The peak between 9 and 10 ppm representing an aldehyde proton was also observed in the reaction samples. The major peak between 4 and 5 ppm represented H2O.
Fig. 2.
Kgd catalyzes the reaction from α-ketoglutarate to SSA. (A) Identification of the Kgd reaction product by TLC. Products were followed at 0, 15, 30, and 60 min. (B) Identification of Kgd reaction product by NMR. [1H]-NMR spectra of samples for reaction mixture with α-ketoglutarate (Top), reaction mixture with α-ketoglutarate and Kgd (Middle), and authentic SSA control (Bottom) are shown. A concentration of 2 μM Kgd was used.
SSADH Activity in Mtb. SSADH, an enzyme of the γ-aminobutyrate shunt, can oxidize SSA to succinate. Because Kgd generated SSA, we hypothesized that Mtb might express SSADH. If so, Kgd and SSADH together could convert α-ketoglutarate to succinate and thereby potentially join the two TCA half-cycles.
Indeed, SSADH activity was detected in lysates of Mtb, M. smegmatis, and M. bovis bacillus Calmette-Guérin (Table 2). The SSADH activity of Mtb was NADP+-dependent. The specific activities from mycobacteria were comparable to those reported for Bradyrhizobium japonicum and potato (17, 23).
Table 2.
SSADH activities in Mtb H37Rv, M. bovis bacillus Calmette-Guèrin (BCG), and M. smegmatis lysates compared to those published for B. japonicum (17) and potato (23)
| Activity Vmax, nmol·min−1·mg−1
|
||
|---|---|---|
| Cell lysates | NADP+ | NAD+ |
| Mtb | 40.7 | 4.7 |
| M. bovis BCG | 32.1 | NM |
| M. smegmatis | 13.3 | NM |
| B. japonicum | 9.4 | 11.1 |
| Potato | NR | 7.8 |
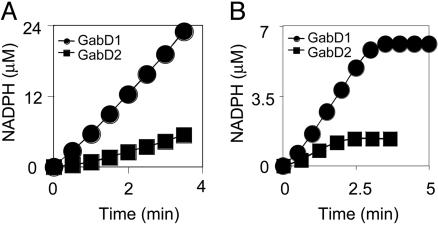
GabD1 and/or GabD2 were considered the most likely sources of SSADH activity in Mtb. The recombinant proteins were produced in E. coli and purified. Both GabD1 and GabD2 displayed NADP+-dependent SSADH activities (Fig. 3A). Both proteins could use NAD+ as a cofactor, but the specific activities were three times lower with NAD+ as compared to NADP+. The Vmax of GabD1 was much higher than that of GabD2, and GabD2 is likely to serve physiologically as a dehydrogenase for a different aldehyde(s). The specific activity of GabD1, 0.8 μmol/min/mg protein (32 min-1), was in the range reported for human and rat protein (24).
Fig. 3.
Kgd together with GabD1 or GabD2 can form a pathway from α-ketoglutarate to succinate in Mtb. (A) SSADH activities are encoded by GabD1 (circles) and GabD2 (squares). The amount of each protein used was 0.2 nmol. (B) Kgd (2 μM) and GabD1 (0.4 μM) (circles) or Kgd (2 μM) and GabD2 (0.4 μM) (squares) can convert α-ketoglutarate to succinate with the production of NADPH followed at A340.
Combined Reaction of Kgd and GabD1 or GabD2. Next, we tested whether Kgd and either GabD1 or GabD2 together could catalyze a coupled reaction from α-ketoglutarate to succinate. In fact, recombinant Kgd and recombinant GabD1 or GabD2 reduced NADP+ to NADPH in a reaction dependent on α-ketoglutarate, TPP, and Mg2+ (Fig. 3B).
TCA Cycle Metabolites in Mtb Lysates. Finally, we tried to quantify SSA and TCA cycle intermediates in Mtb lysates. Succinate, fumarate, malate, and glutamate were readily detected in both logarithmic and stationary phase lysates (Table 4, which is published as supporting information on the PNAS web site). Malate and glutamate concentrations were higher in log phase, whereas succinate and fumarate levels remained comparable in log and stationary phases. Glutamate concentrations were 170- to 880- fold higher than concentrations of succinate, fumarate, or malate. We were not able to detect pyruvate, citrate, aconitate, isocitrate, α-ketoglutarate, succinyl-CoA, and SSA under our conditions, which may reflect the poor stability and low recovery of their authentic standards (Table 3).
Discussion
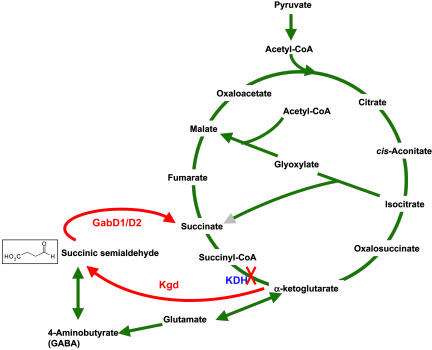
Mtb and certain other mycobacteria appear to lack KDH activity (16). Thus, they might operate separate oxidative and reductive TCA half-cycles. Based on the studies presented here, we propose that Kgd along with GabD1 may form a functional link between the half-cycles, in which α-ketoglutarate is converted to succinate via two reactions that to our knowledge have not been previously observed in mycobacteria: decarboxylation of α-ketoglutarate to SSA and oxidation of SSA to succinate (Fig. 4). The proposed role for Rv1248c may explain why its disruption prevented outgrowth of the resultant mutant strain (25). Future work should attempt to disrupt kgd in both haploid and merodiploid backgounds to confirm its apparent essentiality.
Fig. 4.
TCA cycle with proposed alternative pathway for converting α-ketoglutarate to succinate in Mtb.
Many TCA cycle and related enzymes in Mtb have been studied individually in recent years, such as isocitrate lyase (8), malate dehydrogenase (26), and isocitrate dehydrogenase (26, 27). However, to our knowledge, there have been no reports in which KDH activity was purified from Mtb lysates or reconstituted by using recombinant Mtb proteins. The KDH activity ascribed to Mtb lysates many years ago was NADP+-dependent and CoA-independent (15). Most likely, this represented the combined actions of Kgd plus SSADH, whose coupled reactions convert α-ketoglutarate to succinate with concomitant production of NADPH in a CoA-independent manner, in contrast to the NADH-producing, CoA-dependent activity characteristic of KDH. Reports of KDH in Mycobacterium lepraemurium have been controversial (28-30); when seen, the activities were extremely low, and when the bacteria were studied after recovery from infected hosts, contamination by host enzymes was difficult to exclude. That Mycobacteria cannot utilize many organic substrates led Segal to propose, >20 years ago, that KDH may be absent, leaving a “broken” TCA cycle that subserves only biosynthetic functions (31).
Although unanticipated for Mtb, split TCA cycles with or without interconnecting pathways are common among bacteria. Out of 17 microbial genomes surveyed in ref. 30, only four appeared to encode all of the genes necessary for a complete, canonical TCA cycle. The variant cycles that occur in most species presumably reflect adaptation of their metabolism to diverse environments (32). Lack of KDH often accompanies anaerobic or microaerophilic metabolism (11).
TCA half-cycles can be joined in several ways. Helicobacter pylori lacks KDH, and the oxidative and reductive branches of its TCA pathway are conjoined by α-ketoglutarate ferredoxin oxidoreductase (13). We tested M. smegmatis and M. bovis bacillus Calmette-Guérin for α-ketoglutarate oxidoreductase activity, but were not able to detect it. We cannot exclude its presence, because the enzyme is labile and oxygen-sensitive (33).
To our knowledge, throughout species, only three Kgd activities have been reported: in Euglena gracilis (34), in B. japonicum (17) and in association with a bifunctional protein cloned from E. coli, MenD (35). The activities in Euglena gracilis and the sucA mutant strain of B. japonicum have not been ascribed to specific genes or proteins, so it is not possible to tell whether the enzymes involved are homologs of Mtb's Kgd. Both Euglena gracilis and the sucA mutant strain of B. japonicum lack KDH activities. Their Kgd activities are TPP- and Mg2+-dependent but CoA-independent, as is Mtb's Kgd reaction. MenD, which is involved in biosynthesis of menaquinone (vitamin K2) (36), catalyzes the synthesis of 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate through two reactions, the first being the decarboxylation of α-ketoglutarate (35). The product of α-ketoglutarate decarboxylation is the SSA-TPP anion rather than SSA as in Mtb. Thus, to our knowledge, Mtb's Kgd is the first enzyme of defined sequence shown to produce SSA.
SSADH activity in Mtb is encoded by both gabD1 and gabD2. SSADH belongs to the aldehyde dehydrogenase family, and is well conserved in prokaryotes (37), plants (38), and mammals (24). The requirement of both GabD1 and GabD2 for NADP+ is typical for bacterial SSADH, although Pseudomonas putida has two SSADH's, one dependent on NAD+ and the other on NADP+. Plant SSADH plays a role in antioxidant defense (39). Whether Mtb's GabD1 and GabD2 are engaged in antioxidant defense remains to be addressed.
To maintain carbon flux under oxygen-limiting conditions, bacteria that lack KDH can potentially use several bypass pathways. The glyoxylate shunt has long been known to be operational in Mtb (7, 40). The possibility of a γ-aminobutyric acid shunt in Mtb has not been addressed. We were able to detect high levels of glutamate dehydrogenase activity in M. smegmatis but not in Mtb or M. bovis bacillus Calmette-Guérin (data not shown). In Euglena gracilis (34) and rhizobia such as sucA B. japonicum, Mesorhizobium loti, and Rhizobium leguminosarum, it was suggested that the bypass pathway from α-ketoglutarate to succinate via SSA represents an adaptation to microaerophilic conditions inside legume nodules (17). Similarly the Kgd-GabD1 bypass pathway in Mtb may help the pathogen cope with conditions in host cells that are genuinely hypoxic, or, as appears to be the case in Mtb residing in macrophages in vitro, misperceived as hypoxic, perhaps because Mtb's oxygen-sensing mechanisms have been damaged by host-derived nitric oxide (3). Expression of sucA (that is, the kgd homolog) has been detected in Mycobacterium avium during growth in human macrophages (41).
Of the metabolites whose levels were measured, the most abundant was glutamate. Whether this may be due in part to the high level of glutamate (2.6 mM) in the standard medium used to grow the bacteria is under study. To our knowledge, only one value relating to the free glutamate content of Mtb has been reported, but that value also included glutamine (42). The combined value, which was assessed enzymatically in total cell lysates, was far higher than what we detected by physical methods in the methanol extract of the soluble fraction of the cell lysate. The levels of glutamate detected in Mtb in the present work, which are estimated to correspond to 665 μmol/g dry weight (log phase) and 207 μmol/g dry weight (stationary phase), are within or above the higher values reported for E. coli (18), comparable to those in Bacillus subtilis (43) and in the lower range of values reported for Corynebacterium glutamicum (44). Conversion of these levels to intracellular concentrations depends on measurement of cell water content, which has apparently not been done for Mtb. For purposes of approximation, use of cell volumes estimated for other bacteria such as E. coli (18), Bacillus subtilis (43), and Clostridium perfringens (19) would suggest that Mtb's intracellular glutamate concentration may be on the order of 80 mM. However, Mtb's smaller size (45) and higher lipid content (see Materials and Methods) support estimates of intracellular glutamate concentration ranging from ≈80 mM to the low molar level. Bacteria often respond to a high osmotic environment by accumulating osmoprotectants that can be metabolized, termed “compatible solutes.” Glutamate is among the small number of evolutionarily conserved compatible solutes (46) and serves as the most abundant anion in some bacteria (47). The medium we used to culture Mtb was only 175 mOsm. That Mtb nonetheless contained glutamate in what is estimated to be a high millimolar to molar range suggests that Mtb might accumulate glutamate during growth in culture as a reserve for use under more stringent conditions in the host. Accumulation of glutamate would likely exert feedback inhibition on glutamate dehydrogenase (48), and may account for our inability to detect glutamate dehydrogenase activity in Mtb and M. bovis bacillus Calmette-Guérin lysates.
Thus, we suggest that Mtb and some other mycobacteria may operate the TCA cycle in the half-cyclic mode characteristic of microbes adapted to low-oxygen conditions, leading to α-ketoglutarate and glutamate via the oxidative branch and succinate via the reductive branch. Both branches may be linked by Kgd and SSADH to produce succinate from α-ketoglutarate via SSA. Given that Kgd is lacking in humans, it will be of interest to identify Kgd-selective inhibitors and test their impact on the fate of Mtb during infection of macrophages.
Supplementary Material
Acknowledgments
We thank W. Clay Bracken of the NMR core facility and Gang Lin for help with the NMR experiments and Sabine Ehrt and Ben Gold (Weill Medical College of Cornell University) for lysates from Mtb. This work was supported by National Institutes of Health Grant HL72718 and by the Leading Project for Biosimulation Grant-in-Aid Creative Science Research from the Ministry of Education, Culture, Sports, Science and Technology (Japan). The Department of Microbiology and Immunology acknowledges the support of the William Randolph Hearst Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Mtb, Mycobacterium tuberculosis; PDH, pyruvate dehydrogenase; TCA, tricarboxylic acid; SSA, succinic semialdehyde; SSADH, SSA dehydrogenase; Kgd, α-ketoglutarate decarboxylase; KPi, potassium phosphate; TPP, thiamine pyrophosphate; KDH, α-ketoglutarate dehydrogenase.
References
- 1.Coker, R. J. (2004) Trop. Med. Int. Health 9, 25-40. [DOI] [PubMed] [Google Scholar]
- 2.Andries, K., Verhasselt, P., Guillemont, J., Gohlmann, H. W., Neefs, J. M., Winkler, H., Van Gestel, J., Timmerman, P., Zhu, M., Lee, E., et al. (2005) Science 307, 223-227. [DOI] [PubMed] [Google Scholar]
- 3.Schnappinger, D., Ehrt, S., Voskuil, M. I., Liu, Y., Mangan, J. A., Monahan, I. M., Dolganov, G., Efron, B., Butcher, P. D., Nathan, C. & Schoolnik, G. K. (2003) J. Exp. Med. 198, 693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinney, J. D. & Gomez, J. E. (2003) Nat. Med. 9, 1356-1357. [DOI] [PubMed] [Google Scholar]
- 5.Karakousis, P. C., Yoshimatsu, T., Lamichhane, G., Woolwine, S. C., Nuermberger, E. L., Grosset, J. & Bishai, W. R. (2004) J. Exp. Med. 200, 647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi, L., Jung, Y. J., Tyagi, S., Gemaro, M. L. & North, R. J. (2003) Proc. Natl. Acad. Sci. USA 100, 241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wayne, L. G. & Lin, K. Y. (1982) Infect. Immun. 37, 1042-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honer Zu Bentrup, K., Miczak, A., Swenson, D. L. & Russell, D. G. (1999) J. Bacteriol. 181, 7161-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinney, J. D., Honer zu Bentrup, K., Munoz-Elias, E. J., Miczak, A., Chen, B., Chan, W. T., Swenson, D., Sacchettini, J. C., Jacobs, W. R., Jr., & Russell, D. G. (2000) Nature 406, 735-738. [DOI] [PubMed] [Google Scholar]
- 10.Bryk, R., Lima, C. D., Erdjument-Bromage, H., Tempst, P. & Nathan, C. (2002) Science 295, 1073-1077. [DOI] [PubMed] [Google Scholar]
- 11.Guest, J. R. (1995) Philos. Trans. R. Soc. London B 350, 189-202. [DOI] [PubMed] [Google Scholar]
- 12.Guest, H. (1987) Biochemical Society Symposium 54, 3-16. [PubMed] [Google Scholar]
- 13.Pitson, S. M., Mendz, G. L., Srinivasan, S. & Hazell, S. L. (1999) Eur. J. Biochem. 260, 258-267. [DOI] [PubMed] [Google Scholar]
- 14.Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., Gordon, S. V., Eiglmeier, K., Gas, S., Barry, C. E., III, et al. (1998) Nature 393, 537-544. [DOI] [PubMed] [Google Scholar]
- 15.Murthy, P. S., Sirsi, M. & Ramakrishnan, T. (1962) Biochem. J. 84, 263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian, J., Bryk, B., Shi, S., Erdjument-Bromage, H., Tempst, P. & Nathan, C. (2005) Mol. Microbiol. 57, 859-868. [DOI] [PubMed] [Google Scholar]
- 17.Green, L. S., Li, Y., Emerich, D. W., Bergersen, F. J. & Day, D. A. (2000) J. Bacteriol. 182, 2838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mengin-Lecreulx, D., Flouret, B. & van Heijenoort, J. (1982) J. Bacteriol. 151, 1109-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerlava, P., Izac, V. & Tholozan, J. L. (1998) Curr. Microbiol. 36, 131-135. [DOI] [PubMed] [Google Scholar]
- 20.Neidhardt, F. (1987) in Escherichia coli and Salmonella typhimurium Cellular and Molecular Biology, eds. Neidhardt, F., Ingraham, J., Brooks Low, K., Magasanik, B., Schaechter, M. & Edwin Umbarger, H. (Am. Soc. Microbiol. Press, Washington, DC), Vol. 1, p. 5. [Google Scholar]
- 21.Zinsser, H. & Joklik, W. (1988) Zinsser Microbiology (Appleton and Lange, Norwalk, CT).
- 22.Shigeoka, S. & Nakano, Y. (1991) Arch. Biochem. Biophys. 288, 22-28. [DOI] [PubMed] [Google Scholar]
- 23.Satya Narayan, V. & Nair, P. M. (1989) Arch. Biochem. Biophys. 275, 469-477. [DOI] [PubMed] [Google Scholar]
- 24.Chambliss, K. L., Caudle, D. L., Hinson, D. D., Moomaw, C. R., Slaughter, C. A., Jakobs, C. & Gibson, K. M. (1995) J. Biol. Chem. 270, 461-467. [DOI] [PubMed] [Google Scholar]
- 25.Sassetti, C. M., Boyd, D. H. & Rubin, E. J. (2003) Mol. Microbiol. 48, 77-84. [DOI] [PubMed] [Google Scholar]
- 26.Ohman, R. & Ridell, M. (1996) Tuber Lung Dis. 77, 454-461. [DOI] [PubMed] [Google Scholar]
- 27.Florio, W., Bottai, D., Batoni, G., Esin, S., Pardini, M., Maisetta, G. & Campa, M. (2002) Clin. Diagn. Lab. Immunol. 9, 846-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori, T., Kosaka, K. & Tanaka, Y. (1971) Int. J. Lepr. Other Mycobact. Dis. 39, 796-812. [PubMed] [Google Scholar]
- 29.Ishaque, M., Togola, D. & Sticht-Groh, V. (1994) Int. J. Lepr. Other Mycobact. Dis. 62, 399-403. [PubMed] [Google Scholar]
- 30.Cordwell, S. J. (1999) Arch. Microbiol. 172, 269-279. [DOI] [PubMed] [Google Scholar]
- 31.Segal, W. (1984) Mycobacteria: A Sourcebook, eds. Kubica, G. P. & Wayne, L. G. (Dekker, New York), Part A, pp. 547-573.
- 32.Huynen, M. A., Dandekar, T. & Bork, P. (1999) Trends Microbiol. 7, 281-291. [DOI] [PubMed] [Google Scholar]
- 33.Mai, X. & Adams, M. W. (1996) J. Bacteriol. 178, 5890-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shigeoka, S., Onishi, T., Maeda, K., Nakano, Y. & Kitaoka, S. (1986) FEBS Lett. 195, 43-47. [Google Scholar]
- 35.Palaniappan, C., Sharma, V., Hudspeth, M. E. & Meganathan, R. (1992) J. Bacteriol. 174, 8111-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentley, R. & Meganathan, R. (1982) Microbiol. Rev. 46, 241-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez, M., Alvarez, M. A., Balana, R. & Garrido-Pertierra, A. (1988) Biochim. Biophys. Acta 953, 249-257. [DOI] [PubMed] [Google Scholar]
- 38.Busch, K., Piehler, J. & Fromm, H. (2000) Biochemistry 39, 10110-10117. [DOI] [PubMed] [Google Scholar]
- 39.Bouche, N., Fait, A., Bouchez, D., Moller, S. G. & Fromm, H. (2003) Proc. Natl. Acad. Sci. USA 100, 6843-6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kannan, K. B., Katoch, V. M., Bharadwaj, V. P., Sharma, V. D., Datta, A. K. & Shivannavar, C. T. (1985) Indian J. Lepr. 57, 542-548. [PubMed] [Google Scholar]
- 41.Hou, J. Y., Graham, J. E. & Clark-Curtiss, J. E. (2002) Infect. Immun. 70, 3714-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowley, S., Ko, M., Pick, N., Chow, R., Downing, K. J., Gordhan, B. G., Betts, J. C., Mizrahi, V., Smith, D. A., Stokes, R. W. & Av-Gay, Y. (2004) Mol. Microbiol. 52, 1691-1702. [DOI] [PubMed] [Google Scholar]
- 43.Markuszewski, M. J., Otsuka, K., Terabe, S., Matsuda, K. & Nishioka, T. (2003) J. Chromatogr. A 1010, 113-121. [DOI] [PubMed] [Google Scholar]
- 44.Uy, D., Delaunay, S., Goergen, J. L. & Engasser, J. M. (2004) Bioprocess Biosyst. Eng. 27, 153-162. [DOI] [PubMed] [Google Scholar]
- 45.Bergey, D. H., Holt, J. & Krieg, N. (1993) Bergey's Manual of Determinative Bacteriology (Williams and Wilkins, Baltimore).
- 46.Kempf, B. & Bremer, E. (1998) Arch. Microbiol. 170, 319-330. [DOI] [PubMed] [Google Scholar]
- 47.Csonka, L. N. (1989) Microbiol. Rev. 53, 121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiio, I. & Ozaki, H. (1970) J. Biochem. (Tokyo) 68, 633-647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.