Abstract
A mutant of Mycobacterium tuberculosis defective in the metabolism of l-arginine was constructed by allelic exchange mutagenesis. The argF mutant strain required exogenous l-arginine for growth in vitro, and in the presence of 0.96 mM l-arginine, it achieved a growth rate and cell density in stationary phase comparable to those of the wild type. The mutant strain was also able to grow in the presence of high concentrations of argininosuccinate, but its auxotrophic phenotype could not be rescued by l-citrulline, suggesting that the ΔargF::hyg mutation exerted a polar effect on the downstream argG gene but not on argH. The mutant strain displayed reduced virulence in immunodeficient SCID mice and was highly attenuated in immunocompetent DBA/2 mice, suggesting that l-arginine availability is restricted in vivo.
The variable efficacy of Mycobacterium bovis BCG vaccination in protection against pulmonary tuberculosis in adults has underscored the urgent need to develop new vaccines to control this disease. One strategy being actively pursued involves the construction of attenuated strains of M. tuberculosis which are growth impaired in vivo and unable to reactivate but which retain the ability to prime the immune system (8, 11, 19, 23). Auxotrophic mutants of M. tuberculosis and M. bovis BCG display phenotypes for attenuation of growth in vivo ranging from profound (1, 8, 10, 14, 23) to marginal (8). In addition to their practical utility, auxotrophs also provide a powerful means of probing the range of host cell nutrients that are accessible by mycobacteria residing in membrane-bound vacuoles.
In many microorganisms, l-arginine is used as a source of carbon and/or nitrogen, and in some cases, the anabolic and catabolic pathways for metabolism and utilization of this amino acid are well defined (4, 5). The transport and metabolism of l-arginine have been shown to be essential for the intracellular survival of a number of pathogens (12, 13). Studies in M. bovis BCG have shown that more than one permease is responsible for the uptake of exogenous l-arginine in this organism (22). Although the argF-encoded ornithine carbamoyltransferase has been cloned and purified from M. bovis BCG (24) and an l-arginine biosynthetic cluster has been identified in the genome of M. tuberculosis (3), little is otherwise known about l-arginine metabolism in mycobacteria. In this paper, we report the construction of an l-arginine auxotroph of M. tuberculosis and describe its in vitro and in vivo growth characteristics.
MATERIALS AND METHODS
Construction and in vitro characterization of an ΔargF::hyg mutant of M. tuberculosis.
The vector pARG7S1 was constructed by cloning the hsp60-sacB cassette (where the promoter of the Bacillus subtilis sacB gene [20] was replaced by that of the M. bovis BCG hsp60 gene) in pARG7, which contains a ΔargF::hyg allele and a lacZ marker for identification of single crossovers (17). Allelic exchange was carried out essentially as described by Parish and Stoker (18). M. tuberculosis H37Rv (ATCC 27294) was electroporated with 5 μg of UV-pretreated pARG7S1 (9) and plated on Middlebrook 7H10 agar containing hygromycin (Hyg; 50 μg/ml) and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal; 50 μg/ml). Plating of a blue, single-crossover recombinant colony on medium containing Hyg, X-Gal, sucrose (7, 18), and l-arginine (0.24 mM) produced 184 sucrose-resistant colonies, one of which remained white upon repatching on fresh, l-arginine-supplemented indicator plates.
The growth rate of the mutant strain was compared to that of the wild type by inoculating 30 ml of Middlebrook 7H9 broth into stirred or rolled cultures containing 0, 0.24, or 0.96 mM l-arginine at a bacterial density of 106 CFU/ml. CFU were enumerated by plating duplicate samples of at least 3 serial dilutions on supplemented 7H10 agar. Substrate utilization experiments were performed by streaking equivalent amounts of logarithmic-phase cultures of the wild-type and the argF mutant strains onto 7H10 plates containing l-ornithine, l-citrulline, or argininosuccinate at concentrations of 1 to 10 mM.
Infection of mice.
Median survival times (MSTs) and growth kinetics in SCID and DBA/2 mice infected with M. tuberculosis strains were assessed as previously described (23).
RESULTS AND DISCUSSION
Construction of an argF mutant of M. tuberculosis.
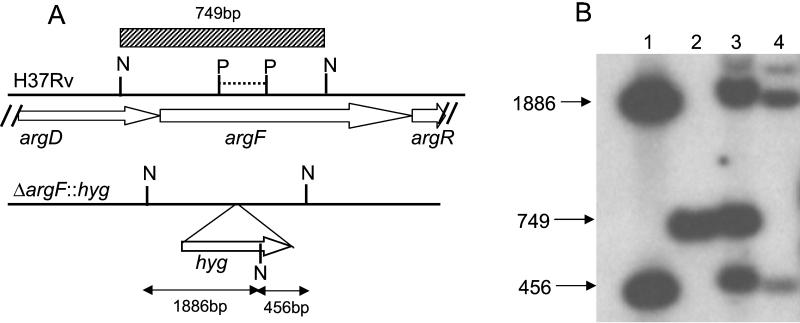
Allelic exchange with the ΔargF::hyg allele was achieved by two-step mutagenesis (18) using the vector pARG7S1. As predicted from earlier work (17), a large number of colonies recovered from the sucrose counterselection step had to be screened before one white, Hyg-resistant colony was obtained, which was suggestive of a double-crossover gene replacement event. Restriction sites used to confirm the mutant genotype are shown for the wild-type and mutant alleles in Fig. 1A along with the Southern blot analysis (Fig. 1B). The 749-bp fragment observed in the wild type and partial merodiploids (Fig. 1B, lanes 2 and 3) was lost in the knockout mutant (lane 4) and replaced by two cross-hybridizing fragments (456 and 1,886 bp) by the introduction of a new NotI site from the hyg gene.
FIG. 1.
Construction of the argF mutant of M. tuberculosis. (A) Genotypic analysis of the ΔargF::hyg mutation. Maps of the argF locus of H37Rv (3) and the corresponding ΔargF::hyg allele are shown. The dashed horizontal line represents the 156-bp PstI (P) fragment deleted in pARG7S1. N, NotI. (B) Southern blot analysis of the argF mutant. NotI digests of pARG7S1 (lane 1), wild-type H37Rv (lane 2), a single-crossover recombinant (lane 3), and the argF mutant (lane 4) were probed using a 749-bp NotI fragment derived from the argDF region (hatched block in panel A). Chromosomal DNA extraction and Southern blotting were carried out as previously described (6).
In vitro characterization of the argF mutant.
To demonstrate its auxotrophic phenotype, the mutant strain was grown in axenic culture alongside wild-type H37Rv in Middlebrook 7H9 medium supplemented with 0, 0.24, or 0.96 mM l-arginine (Fig. 2A). In the absence of this supplement, the mutant strain showed a steady decline from 106 CFU/ml to an undetectable level after 14 days in culture. In the presence of 0.24 mM l-arginine, the growth rate of the mutant strain was significantly lower than that of its parental wild type and the maximum cell density achieved was approximately 10-fold lower. However, the mutant strain grew at a rate approaching that of the wild type and achieved a comparable bacterial density in stationary phase in the presence of 0.96 mM l-arginine, although it did display a longer lag phase, reaching its peak of growth at 15 days compared to 10 days for the wild type. By comparison, the wild-type strain displayed little difference in growth rate or maximum cell density in the presence of 0.24 mM or 0.96 mM l-arginine compared to that of the l-arginine-free control (Fig. 2B). The high concentration of supplement required to restore optimal growth rates in the mutant suggests that the normal demand for l-arginine in M. tuberculosis is high.
FIG. 2.
In vitro growth kinetics of the wild type versus the argF mutant strain in axenic culture. (A) Growth of the wild-type (wt) strain without (0) l-arginine supplement and of the argF mutant (mut) strain supplemented with 0, 0.24, or 0.96 mM l-arginine. (B) Growth of H37Rv supplemented with exogenous l-arginine at 0, 0.24, or 0.96 mM. In all experiments, Middlebrook 7H9 medium supplemented with the various concentrations of l-arginine was inoculated with either the wild-type or the mutant strain at 1 × 106 to 4 × 106 CFU/ml. Growth was assessed by plating on 7H10 agar with or without l-arginine and scoring for viable CFU after 21 to 25 days. The data show the means and standard errors of three dilutions spread in duplicate for each time point and are representative of three independent experiments.
In a further set of experiments in vitro, argF mutant bacteria were found to survive within bone marrow-derived macrophages cultured as described by Smith et al. (23) in medium containing 0.4 mM l-arginine (Dulbecco’s modified Eagle’s medium) and to elicit the secretion of NO•, tumor necrosis factor α, interleukin-10, and interleukin-12 to the same extent as wild-type bacteria (data not shown).
Substrate utilization.
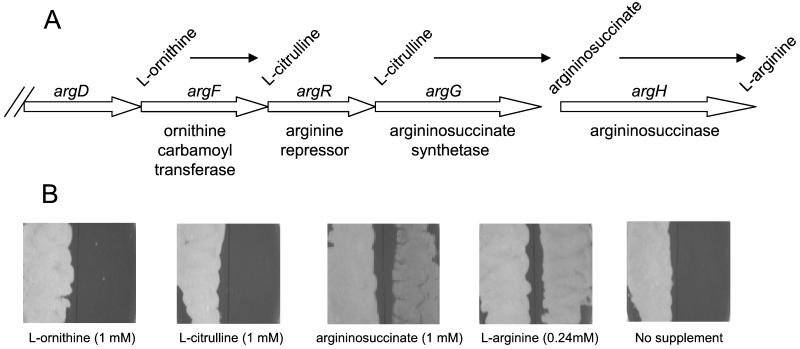
In the M. tuberculosis genome (3), argF is located within the argCJBDFRGH gene cluster (Fig. 3A). To investigate the effect of the ΔargF::hyg mutation on the function of downstream genes, substrate utilization by the argF mutant strain was analyzed by monitoring its growth when streaked on medium containing l-ornithine, l-citrulline, argininosuccinate, or l-arginine (Fig. 3B). Since disruption of argF would abolish ornithine carbamoyltransferase activity, the failure of the mutant strain to grow on medium containing 1 to 10 mM l-ornithine was as expected. However, the failure of 1 to 10 mM l-citrulline to support its growth indicated that the argF mutant was also defective in argG function. This observation suggests that the ΔargF::hyg mutation exerted polar effects on argR and argG, which is consistent with the operonic arrangement of the argCJBDFRG cluster (3). In contrast, the mutant bacteria grew as well as wild-type bacteria with 10 mM argininosuccinate supplement (data not shown), suggesting that the argH gene is expressed. However, the growth supported by 1 mM argininosuccinate was markedly poorer than that observed with 0.24 mM l-arginine, suggesting that the uptake of argininosuccinate may be relatively inefficient.
FIG. 3.
Substrate utilization. (A) Genetic organization of part of the arg biosynthetic gene cluster of M. tuberculosis (3), showing the last three steps in the biosynthesis of l-arginine (from l-ornithine to l-arginine). (B) Substrate utilization by the argF mutant compared to its parental wild type. Fifty microliters of a logarithmic-phase culture of either the wild type (left half of each panel) or the mutant strain (right half of each panel) was streaked onto 7H10 agar supplemented with the various substrates at the concentrations shown in parentheses. Plates were scored for growth after 21 to 25 days.
Virulence of the argF mutant in immunodeficient mice.
To assess the effect of the ΔargF::hyg mutation on bacterial virulence in the absence of specific immunity, SCID mice were infected with either wild-type H37Rv or the argF mutant strain. SCID mice are highly susceptible to M. tuberculosis infection (16), and as previously observed (23), they succumbed to infection approximately 29 days after infection with wild-type bacteria (Fig. 4A). Mice infected with the mutant strain survived significantly longer, with an MST of 83 days (P < 0.0004), suggesting that this strain is less virulent than its wild-type parent. However, the l-arginine auxotroph of M. tuberculosis is significantly more virulent than the corresponding proline and tryptophan auxotrophic mutants, with the former displaying an MST of 130 days and 80% of the experimental group of the latter surviving to more than 300 days (23). These observations presumably reflect differences in the availability of arginine compared with proline and tryptophan to tubercle bacilli in vivo. The concentration of l-arginine has been reported to be 0.1 to 0.3 mM in the plasma of various animal species (2, 25). Although these levels are sufficient to allow the mutant to grow in SCID mice, the attenuation observed in this model suggests that access of the mutant to l-arginine is restricted in the animal.
FIG. 4.
Virulence of wild-type H37Rv versus the argF mutant strain in mice. Immunodeficient SCID mice (A) or immunocompetent DBA/2 mice (B) were infected intravenously with 106 CFU of viable H37Rv (solid circle)/ml or of the argF mutant (open circle)/ml or with pyrogen-free saline as a control (solid square), and survival was monitored over 250 days. Six mice were included in each group.
Virulence and growth kinetics of the argF mutant in immunocompetent mice.
To assess whether l-arginine auxotrophy affected the growth rate of M. tuberculosis in an immunocompetent host, DBA/2 mice were infected with either H37Rv or the argF mutant strain. Mice infected with H37Rv died with an MST of 95.5 days (Fig. 4B), whereas those infected with the mutant strain survived significantly longer (P > 0.0006). The increased survival of mice infected with the mutant strain correlated with the very low numbers of bacteria recoverable from the organs of immunocompetent mice (Fig. 5). Whereas bacterial growth of the wild-type strain rose 10-fold in the lung over the course of infection and the bacillary load remained above 5 logs in both the liver and spleen, the mutant was attenuated in DBA/2 mice, with bacillary loads in the liver, spleen, and lung of infected mice being at or below the limit of detection at time points up to 60 days. In accordance with this, analysis of histological responses in the tissues revealed little evidence of inflammatory responses in mutant-strain-infected mice (data not shown). There was evidence of low numbers of bacteria in the spleen at day 60, and by 99 days of infection, some bacterial outgrowth of the mutant strain was seen in the livers of infected mice. The rapid decline in the numbers of argF mutant bacteria in DBA/2 mice after high-dose intravenous infection suggests that sufficient l-arginine is unavailable during initial stages of infection in this mouse strain, but the growth observed in the liver at later time points suggests that l-arginine availability may be altered at later stages of infection. However, the survival data suggest that the mutant numbers were nonetheless maintained at extremely low levels.
FIG. 5.
Survival and multiplication of wild-type H37Rv versus the argF mutant strain in the tissues of DBA/2 mice. Mice were infected intravenously with 106 CFU of viable H37Rv (solid circle) or of the argF mutant (open circle)/ml. CFU were analyzed at various intervals on 7H10 agar with (mutant) or without (wild type) l-arginine supplement. The results represent means and standard errors for three mice per group. A reliable level of detection by plating was defined as ≥100 bacteria per organ (broken line). Therefore, zero means that for each of the three mice, no bacteria were detected in 100 μl of undiluted tissue homogenate and that the inability to detect mycobacteria under these conditions does not necessarily mean that sterilization had occurred.
Conclusions.
The data presented herein suggest that l-arginine auxotrophy does confer on M. tuberculosis some of the properties sought for a rationally attenuated vaccine candidate. This study has also raised interesting questions regarding the supply of l-arginine in vivo and the role of the immune system in the control of this auxotroph. In the context of macrophage function, l-arginine is unique among amino acids as it is the substrate for nitric oxide synthase, the inducible isoform of which plays a central role in the antimicrobial activity of activated macrophages against intracellular parasites (15). The l-arginine auxotroph of M. tuberculosis could thus provide a useful tool for investigating the interplay between mycobacterial infection, l-arginine uptake and distribution between host and pathogen, and NO• production in macrophages (21).
Acknowledgments
B.G.G. and D.A.S. contributed equally to this work.
B.G.G., D.A.S., and H.A. were supported by the GlaxoSmithKline Action TB Initiative. V.M. was supported by the South African Medical Research Council, the University of the Witwatersrand, and an International Research Scholars grant from the Howard Hughes Medical Institute.
We are particularly grateful to Tanya Parish for providing pARG7 and for her invaluable advice on methodologies for _targeted gene knockout. We also thank Selwyn Quan and Pelle Stolt for providing vectors, Neil Stoker and Katrina Downing for helpful discussions, and Nancy Connell for communicating data prior to publication. We are grateful for the help of the staff of the Biological Services Facility at LSHTM.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bange, F. C., A. M. Brown, and W. R. Jacobs, Jr. 1996. Leucine auxotrophy restricts growth of Mycobacterium bovis BCG in macrophages. Infect. Immun. 64:1794-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, C., J. C. Liao, and L. Kuo. 1998. Arginase modulates nitric oxide production in activated macrophages. Am. J. Physiol. 274:H342-H348. [DOI] [PubMed]
- 3.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 4.Cunin, R., N. Glansdorff, A. Piérard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glansdorff, N. 1996. Biosynthesis of arginine and polyamines, p. 408-433. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 6.Gordhan, B. G., S. J. Andersen, A. R. De Meyer, and V. Mizrahi. 1996. Construction by homologous recombination and phenotypic characterization of a polA mutant of Mycobacterium smegmatis. Gene 178:125-130. [DOI] [PubMed] [Google Scholar]
- 7.Gordhan, B. G., and T. Parish. 2001. Gene replacement using pretreated DNA. Methods Mol. Med. 54:77-92. [DOI] [PubMed]
- 8.Guleria, I., R. Teitelbaum, R. A. McAdam, G. Kalpana, W. R. Jacobs, Jr., and B. R. Bloom. 1996. Auxotrophic vaccines for tuberculosis. Nat. Med. 2:334-337. [DOI] [PubMed] [Google Scholar]
- 9.Hinds, J., E. Mahenthiralingam, K. E. Kempsell, K. Duncan, R. W. Stokes, T. Parish, and N. G. Stoker. 1999. Enhanced gene replacement in mycobacteria. Microbiology 145:519-527. [DOI] [PubMed] [Google Scholar]
- 10.Hondalus, M. K., S. Bardarov, R. Russell, J. Chan, W. R. Jacobs, Jr., and B. R. Bloom. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 68:2888-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson, M., S. W. Phalen, M. Lagraderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klarsfels, A. D., P. L. Goossens, and P. Cossart. 1994. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE, and an arginine ABC transporter, argJ. Mol. Microbiol. 13:585-597. [DOI] [PubMed] [Google Scholar]
- 13.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 14.McAdam, R. A., T. R. Weisbrod, J. Martin, J. D. Scuderi, A. M. Brown, J. D. Cirillo, B. R. Bloom, and W. R. Jacobs, Jr. 1995. In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect. Immun. 63:1004-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan, C. 1992. Nitric oxide as a secretory product of mammalian cells. FASEB J. 6:3051-3064. [PubMed] [Google Scholar]
- 16.North, R. J., and A. A. Izzo. 1993. Mycobacterial virulence: virulent strains of Mycobacterium tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J. Exp. Med. 177:1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parish, T., B. G. Gordhan, R. A. McAdam, K. Duncan, V. Mizrahi, and N. G. Stoker. 1999. Production of mutants in amino acid biosynthetic genes of Mycobacterium tuberculosis by homologous recombination. Microbiology 145:3497-3503. [DOI] [PubMed] [Google Scholar]
- 18.Parish, T., and N. G. Stoker. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146:1969-1975. [DOI] [PubMed] [Google Scholar]
- 19.Pavelka, M. S., Jr., and W. R. Jacobs, Jr. 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis Bacillus Calmette-Guérin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 181:4780-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelicic, V., J. M. Reyrat, and B. Gicquel. 1996. Generation of unmarked directed mutations in mycobacteria, using sucrose counter-selectable suicide vectors. Mol. Microbiol. 20:919-925. [DOI] [PubMed] [Google Scholar]
- 21.Peteroy-Kelly, M., V. Venketaraman, and N. D. Connell. 2001. Effects of Mycobacterium bovis BCG infection on regulation of l-arginine uptake and synthesis of reactive nitrogen intermediates in J774.1 murine macrophages. Infect. Immun. 69:5823-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seth, A., and N. D. Connell. 2000. Amino acid transport and metabolism in mycobacteria: cloning, interruption, and characterization of an l-arginine/γ-aminobutyric acid permease in Mycobacterium bovis BCG. J. Bacteriol. 182:919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, D. A., T. Parish, N. G. Stoker, and G. J. Bancroft. 2001. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 69:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timm, J., I. Van Rompaey, C. Tricot, M. Massaer, F. Haeseleer, A. Fauconnier, V. Stalon, A. Bollen, and P. Jacobs. 1992. Molecular cloning, characterization and purification of ornithine carbamoyltransferase from Mycobacterium bovis BCG. Mol. Gen. Genet. 234:475-480. [DOI] [PubMed] [Google Scholar]
- 25.Wu, G., and S. M. Morris. 1998. Arginine metabolism: nitric oxide and beyond. Biochem. J. 336:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]