Abstract
Farnesol is a quorum-sensing molecule that inhibits filamentation in Candida albicans. Both filamentation and quorum sensing are deemed to be important factors in C. albicans biofilm development. Here we examined the effect of farnesol on C. albicans biofilm formation. C. albicans adherent cell populations (after 0, 1, 2, and 4 h of adherence) and preformed biofilms (24 h) were treated with various concentrations of farnesol (0, 3, 30, and 300 μM) and incubated at 37°C for 24 h. The extent and characteristics of biofilm formation were then assessed microscopically and with a semiquantitative colorimetric technique based on the use of 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide. The results indicated that the effect of farnesol was dependent on the concentration of this compound and the initial adherence time, and preincubation with 300 μM farnesol completely inhibited biofilm formation. Supernatant media recovered from mature biofilms inhibited the ability of planktonic C. albicans to form filaments, indicating that a morphogenetic autoregulatory compound is produced in situ in biofilms. Northern blot analysis of RNA extracted from cells in biofilms indicated that the levels of expression of HWP1, encoding a hypha-specific wall protein, were decreased in farnesol-treated biofilms compared to the levels in controls. Our results indicate that farnesol acts as a naturally occurring quorum-sensing molecule which inhibits biofilm formation, and we discuss its potential for further development and use as a novel therapeutic agent.
Candida albicans is the most frequently isolated human fungal pathogen (5). The most recent surveys have shown that Candida is the third or fourth most commonly isolated bloodstream pathogen in United States hospitals, having surpassed gram-negative rods in frequency (2, 13, 20, 21). Notably, yeasts (mainly C. albicans) are the third leading cause of catheter-related infections, and they have the second highest colonization to infection rate and the highest crude mortality rate overall (8, 22).
Structured microbial communities attached to surfaces, commonly referred to as biofilms, have increasingly been found to be sources of infection by C. albicans, especially in view of the vast number of biomaterials that are now being used in the medical industry. Biomaterials, such as stents, catheters, and orthopedic joints, for example, serve as excellent substrates for microbial adhesion and subsequent biofilm formation (6, 10, 15). Biofilms are specific and organized communities of cells under the control of signaling molecules rather than random accumulations of cells resulting from cell division (9). Cell-cell signaling, particularly quorum sensing, has been the focus of much research over the past decade in the microbiological arena. It has been demonstrated that quorum-sensing molecules are essential for bacterial biofilm formation and that homoserine lactones act in a concentration-dependent manner; a threshold concentration triggers the formation of a biofilm (17, 27). Recently, it has been reported that a quorum-sensing molecule is produced by planktonic cultures of C. albicans (12). This molecule, farnesol, was shown to prevent the germination of yeast cells into mycelia, a phenomenon that may be pertinent to C. albicans biofilm formation.
C. albicans has the capacity to switch from a yeast morphology to a hyphal morphology, one of its major virulence determinants (16). The morphological transition from the yeast form to the mycelial form (dimorphic switching) is induced by many different environmental factors, such as mammalian serum, high temperatures (37°C), and neutral pH (3, 4, 14). Our group and others have demonstrated that the dimorphic transition from a yeast form to a hyphal form is a pivotal factor for C. albicans biofilm development (1; K. VandeWalle, G. Ramage, J. L. López-Ribot, and B. L. Wickes, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr F-50, p. 364, 2001).
Farnesol, which is associated with mycelial development, may be an important regulatory (quorum-sensing) molecule in C. albicans biofilm formation. Here we investigated the effects that farnesol has upon C. albicans biofilm formation in order to ascertain its importance to biofilms. This study provided potential insight into cell-cell signaling mechanisms that occur in C. albicans biofilms, and the results could also lead to novel therapeutic strategies to counter biofilm formation.
MATERIALS AND METHODS
Organisms.
C. albicans collection strains 3153A and SC5314 were used in this study and were stored on Sabouraud dextrose slopes (BBL, Cockeysville, Md.) at −70°C. These C. albicans strains were propagated in yeast peptone dextrose (YPD) medium (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] dextrose [US Biological, Swampscott, Mass.]). Batches of medium (20 ml in 250-ml Erlenmeyer flasks) were inoculated with material from YPD agar plates containing freshly grown C. albicans and incubated overnight in an orbital shaker (200 rpm) at 30°C under aerobic conditions. Both C. albicans strains grew in the budding-yeast phase under these conditions. Cells were harvested and washed twice in sterile phosphate-buffered saline (PBS) (10 mM phosphate buffer, 2.7 mM potassium chloride, 137 mM sodium chloride; pH 7.4) (Sigma Chemical Co., St. Louis, Mo.). Cells were resuspended in RPMI 1640 supplemented with l-glutamine and buffered with morpholinepropanesulfonic acid (MOPS) (Angus Buffers and Chemicals, Niagara Falls, N.Y.) and adjusted to the desired density after counting with a hematocytometer (see below).
Effect of farnesol on adherent cells and subsequent biofilm formation on the surfaces of wells of microtiter plates.
Farnesol (Sigma Chemical Co.) was obtained as a 3 M stock solution and then diluted to obtain a 30 mM working stock solution in 100% (vol/vol) methanol. Working concentrations of farnesol were prepared in RPMI 1640 by using the 30 mM stock solution. All experiments were performed in presterilized, polystyrene, flat-bottom, tissue culture-treated, 96-well microtiter plates (Corning Incorporated, Corning, N.Y.). Standardized cell suspensions (100-μl portions of suspensions containing 1.0 × 106 cells/ml in RPMI 1640) were seeded into selected wells of the microtiter plates and incubated for 0, 1, 2, and 4 h at 37°C. Following the initial incubation the medium was aspirated, and nonadherent cells were removed by thoroughly washing the preparations three times in sterile PBS. Farnesol (in RPMI 1640) was then added at different concentrations (0, 3, 30, and 300 μM) to the adherent cells. At zero time (preincubation), farnesol was also added to a standardized suspension before it was added to a microtiter plate. A semiquantitative measure of biofilm formation was calculated by using a 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay, as previously described (25). Briefly, XTT (Sigma Chemical Co.) was prepared as a saturated solution at a concentration of 0.5 mg/ml in Ringer's lactate. This solution was filter sterilized through a 0.22-μm-pore-size filter, divided into aliquots, and then stored at −70°C. Prior to each assay, an aliquot of the XTT stock solution was thawed, and menadione (10 mM prepared in acetone; Sigma Chemical Co.) was added to a final concentration of 1 μM. A 100-μl aliquot of XTT-menadione was then added to each prewashed biofilm and to control wells to measure background XTT levels. The plates were then incubated in the dark for 2 h at 37°C, and the colorimetric change at 490 nm (a reflection of the metabolic activity of the biofilm) was measured with a microtiter plate reader (Benchmark microplate reader; Bio-Rad, Hercules, Calif.). Light microscopic examination of biofilms formed in microtiter plates was performed in parallel by using an inverted microscope and selected wells of microtiter plates.
Effect of farnesol on preformed biofilms on the surfaces of wells of microtiter plates.
C. albicans biofilms were formed for 24 h at 37°C on the surfaces of microtiter plates by using the protocol described above. Following biofilm formation the medium was aspirated, and nonadherent cells were removed by washing the biofilms three times in sterile PBS. Residual PBS was removed by blotting with paper towels before addition of farnesol at different concentrations (0, 3, 30 and 300 μM) in RPMI 1640 to selected wells of the microtiter plates. The plates were then incubated for 24 h at 37°C. The effect of farnesol on preformed biofilms was then estimated by using the XTT reduction assay, as described above.
Effect of farnesol on planktonically grown cells.
A range of farnesol concentrations (3, 30, and 300 μM) were added to standardized cellular suspensions of C. albicans (1 × 106 cells/ml in RPMI 1640), which were then incubated at 37°C overnight in an orbital incubator. Suitable farnesol-free controls were also included. Macroscopic appearance and microscopic appearance were monitored, and a quantitative assessment of cellular morphology was performed with a light microscope.
Effects of biofilm culture supernatants on planktonic growth morphology.
Hornby et al. (12) demonstrated that planktonically grown C. albicans produced a quorum-sensing molecule, which could be isolated from the supernatant. Therefore, we designed an experiment to determine if culture supernatants from C. albicans biofilms also displayed this activity. Biofilms were formed on the surfaces of 75-cm2 tissue culture flasks. Briefly, C. albicans cells were washed and resuspended in RPMI 1640 at a density of 1.0 × 106 cells/ml. Cells were allowed to adhere for 1 h, after which the culture supernatant was decanted and fresh RPMI 1640 was added. Biofilms were then formed for selected time intervals (24, 48, 72, and 96 h). Following biofilm formation the supernatant fractions were decanted and filter sterilized with a 0.22-μm-pore-size filter. These biofilm supernatants were then diluted 1:1 with 2× RPMI 1640 and stored at 4°C until they were used. C. albicans SC5314 was then grown overnight in YPD, washed, and counted. A standardized suspension (1.0 × 106 cells/ml) was prepared in the stored supernatant medium. Planktonic cultures were then grown overnight at 37°C in an orbital shaker. A farnesol control (30 μM farnesol) and a negative control were included. Macroscopic evaluation and microscopic evaluation of the cell morphology were then performed.
SEM.
For scanning electron microscopy (SEM), biofilm formation was initiated on sterile plastic coverslip discs (diameter, 15 mm; Nalge Nunc International) in 24-well cell culture plates (Corning Inc.) by dispensing a standardized cell suspension (2 ml of a suspension containing 1.0 × 106 cells/ml in RPMI 1640) onto appropriate discs at 37°C. Cells were pretreated with farnesol at various concentrations (0, 3, 30, and 300 μM) and incubated at 37°C, as described above. The discs were removed after 24 h and washed three times in sterile PBS. The biofilms were placed in fixative (4% [vol/vol] formaldehyde and 1% [vol/vol] glutaraldehyde in PBS) overnight. The samples were rinsed twice (3 min each) in 0.1 M phosphate buffer and then placed in 1% Zetterquist's osmium for 30 min. The samples were subsequently dehydrated in a series of ethanol washes (70% ethanol for 10 min, 95% ethanol for 10 min, 100% ethanol for 20 min), and then they were treated twice (5 min each) with hexamethyldisilizane (Polysciences Inc., Warrington, Pa.) and finally air dried in a desiccator. The specimens were then coated with 40% gold-60% palladium and observed with a scanning electron microscope (Leo 435 VP) in high-vacuum mode at 15 kV. The images were processed for display by using Photoshop software (Adobe, Mountain View, Calif.).
Extraction of RNA from farnesol-treated cells and Northern blot analysis.
Biofilm RNA was obtained as described previously (23). Briefly, standardized C. albicans cells were prepared and added to 25-ml portions of RPMI 1640 in 75-cm2 vent cap tissue culture flasks at a density of 1.0 × 106 cells/ml. The flasks were incubated statically for 1 h to allow initial adherence of the cells, after which the medium was decanted and replaced with 50-ml portions of prewarmed (37°C) RPMI 1640 containing 30 μM farnesol. This farnesol concentration was chosen since it still had an effect on biofilm formation but allowed recovery of sufficient cellular mass for RNA extraction. The flasks were then gently rocked to promote biofilm formation at 37°C for 24 and 48 h. Farnesol-free controls were also included. The cells were washed in ice-cold sterile PBS in the flasks and then removed from the flask surfaces with a sterile scraper. The cells were pelleted and resuspended in TRI reagent (Molecular Research Centre Inc., Cincinnati, Ohio). The cells were then mechanically disrupted with 0.5-mm-diameter glass beads in a mini bead beater (Biospec Products, Bartlesville, Okla.). RNA was separated from other cellular debris with bromochloropropane and precipitated with isopropanol by following manufacturer's instructions. Equal quantities (approximately 5 μg) of total RNA, as determined by A260 measurement, were separated by electrophoresis and subsequently transferred to nylon membranes (Nytran; Schleicher & Schuell) by using a Turboblotter apparatus (Schleicher & Schuell). A probe for HWP1, a hypha-specific gene (29), was prepared by PCR and labeled by random priming (Random Primers DNA labeling system; Gibco-BRL), and hybridization was performed by using Rapid-Hyb buffer (Amersham Life Science Inc., Arlington Heights, Ill.) and the manufacturer's instructions. After hybridization, the blots were washed under high-stringency conditions and exposed to autoradiography film. For preparation of figures, digital images were processed with the Adobe Photoshop program.
RESULTS
Effect of farnesol on biofilm development and formation.
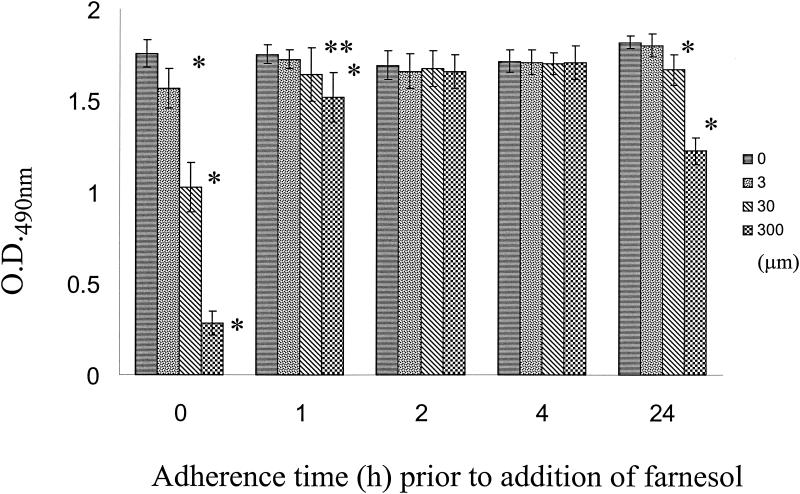
Farnesol was used to treat adherent cell populations at 0, 1, 2, and 4 h to determine whether different concentrations (3, 30, and 300 μM) could adversely affect C. albicans biofilm formation (initial experiments showed that methanol did not have an effect on C. albicans cell viability at the concentrations used in these experiments, and the same was true for the different farnesol concentrations). The results of these experiments, as assessed by a colorimetric assay and microscopy, demonstrated that preincubation with the highest concentration of farnesol (300 μM) prevented successful germination of the adherent yeast cells, resulting in scant or nonexistent biofilms. When the concentration was decreased 10-fold and 100-fold, pseudohyphae (30 μM farnesol) and true hyphae (3 μM farnesol) were observed. This was reflected in the A490 values, which showed the lowest XTT readings at the highest farnesol concentration and progressively higher readings as the farnesol concentration was decreased (Fig. 1). The negative controls (biofilms formed in the absence of farnesol) for both C. albicans SC5314 and 3153A exhibited typical biofilm architecture, with biofilms composed of intertwining mycelial structures and a basal layer of blastospores. When the farnesol was decanted, the cells were washed twice with PBS, and fresh medium was added, reincubation of the cells at 37°C resulted in the formation of a typical biofilm (results not shown). As the initial adherence time increased, the effect of farnesol on subsequent biofilm development decreased, as indicated by smaller differences in XTT colorimetric readings (Fig. 1). Microscopic examination demonstrated that once hyphal formation had been initiated, it was not inhibited by the addition of farnesol, and the subsequent biofilm formation was generally unaffected. Interestingly, significantly lower XTT readings were obtained for farnesol with mature (24-h) biofilms (Fig. 1).
FIG. 1.
Effect of farnesol on C. albicans biofilm formation. Different farnesol concentrations (0, 3, 30, and 300 μm) were added to C. albicans cells at different times after attachment (0, 1, 2, and 4 h), and the cells were incubated under biofilm-growing conditions; farnesol was also added to preformed (24-h) biofilms, which were incubated for an additional 24 h. The extent of biofilm formation was estimated by the XTT reduction assay. The values are mean absorbance values and standard deviations for 10 independent biofilms. Statistically significant differences (as determined by Student's t test, compared to biofilms formed in the absence of farnesol) are indicated as follows: one asterisk, P < 0.01; two asterisks, P < 0.05. O.D.490nm, optical density at 490 nm.
SEM visualization of C. albicans biofilms formed in the presence of farnesol.
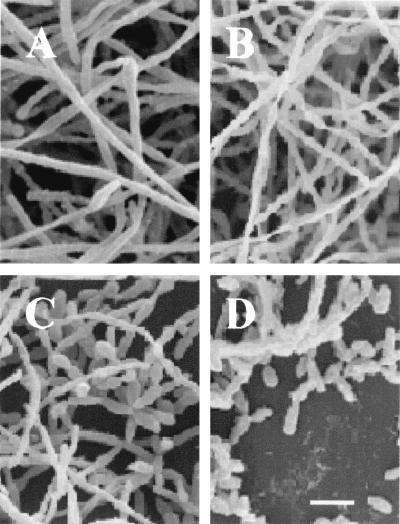
Biofilm formation by C. albicans on plastic coverslips was monitored by SEM (Fig. 2). Pretreatment of C. albicans SC5314 with various farnesol concentrations showed that there was a dose-dependent effect on biofilm formation. Cells treated with farnesol at a concentration of 300 μM produced scant biofilms, which were composed predominantly of yeast cells and pseudohyphae. True hyhae were rarely observed, a factor which contributed to the poor biofilm architecture. As shown in Fig. 2, biofilms formed in the presence of 3 and 30 μM farnesol were more dense and composed of yeasts, pseudohyphae, and true hyphae. The farnesol-free control biofilm was composed mainly of true hyphae.
FIG. 2.
SEM showing the effects of different concentrations of farnesol on C. albicans biofilm formation. (A) Control (no farnesol); (B) 3 μM farnesol; (C) 30 μM farnesol; (D) 300 μM farnesol. Bar = 10 μm.
Effect of biofilm supernatants on the growth of planktonic cultures.
Supernatants of biofilms grown for 24, 48, 72, and 96 h were collected, filter sterilized, and refrigerated. Following 24 h of growth of C. albicans SC5314 in 1× RPMI 1640 with each of the supernatants, as well as a farnesol control (300 μM farnesol) and an RPMI 1640 control, cells were counted, and the numbers of yeast cells, pseudohyhae, and true hyphae were determined. The RPMI 1640 control contained more than 95% true hyphae, and the farnesol control contained more than 95% yeast cells and pseudohyphae. Cultures treated with biofilm supernatants from 24-, 48-, 72-, and 96-h cultures contained approximately 80, 83, 85, and 93% yeast cells and pseudohyphae, respectively. The value given here are based on microscopic evaluation of 20 fields on two separate occasions.
Northern blot analysis of farnesol-treated cells.
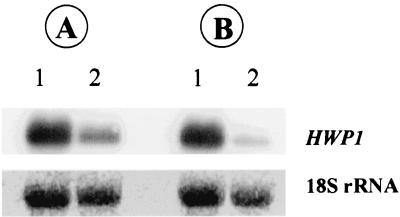
The results of the Northern blot analysis of biofilms grown in the presence of farnesol are shown in Fig. 3. Sessile cells were treated with a subinhibitory concentration of farnesol (30 μM) and grown for 24 and 48 h. The RNA extracted from the cells was probed with an amplified fragment of HWP1, a gene encoding a hyphal wall protein. The results demonstrated that farnesol-treated cells expressed lower levels of HWP1 mRNA than cells grown in the absence of farnesol expressed, which correlated with the morphology (yeast or hyphal) exhibited by the cells. We also noted that the level of expression of HWP1 was lower in the 48-h biofilm than in the 24-h biofilm (Fig. 3, compare lanes 2).
FIG. 3.
Northern blots of total RNA obtained from farnesol-treated (30 μM) (lanes 2) and untreated (control) (lanes 1) C. albicans biofilms grown for 24 (A) or 48 h (B) and probed with HWP1, which encodes a hypha-specific wall protein. Hybridization was performed as described in Materials and Methods. The bottom panel shows the amounts of 18S rRNA used to standardize signal levels according to lane-loading parameters.
DISCUSSION
Biofilms possess unique developmental characteristics that are in stark contrast to the characteristics of free-floating planktonic cells, and biofilms are much more difficult to treat chemotherapeutically (24, 26, 30). Biofilms are highly organized communities of cells (7). Like the cells of a tissue that communicate via autocrine and paracrine stimulation, cells of microbial biofilms release chemical compounds that act in concert, reaching threshold densities that signal the initiation of coordinated cellular differentiation events (9, 17, 19, 28). In essence, biofilms may represent the foundation of multicellular life. Understanding the way in which these complex structures form should provide insights into biofilm prevention strategies.
It was recently reported by Hornby et al. (12) that an extracellular compound released by C. albicans, identified as farnesol, inhibited the yeast-to-mycelium conversion, a differentiation process which is fundamental for the opportunistic pathogen to thrive, disseminate, and initiate infection (5, 18). These workers described farnesol as a quorum-sensing molecule, and it may be similar to the morphogenic autoregulatory substance in C. albicans previously described by Hazen and Cutler (11). Here we confirmed that farnesol is a molecule with properties that suggest that there is a quorum-sensing ability in C. albicans cells when they are growing as biofilms. The dimorphic transition from a yeast form to a hyphal form appears to be a pivotal biological process required for biofilm formation. We demonstrated previously that C. albicans mutant strains (Δefg1 and Δcph1/Δefg1) that were defective in the ability to germinate were unable to form biofilms (VandeWalle et al., Abstr. 101st Gen. Meet. Am. Soc. Microbiol.). Similar conclusions were drawn by another group of workers, who used two nongenetically defined mutant C. albicans strains that were defective in the ability to form either yeast cells or hyphae (1). Here we found that C. albicans biofilm density and morphology were drastically altered by high concentrations of farnesol, most likely as a direct consequence of the inhibitory effect of farnesol on the morphogenetic process. As the concentration of farnesol was decreased, the morphology of the biofilms changed in a dose-dependent manner to a typical hyphal morphology. Furthermore, we noted that the initial adherence time, prior to the addition of farnesol, was important in terms of the ability of farnesol to inhibit biofilm formation. Adherent cell populations that began to germinate naturally were not inhibited by a subsequent addition of farnesol. In fact, the germinating cells were able to form hyphae quite readily, which progressed to nearly typical biofilms (Fig. 1). Therefore, the antibiofilm properties of farnesol are better suited to preventive strategies than to treatment strategies.
We noted that newly budded yeast cells formed in mature biofilms could not germinate in the presence of high concentrations of farnesol, which may explain why the absorbance values for 24-h biofilms treated with 30 and 300 μM farnesol were significantly lower than those of the untreated controls. We hypothesize that once the farnesol concentration reaches a certain threshold, mycelial development, which is pertinent to biofilm formation, cannot occur in newly produced yeast cells. Instead, these cells may detach and colonize a new substrate area that is not nutrient deprived, where a new biofilm can develop unhindered. Since farnesol is produced in situ as planktonic cultures age (12), we hypothesize that farnesol is also produced in aging biofilms and is responsible for the quorum-sensing effect exhibited by biofilm supernatants. Indeed, additional experiments in our laboratory have indicated that C. albicans biofilms formed over extended periods of time without replenishment of nutrients detach from the substrate (G. Ramage, B. L. Wickes, and J. L. Lopez-Ribot, unpublished observations). This strategy of cell-cell communication benefits the biofilm community by preventing and controlling unnecessary overpopulation and competition for nutrients and has important implications for the infectious process, especially for dissemination and establishment at distal sites of infection.
In summary, we provide evidence which demonstrates that biofilms are not simply amalgamations of randomly dividing cells. Biofilms are precisely organized communities that are dependent on the quorum-sensing abilities of microorganisms. To our knowledge, the results reported here provide the first example of a quorum-sensing event in fungal biofilms. Farnesol plays a crucial role in biofilm development and survival. Biomaterial infections are an increasingly alarming problem, and due to their intrinsic recalcitrance to conventional therapy new methods of dealing with these infections must be explored. Farnesol may be an interesting prospect as an anti-infective strategy in this setting.
Acknowledgments
This work was supported by grant ATP 3659-0080 from the Texas Higher Education Coordinating Board (Advance Technology Program, Biomedicine). J.L.L.-R. is the recipient of a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund.
We thank Peggy Miller for assistance with SEM experiments.
REFERENCES
- 1.Baillie, G. S., and L. J. Douglas. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48:671-679. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, S. N., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, and W. J. Martone. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. National Nosocomial Infections Surveillance System. Am. J. Med. 91:86S-89S. [DOI] [PubMed] [Google Scholar]
- 3.Brown, A. J., and N. A. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 4.Brown, D. H., Jr., A. D. Giusani, X. Chen, and C. A. Kumamoto. 1999. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol. Microbiol. 34:651-662. [DOI] [PubMed] [Google Scholar]
- 5.Calderone, R. A. (ed.) 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 6.Cardinal, E., E. M. Braunstein, W. N. Capello, and D. A. Heck. 1996. Candida albicans infection of prosthetic joints. Orthopedics 19:247-251. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W. 1995. Overview of microbial biofilms. J. Ind. Microbiol. 15:137-140. [DOI] [PubMed] [Google Scholar]
- 8.Crump, J. A., and P. J. Collignon. 2000. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1-8. [DOI] [PubMed] [Google Scholar]
- 9.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 10.Ell, S. R. 1996. Candida, ′the cancer of silastic.' J. Laryngol. Otol. 110:240-242. [PubMed] [Google Scholar]
- 11.Hazen, K. C., and J. E. Cutler. 1983. Isolation and purification of morphogenic autoregulatory substance produced by Candida albicans. J. Biochem. (Tokyo) 94:777-783. [DOI] [PubMed] [Google Scholar]
- 12.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, R. N., S. A. Marshall, M. A. Pfaller, W. W. Wilke, R. J. Hollis, M. E. Erwin, M. B. Edmond, and R. P. Wenzel. 1997. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. SCOPE Hospital Study Group. Diagn. Microbiol. Infect. Dis. 29:95-102. [DOI] [PubMed] [Google Scholar]
- 14.Kohler, J. R., and G. R. Fink. 1996. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc. Natl. Acad. Sci. USA 93:13223-13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonhardt, A., S. Renvert, and G. Dahlen. 1999. Microbial findings at failing implants. Clin. Oral Implants Res. 10:339-345. [DOI] [PubMed] [Google Scholar]
- 16.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 17.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 18.Molero, G., R. Diez-Orejas, F. Navarro-Garcia, L. Monteoliva, J. Pla, C. Gil, M. Sanchez-Perez, and C. Nombela. 1998. Candida albicans: genetics, dimorphism and pathogenicity. Int. Microbiol. 1:95-106. [PubMed] [Google Scholar]
- 19.Parsek, M. R., and E. P. Greenberg. 1999. Quorum sensing signals in development of Pseudomonas aeruginosa biofilms. Methods Enzymol. 310:43-55. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller, M., and R. Wenzel. 1992. Impact of the changing epidemiology of fungal infections in the 1990s. Eur. J. Clin. Microbiol. Infect. Dis. 11:287-291. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 31:327-332. [DOI] [PubMed] [Google Scholar]
- 22.Raad, I. 1998. Intravascular-catheter-related infections. Lancet 351:893-898. [DOI] [PubMed] [Google Scholar]
- 23.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 24.Ramage, G., K. VandeWalle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163-170. [PubMed] [Google Scholar]
- 25.Ramage, G., K. VandeWalle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramage, G., B. L. Wickes, and J. L. Lopez-Ribot. 2001. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am. Clin. Lab. 20:42-44. [PubMed] [Google Scholar]
- 27.Riedel, K., M. Hentzer, O. Geisenberger, B. Huber, A. Steidle, H. Wu, N. Hoiby, M. Givskov, S. Molin, and L. Eberl. 2001. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 147:3249-3262. [DOI] [PubMed] [Google Scholar]
- 28.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 29.Staab, J. F., and P. Sundstrom. 1998. Genetic organization and sequence analysis of the hypha-specific cell wall protein gene HWP1 of Candida albicans. Yeast 14:681-686. [DOI] [PubMed] [Google Scholar]
- 30.Stephens, C. 2002. Microbiology: breaking down biofilms. Curr. Biol. 12:R132-R134. [DOI] [PubMed] [Google Scholar]