Abstract
The events involved in the establishment of a latent infection with Mycobacterium tuberculosis are not fully understood, but hypoxic conditions are generally believed to be the environment encountered by the pathogen in the central part of the granuloma. The present study was undertaken to provide insight into M. tuberculosis protein expression in in vitro latency models where oxygen is depleted. The response of M. tuberculosis to low-oxygen conditions was investigated in both cellular and extracellular proteins by metabolic labeling, two-dimensional electrophoresis, and protein signature peptide analysis by liquid chromatography-mass spectrometry. By peptide mass fingerprinting and immunodetection, five proteins more abundant under low-oxygen conditions were identified from several lysates of M. tuberculosis: Rv0569, Rv2031c (HspX), Rv2623, Rv2626c, and Rv3841 (BfrB). In M. tuberculosis culture filtrates, two additional proteins, Rv0363c (Fba) and Rv2780 (Ald), were found in increased amounts under oxygen limitation. These results extend our understanding of the hypoxic response in M. tuberculosis and potentially provide important insights into the physiology of the latent bacilli.
Mycobacterium tuberculosis is the bacterium responsible for human tuberculosis, and epidemiological data suggest that possibly up to one-third of the world's population is latently infected with this microorganism (27). After infection, clinical disease is uncommon. Instead, the infection is typically controlled by the immune system, leading to the clearance or containment of most viable mycobacteria. The resulting latent stage of infection is normally associated with a few bacteria surviving in the granulomas for years in a so-called dormant state with low or no metabolic activity (28). These bacteria appear to be resistant to ordinary chemotherapy and are capable of reactivating at a later time point.
One of the primary host defenses against mycobacterial disease involves the formation of a heterogeneous cluster of macrophages in a granuloma-like structure. These granulomas appear to be initially solid, or caseous during a latent infection, but often change considerably when the immune pressure wanes and liquefy coincident with rapid bacterial replication and lung damage. Immune containment by granuloma formation creates a physical microenvironment that has not been characterized in detail, but nutrient limitation, low pH, hydrolytic enzymes, reactive nitrogen and oxygen species, and reduced oxygen tension are believed to be factors that coincide with the establishment of latent infection (9). In vitro low-oxygen culture models have therefore attracted interest as tools for identifying proteins that are differentially expressed by the bacteria in this metabolic state. The best-studied model is based on controlled agitation of sealed liquid cultures exposed to limited headspace volumes of air (29). In this model, oxygen is gradually depleted by bacterial growth, and two nonreplicating stages are observed: a microaerophilic stage followed by an anaerobic stage. Both of these stages are characterized by a phenotypic form of drug resistance that extends to otherwise cidal agents such as isoniazid and rifampin that require replication. Microaerophilically and anaerobically cultured M. tuberculosis and M. bovis BCG (an attenuated strain of M. bovis used as a vaccine against tuberculosis) develop a thickened cell wall that may be important for adaptation to low-oxygen conditions. At the protein level, the 16-kDa small heat shock protein or α-crystallin homolog (HspX, Rv2031c) was identified as a highly expressed protein under low-oxygen conditions (7, 30), and a knockout strain of M. tuberculosis lacking HspX showed reduced growth in macrophages (31).
Recently, two studies of the response to oxygen limitation in M. bovis BCG were published (4, 10). Using either the Wayne dormancy model (29) or standing cultures combined with two-dimensional electrophoresis (2-DE), the two studies identified a total of six proteins that were more abundant at reduced oxygen tensions.
Although M. tuberculosis and M. bovis BCG are closely related, more than 100 genes are deleted in the BCG strains (3, 16), several of which may be involved in complex responses such as the hypoxic gene response. In the present study, M. tuberculosis was therefore selected for a proteomic study of the hypoxic response, and we monitored changes of both cellular and extracellular proteins. We took advantage of two different models for low-oxygen conditions: de novo protein synthesis was studied in the Wayne model by the addition of [35S]methionine and [35S]cysteine, and steady-state levels of M. tuberculosis proteins were studied under different, defined oxygen tensions.
In addition, signature peptide analysis via liquid chromatography-mass spectrometry (LC-MS) was used to confirm the results obtained by 2-DE for whole-cell lysates. Quantification of proteins via characteristic ions after protein digestion is routinely performed with automated LC-MS systems (13). Their accuracy and specificity, however, need to be evaluated by comparison with traditional proteomic methods. LC-MS can be employed directly to complex mixtures of proteins and/or peptides in some cases to yield quantitative information directly. M. tuberculosis, with 3,924 open reading frames, offers an approachable system for the direct analysis of peptide differential expression without prior electrophoretic separation of parent proteins. We report here the identification of seven M. tuberculosis proteins found in higher levels under low-oxygen conditions by classical protein analysis systems and the confirmation of increased abundance by signature peptide LC-MS analysis.
MATERIALS AND METHODS
Metabolic labeling.
A starter culture of M. tuberculosis H37Rv was grown at 37°C in modified Sauton medium to an optical density at 580 nm (OD580) of ca. 1.0 (6). Sterile, 10-ml polystyrene tubes (Nunc, Roskilde, Denmark) or 125-ml polycarbonate Erlenmeyer flasks (Corning, Acton, Mass.) containing 6.7 or 20 ml of modified Sauton medium, respectively, were inoculated with 8 × 106 bacteria per ml from the starter culture. Erlenmeyer flasks were placed in a standard shaking incubator at 37°C (normal cultures), whereas tubes with tightly screwed caps (low-oxygen cultures) were placed at 37°C under magnetic stirring at ∼100 rpm. Metabolic labeling was performed by the addition of 10 μCi of l-[35S]methionine and l-[35S]cysteine (Redivue Promix, Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom)/ml. After 20 h, bacteria were harvested by centrifugation, and the medium was also collected. The bacterial pellet was washed once in phosphate-buffered saline (PBS; pH 7.4) and resuspended in 300 μl of a suspension containing equal volumes of 0.1-mm glass beads and PBS with 0.1% sodium dodecyl sulfate (SDS) and 1 mM phenylmethylsulfonyl fluoride. The bacteria were lysed for 5 min at maximum speed on an MS2 minishaker (IKA Works, Inc., Wilmington, N.C.). The harvested medium (culture filtrate) was sterile filtered and concentrated ca. 20 times in a Centricon-3 ultrafiltration unit (Amicon, Danvers, Mass).
Growth under defined oxygen tensions.
M. tuberculosis H37Rv was grown in Middlebrook medium in insect cell culture flasks under a continuous flow of nitrogen containing oxygen tensions of 1, 5, and 20% as previously described (31). Culturing was continued until an OD650 of ca. 0.5 was reached. Bacteria were harvested by centrifugation and lysed by using 0.1-mm glass beads.
2-DE analysis.
During rehydration, 40 μl of each lysate or culture filtrate was applied to 13-cm IPG pH 4-7L strips (Amersham Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. Focusing started at 500 V (1 h), was increased to 1,000 V (1 h), and finally was increased to 8,000 V (2 h) in an IPGphor unit (Amersham Pharmacia Biotech). The second-dimensional separation was performed in SDS-10 to 20% polyacrylamide gel electrophoresis gradient gels in the Protean IIxi system (Bio-Rad, Richmond, Calif.). The gel was either silver stained (26) or Coomassie blue stained (Gelcode Blue stain reagent; Pierce, Rockford, Ill.) for protein identification by MS, or the gel was blotted to polyvinylidene difluoride (PVDF) membrane, and the membrane was exposed to Biomax MR film (Kodak, Rochester, N.Y.) for 3 to 42 days to make autoradiographs.
Image analysis of 2-DE gels.
Autoradiographs and silver-stained gels were scanned with a Umax Powerlook III scanner (Umax Systems GmbH, Willich, Germany) in transparency mode and analyzed with the Phoretix 2D gel analysis software (version 5.01; Non Linear Dynamics, Newcastle upon Tyne, United Kingdom). Spot volumes were normalized to the total spot volume, and spots from lysates or culture filtrates of M. tuberculosis cultured under stress conditions were matched to the unstressed control. Spots were considered to be more intense when they showed at least twofold-increased normalized volume compared to the respective control experiment. The changes in the protein levels were considered valid only if they were observed in at least two independent experiments.
Antibodies.
Mouse monoclonal antibodies HYB 76-5 and HBT10 specific for the M. tuberculosis proteins HspX and Ald, respectively, have been described elsewhere (1, 15). A rabbit monospecific polyclonal antibody K2050 directed against M. tuberculosis protein Rv0569 was raised by immunizing rabbits with the corresponding recombinant, His-tagged protein obtained as previously described (22).
Identification of 2-DE spots.
After PVDF membranes were exposed to films to prepare autoradiographs, the membrane was used for protein identification by immunodetection. The same blot was stained by consecutive reactions with different antibodies as previously described (23).
For identification by MS, silver- or Coomassie blue-stained spots were excised from the 2-DE gel and digested with trypsin by a protocol essentially as described previously (26). Briefly, aliquots of the generated tryptic peptide mixtures were analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) MS on a REFLEX III instrument (Bruker Daltonik, Bremen, Germany). For low-amount samples, the tryptic peptide mixtures were concentrated and desalted on a Poros R2 perfusion chromatography column and eluted with 1 μl of 2,5-dihydroxybenzoic acid matrix solution directly onto the MALDI _target. For protein identification, the peptide masses obtained by MALDI-TOF MS were used to query the H37Rv protein database and the NRDB sequence database (European Molecular Biology Laboratory). The H37Rv database contains proteins derived from the 3,924 predicted open reading frames of the M. tuberculosis H37Rv genome (5).
LC-MS.
Cells were lysed and the crude total lysate was subjected to tryptic digestion for 16 h at 37°C in 0.2 M ammonium bicarbonate at an enzyme/protein ratio of 1:20. Upon completion, the samples were dried, resuspended in 5% acetonitrile, and analyzed by LC-MS with a Finnigan LCQ mass spectrometer (Thermo Finnigan, San Jose, Calif.). The peptides resulting from the proteolytic digest were separated on a Magic C18 reversed-phase chromatography column (5μ 200A, 0.2 by 50 mm; Michrom Bioresources, Inc., Auburn, Calif.) and eluted with a gradient of 5 to 48% acetonitrile for 30 min followed by 48 to 64% for 10 min. The eluted peptides were directly analyzed by electrospray ionization MS for each growth state on a “total-ion” basis. RplL (Ribosomal protein L7/L12; Rv0652) was used as an internal standard to determine the relative abundance of the proteins of interest rather than only the total protein concentration to determine the relative abundances of proteins in complex mixtures. This technique is based on the monitoring of a predictably stable known protein and determining relative abundance changes based on its stability. Confirmation analysis of the proteins identified by 2-DE was done by LC-MS analysis by direct comparison of the 1 and 5% oxygen protein lysates with that of 20% protein lysate. In silico, trypsin digestion of the protein of interest was performed by using the Protein Prospector program MS-Digest (http://prospector.ucsf.edu/). Specifically, signature peptide analysis consisted of direct comparison of three unique peptides and their corresponding multiply charged states. We chose to extract three noncomigrating peptides to quantify each protein. The mass of each peptide was confirmed by comigration of multiply charged state ions. We selected three independent peptides to monitor for each protein since a single peptide would only uniquely identify between 96 and 97.5% of the proteome, two peptides considered together uniquely identify between 99.29 and 99.88% of the proteome, and three peptides increases the resolution to >99.9% or a single protein. The relative abundance was determined from the average of each of the representing peptides for each protein.
RESULTS AND DISCUSSION
Steady-state levels of M. tuberculosis proteins under defined oxygen tensions.
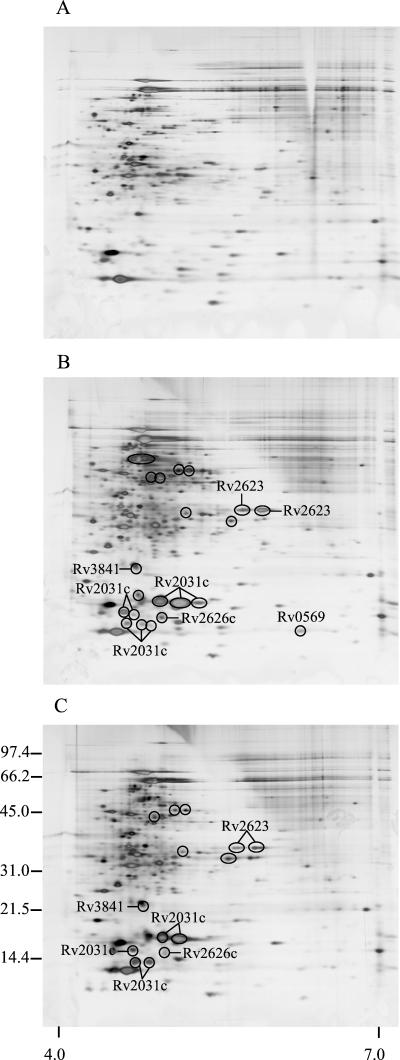
In the study by Yuan et al. (31), 2-DE of M. tuberculosis lysates grown under 1.3 and 20% oxygen demonstrated much higher levels of HspX under oxygen limitation. A detailed comparison of the gels suggested that other protein spots were also more intense under hypoxia. To identify differences in protein levels between aerobically grown M. tuberculosis and that grown (under limited-oxygen conditions), bacteria were cultured in flasks with defined oxygen atmospheres of 1, 5, and 20% oxygen, respectively, and the steady-state levels of proteins were analyzed by 2-DE and silver staining (Fig. 1 ). The protein patterns obtained were analyzed by 2-DE gel evaluation software, and the investigations were focused on the more intense spots. At 5% oxygen, 21 spots showed at least a twofold increase (encircled) compared to normal conditions at 20% oxygen. The same comparison with lysate proteins from 1 and 20% oxygen cultures showed that 14 spots were more abundant at 1% oxygen. The overall 2-DE pattern observed at 1% oxygen was similar to those of the previously reported 2-DE gels from M. tuberculosis cultures grown at 1.3% oxygen (31).
FIG. 1.
2-DE of the steady-state levels of the lysate proteins of M. tuberculosis grown at different oxygen concentrations. The bacteria were cultured with an oxygen supply of 20% (A), 5% (B), or 1% (C) oxygen and then harvested. Lysate proteins were analyzed by 2-DE and silver staining. The numbers on the left indicate molecular mass markers in kilodaltons, and the numbers below indicate the pH range. Spots that are more intense under low-oxygen conditions are encircled.
Metabolic labeling of M. tuberculosis proteins under oxygen limitation.
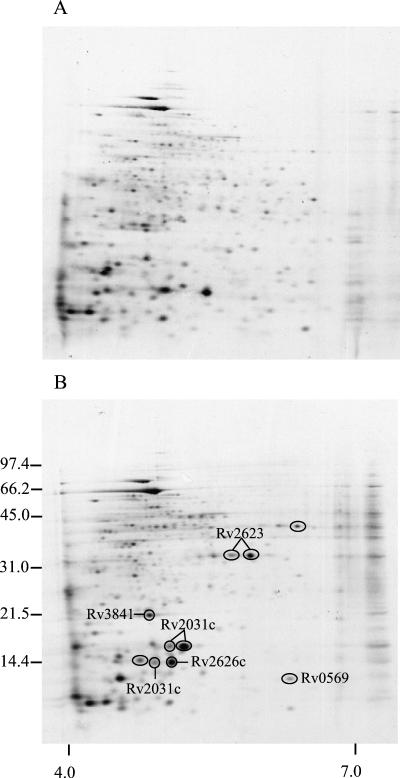
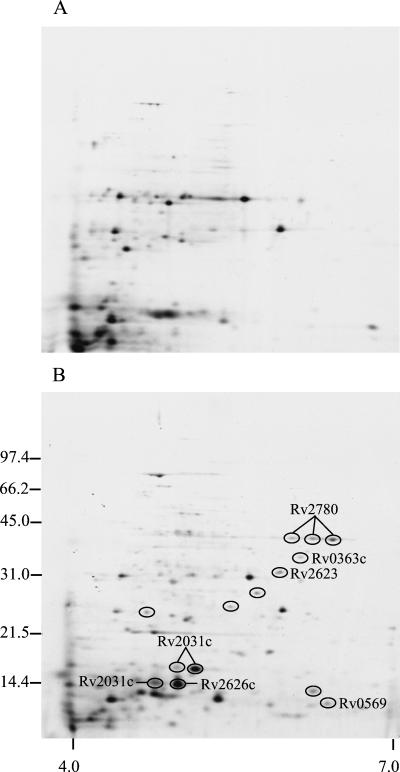
To examine the de novo synthesis of M. tuberculosis proteins, cultures were radiolabeled with [35S]methionine and [35S]cysteine. Aerobic growth was obtained by shaking, and microaerophilic growth was achieved by culture in sealed, agitated tubes (i.e., the Wayne model [29]). To allow characterization of the extracellular proteins, the medium was collected after harvest of the bacteria, sterile filtered, and concentrated by ultrafiltration. Both cellular (lysates) and extracellular proteins (culture filtrates) were then analyzed by 2-DE, followed by transfer to PVDF membrane and autoradiography (Fig. 2 and 3). The investigations were again focused on the spots that were more abundant under hypoxia, and 10 spots were found to be induced in lysates. 2-DE analysis of the culture filtrate proteins showed that 14 spots were more abundant under low-oxygen conditions. It should be noted that spots identified as more intense in metabolically labeled culture filtrates may represent proteins with increased expression, but increased secretion or bacterial lysis could also explain the enhanced levels of a protein in culture filtrates during oxygen limitation.
FIG. 2.
2-DE autoradiograph of the cellular proteins of M. tuberculosis grown under aerobic and low-oxygen conditions. [35S]Methionine- and [35S]cysteine-labeled cellular proteins of M. tuberculosis were grown in shaking culture at 37°C (Control, A) and in sealed, agitated tubes (low oxygen, B). The numbers on the left indicate molecular mass markers in kilodaltons, and the numbers below indicate the pH range. Spots induced under low-oxygen conditions are encircled.
FIG. 3.
2-DE autoradiograph of the extracellular proteins of M. tuberculosis grown under aerobic and low-oxygen conditions. [35S]Methionine- and [35S]cysteine-labeled culture filtrate proteins of M. tuberculosis were grown in shaking culture at 37°C (Control, A) and in sealed, agitated tubes (low oxygen, B). The numbers on the left indicate molecular mass markers in kilodaltons, and the numbers below indicate the pH range. Spots that are more intense under low-oxygen conditions are encircled.
Identification of M. tuberculosis proteins more abundant under low-oxygen conditions.
Protein spots found to be more intense under oxygen limitation were identified either by immunodetection or by tryptic digestion of excised spots from silver- or Coomasie blue-stained gels, followed by MALDI-TOF MS analysis of the resulting digestion mixture (Table 1).
TABLE 1.
M. tuberculosis proteins that are more abundant in 2-DE under low-oxygen conditions
| Protein | Gene | Theoretical mass (kDa)a | pIa | Identification method | Steady-state protein levels under defined oxygen conditions inb:
|
De novo protein synthesis in the Wayne model inb:
|
||
|---|---|---|---|---|---|---|---|---|
| Lysates (5% oxygen)c | Lysates (1% oxygen)d | Lysatese | Culture filtratesf | |||||
| Rv0363c | fba | 36.6 | 5.59 | MS | NI | NI | NI | ↑ |
| Rv0569 | 9.5 | 6.08 | MS, immunodetection | ↑ | - | ↑ | ↑ | |
| Rv2031c | hspX | 16.2 | 4.75 | MS, immunodetection | ↑ | ↑ | ↑ | ↑ |
| Rv2623 | 31.6 | 5.56 | MS | ↑ | ↑ | ↑ | ↑ | |
| Rv2626c | 15.5 | 4.77 | MS | ↑ | ↑ | ↑ | ↑ | |
| Rv2780 | ald | 38.7 | 6.18 | Immunodetection | - | - | - | ↑ |
| Rv3841 | bfrB | 18.3 | 4.23 | MS | ↑ | ↑ | ↑ | NI |
As predicted from TubercuList (http://genolist.pasteur.fr/TubercuList/).
↑, More intense spot compared to cultures at normal oxygen tension. -, no increased level of this protein observed compared to cultures at normal oxygen tension; NI, not identified.
See Fig. 1B.
See Fig. 1C.
See Fig. 2.
See Fig. 3.
(i) HspX.
Increased steady-state level of this protein in lysates was identified at 5% oxygen (eight spots) and at 1% oxygen (five spots) by immunodetection with monoclonal antibody HYB 76-5 and by MALDI-TOF MS analysis (Fig. 1). By metabolic labeling of M. tuberculosis proteins, induced protein synthesis of HspX was demonstrated in lysates (three spots, Fig. 2), and the protein was also more abundant in culture filtrates (three spots, Fig. 3). HspX is a member of the small heat shock protein family of chaperones, and as many as eight spots representing different isoforms of HspX were observed in the lysate obtained from culturing at 5% oxygen. This apparent heterogeneity of HspX was also observed in normal aerated cultures in another study (18).
Under hypoxic conditions, increased levels of HspX were found in M. tuberculosis and M. bovis BCG (4, 7, 8, 10, 25, 31). In addition, during in vitro infection of macrophages, expression of HspX is induced (19, 31). Among the induced genes identified in a microarray study of the hypoxic response of M. tuberculosis by Sherman et al. (25) is Rv3133c encoding a two-component response regulator. _targeted disruption experiments suggest a role for the Rv3133c protein in regulation of HspX expression in response to hypoxia (25). In agreement with this, Rv3133c was found to be induced in M. bovis BCG under oxygen starvation (4). In the present study, Rv3133c was not among the identified proteins, and a close inspection of the relevant section of the gels did not reveal any obvious more-intense spots in this region. Additional studies are needed to explain this apparent discrepancy.
(ii) Rv2623.
Increased steady-state levels of Rv2623 in lysates were identified at 5% oxygen (two spots) and at 1% oxygen (two spots) by MALDI-TOF MS analysis (Fig. 1). By metabolic labeling of M. tuberculosis proteins synthesis of Rv2623 was found to be induced in lysates (two spots, Fig. 2), and the protein was also more abundant in culture filtrates (one spot, Fig. 3). Elevated levels of Rv2623 are also found under reduced oxygen tension when the M. bovis BCG strain is studied (4, 10) and when the human macrophage cell line THP-1 is infected with M. bovis BCG (19), suggesting that Rv2623 could be important for intracellular survival. Interestingly, Rv2623 contains two domains unique for a family of universal stress proteins. It has been suggested that UspA (for “universal stress protein A”) from Escherichia coli has a function related to the growth arrest state caused by starvation (20), and it is tempting to speculate that Rv2623 may play a similar role in M. tuberculosis.
(iii) Rv2626c.
Increased levels of Rv2626c in lysates were found in single spots at 1 and 5% oxygen by MALDI-TOF MS (Fig. 1). The protein was also more abundant in metabolically labeled M. tuberculosis lysates (one spot, Fig. 2) and culture filtrates (one spot, Fig. 3) under low-oxygen conditions. The increased levels of Rv2626c observed in the present study are in agreement with the microarray study (25) and the study of M. bovis BCG in the Wayne model (4). Rv2626c shows similarity to B. subtilis YlbB protein, a homolog of IMP dehydrogenase (5).
(iv) BfrB (Rv3841, bacterioferritin).
BrfB was identified by MALDI-TOF MS in a single spot in lysates from both experimental models (Fig. 1 and 3). Bacterioferritins are involved in intracellular storage of iron, and in the microarray study bfrB was also found to be induced at reduced oxygen tensions (25).
(v) Rv0569 (a conserved hypothetical protein).
Rv0569 was identified by MALDI-TOF MS and immunodetection as a more abundant protein in metabolically labeled lysates and culture filtrates (Fig. 2 and 3); increased levels were also found in cultures at 5% oxygen tension, whereas no significant increase was observed at 1% oxygen. The microarray study also identified Rv0569 as induced under hypoxia (25), but it was not identified in the proteome studies of M. bovis BCG under oxygen-limiting conditions (4, 10). This may reflect a difference in the hypoxic response in M. tuberculosis and M. bovis BCG or simply that low-mass proteins (<15 kDa) were not included in the M. bovis BCG studies which used 10% (10) and 12.5% (4) SDS-polyacrylamide gel electrophoresis gels.
(vi) Fba.
The level of Fba (for “fructose biphosphate aldolase”), an enzyme of the glycolytic pathway, was found to be increased only in culture filtrates of early microaerophilic cultures (one spot, Fig. 3). Interestingly, Bai et al. (2) observed increased activity of this type of aldolase sensitive to EDTA in cell extracts from M. tuberculosis H37Rv under conditions of low-oxygen tension. In Bacillus subtilis and Lactobacillus brevis, increased levels of this enzyme were also observed under anaerobic conditions (17, 24). Fba was not identified in the microarray study or in the proteome studies of the M. bovis BCG response to low-oxygen tension that did not include culture filtrates (4, 10, 25). The presence of this housekeeping enzyme in the extracellular environment of the mycobacterium is surprising, although this has been observed previously (14, 21).
(vii) Ald.
By immunodetection with monoclonal antibody HBT10, increased levels of l-alanine dehydrogenase (or 40-kDa antigen) were identified only in culture filtrates of metabolically labeled cultures (three spots, Fig. 3). In M. smegmatis, increased alanine dehydrogenase activity in extracts derived from bacteria grown in the Wayne model was also reported (11). It has been suggested that Ald is involved in peptidoglycan synthesis; its localization in the cell wall and the extracellular environment supports this hypothesis (12). However, Ald is not essential since M. bovis and M. bovis BCG strains lack this enzyme (1, 12). Importantly, material (available at http://schoolniklab.stanford.edu/projects/tb.html) supplementary to the microarray study (25) also indicates that Ald expression is induced by hypoxia.
Differences in steady-state levels of M. tuberculosis proteins under defined oxygen tensions by the signature peptide analysis.
LC-electrospray ionization MS was employed as a method to confirm the results obtained by 2-DE protein analysis. Our approach is to convert a crude lysate to peptides by tryptic digestion and then separate the peptides by LC before subjecting them to MS analysis. The method relies on the identification and analysis of a unique signature of peptides derived from the tryptic digestion of a specific protein. By quantitative comparison of ion intensity with that of ions derived from RplL peptides as an internal control, we were able to evaluate the relative abundance of specific peptides from the proteins of interest. We focused on the seven proteins identified by 2-DE (Table 1) and performed in silico trypsin digestion of each protein. The resulting peptides were compared to the predicted M. tuberculosis proteome to identify unique peptide masses. This information allowed us to perform the analysis by extracting three ions corresponding to three signature peptides for each protein from the total ion chromatogram resulting from LC-MS analysis of bacteria grown under 1, 5, or 20% oxygen. Quantitation of the signature peptides in comparison with fragments from RplL yielded three independent measurements of the relative abundance based on absolute ion intensity for each protein, and the average relative abundance was then calculated. The ions corresponding to each protein in the positive scan mode are indicated in Table 2. Signature peptide analysis confirmed the increased production of five of the seven proteins identified by 2-DE. The two proteins which did not show significantly increased levels in hypoxic lysates by LC-MS analysis were the proteins Fba (Rv0363c) and Ald (Rv2780), which showed increased levels only in culture filtrates.
TABLE 2.
Signature peptide analysis by LC-MS of crude M. tuberculosis lysate proteins under hypoxic conditions
| Protein | Gene | m/z of signature peptidesa | Relative abundance of proteins at defined oxygen concentrations ofb:
|
|
|---|---|---|---|---|
| 1% | 5% | |||
| Rv0363c | fba | 947.0 (1), 474.0 (2) | 0.98 | 1.72 |
| 1,493.8 (1), 747.3 (2) | ||||
| 1,703.0 (1), 852.0 (2) | ||||
| Rv0569 | 1,164.2 (1), 582.6 (2) | 1.33 | 7.31 | |
| 1,270.4 (1), 635.7 (2) | ||||
| 1,617.7 (2), 1,078.4 (3) | ||||
| Rv2031c | hspX | 1,163.2 (1), 582.1 (2) | 4.06 | 6.87 |
| 1,459.6 (1), 730.3 (2) | ||||
| 1,715.9 (1), 858.4 (2) | ||||
| Rv2623 | 1,102.3 (1), 551.6 (2) | 4.76 | 5.28 | |
| 1,387.6 (1), 694.3 (2) | ||||
| 1,937.2 (1), 969.1 (2) | ||||
| Rv2626c | 943.1 (1), 472.0 (2) | 2.81 | 4.88 | |
| 1,698.8 (1), 849.9 (2) | ||||
| 1,257.5 (1), 629.2 (2) | ||||
| Rv2780 | ald | 1,298.5 (1), 649.7 (2) | 1.28 | 2.29 |
| 1,317.4 (1), 659.2 (2) | ||||
| 1,712.0 (1), 856.5 (2) | ||||
| Rv3841 | bfrB | 1,266.3 (1), 633.6 (2) | 4.27 | 5.19 |
| 1,578.9 (1), 789.9 (2) | ||||
| 1,633.7 (1), 817.3 (2) | ||||
m/z values were predicted by in silico trypsin digestion by using the Protein Prospector program MS-Digest (http://prospector.ucsf.edu/). The charge state is indicated in parentheses after each value.
Relative to the abundance at 20% oxygen.
The data presented here describe results from two different models in which the hypoxic response of M. tuberculosis can be studied by proteomics. Interestingly, in both models we found that the same five lysate proteins were more abundant, although two different parameters are measured: the de novo protein synthesis in an early microaerophilic culture versus the steady-state levels in stationary, low-oxygen cultures.
By LC-MS signature peptide analysis, we validated the concept that the steady-state level data for the five lysate proteins that were found to be more abundant by 2-DE were contained within the total ion chromatogram of the bacterial crude lysate. Therefore, it is likely that the expression status of the entire proteome can be assessed by a direct examination of such complex data with enhanced algorithms for extraction of multiple ion data arising from individual discrete proteins.
Interestingly, the relative abundance of all seven proteins investigated by LC-MS signature peptide analysis was lower at 1% oxygen compared to that seen at 5% oxygen; notably, Rv0569 showed increased levels only at 5% oxygen but not at 1% oxygen (7.31 versus 1.33-fold, respectively). The reason for these differences is not clear; they may reflect that the cultures undergo different stages during adaptation to oxygen depletion, but different growth rates at 1 and 5% oxygen could also influence protein expression.
In conclusion, the present study demonstrates the power of combining 2-DE analysis, MALDI-TOF MS protein identification, and LC-MS signature peptide analysis to investigate how M. tuberculosis responds to an environmental change. It also supports and complements other studies of the mycobacterial hypoxic response (4, 10, 25). To further enhance our understanding of latent M. tuberculosis, the role of the seven identified proteins in response to hypoxia must be elucidated, and the response of M. tuberculosis to other latency related changes such as low pH and reactive nitrogen intermediates should also be characterized.
Acknowledgments
We are grateful to John Belisle and Benjamin Espinosa for their help and expertise with LC-MS signature peptide analysis. We thank Tove Slotved Simonsen, Carsten Stokkebye Andersen, and Vivi Andersen for excellent technical assistance.
REFERENCES
- 1.Andersen, Å. B., P. Andersen, and L. Ljungqvist. 1992. Structure and function of a 40,000-molecular-weight protein antigen of Mycobacterium tuberculosis. Infect. Immun. 60:2317-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, N. J., M. R. Pai, P. S. Murthy, and T. A. Venkitasubramanian. 1974. Effect of oxygen tension on the aldolases of Mycobacterium tuberculosis H37Rv. FEBS Lett. 45:68-70. [DOI] [PubMed] [Google Scholar]
- 3.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 4.Boon, C., R. Li, R. Qi, and T. Dick. 2001. Proteins of Mycobacterium bovis BCG induced in the Wayne dormancy model. J. Bacteriol. 183:2672-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Collins, F. M., J. R. Lamb, and D. B. Young. 1988. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect. Immun. 56:1260-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J. Bacteriol. 180:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desjardin, L. E., L. G. Hayes, C. D. Sohaskey, L. G. Wayne, and K. D. Eisenach. 2001. Microaerophilic induction of the alpha-crystallin chaperone protein homologue (HspX) mRNA of Mycobacterium tuberculosis. J. Bacteriol. 183:5311-5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton, M. J., and M. W. Vermeulen. 1996. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect. Immun. 64:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florczyk, M. A., L. A. McCue, R. F. Stack, C. R. Hauer, and K. A. McDonough. 2001. Identification and characterization of mycobacterial proteins differentially expressed under standing and shaking culture conditions, including Rv2623 from a novel class of putative ATP-binding proteins. Infect. Immun. 69:5777-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutter, B., and T. Dick. 1998. Increased alanine dehydrogenase activity during dormancy in Mycobacterium smegmatis. FEMS Microbiol. Lett. 167:7-11. [DOI] [PubMed] [Google Scholar]
- 12.Hutter, B., and M. Singh. 1999. Properties of the 40-kDa antigen of Mycobacterium tuberculosis, a functional l-alanine dehydrogenase. Biochem. J. 343 Pt 3:669-672. [PMC free article] [PubMed] [Google Scholar]
- 13.Ji, J., A. Chakraborty, M. Geng, X. Zhang, A. Amini, M. Bina, and F. Regnier. 2000. Strategy for qualitative and quantitative analysis in proteomics based on signature peptides. J. Chromatogr. B 745:197-210. [DOI] [PubMed] [Google Scholar]
- 14.Jungblut, P. R., U. E. Schaible, H. Mollenkopf, U. Zimny-Arndt, B. Raupach, J. Mattow, P. Halada, S. Lamer, K. Hagens, and S. H. Kaufmann. 1999. Comparative proteome analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG strains: towards functional genomics of microbial pathogens. Mol. Microbiol. 33:1103-1117. [DOI] [PubMed] [Google Scholar]
- 15.Klausen, J., M. Magnusson, A. B. Andersen, and C. Koch. 1994. Characterization of purified protein derivative of tuberculin by use of monoclonal antibodies: isolation of a delayed-type hypersensitivity reactive component from M. tuberculosis culture filtrate. Scand. J. Immunol. 40:345-349. [DOI] [PubMed] [Google Scholar]
- 16.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marino, M., T. Hoffmann, R. Schmid, H. Mobitz, and D. Jahn. 2000. Changes in protein synthesis during the adaptation of Bacillus subtilis to anaerobic growth conditions. Microbiology 146:97-105. [DOI] [PubMed] [Google Scholar]
- 18.Mattow, J., P. R. Jungblut, E. C. Muller, and S. H. Kaufmann. 2001. Identification of acidic, low molecular mass proteins of Mycobacterium tuberculosis strain H37Rv by matrix-assisted laser desorption/ionization and electrospray ionization mass spectrometry. Proteomics 1:494-507. [DOI] [PubMed] [Google Scholar]
- 19.Monahan, I. M., J. Betts, D. K. Banerjee, and P. D. Butcher. 2001. Differential expression of mycobacterial proteins following phagocytosis by macrophages. Microbiology 147:459-471. [DOI] [PubMed] [Google Scholar]
- 20.Nystrom, T., and F. C. Neidhardt. 1994. Expression and role of the universal stress protein, UspA, of Escherichia coli during growth arrest. Mol. Microbiol. 11:537-544. [DOI] [PubMed] [Google Scholar]
- 21.Rosenkrands, I., A. King, K. Weldingh, M. Moniatte, E. Moertz, and P. Andersen. 2000. Towards the proteome of Mycobacterium tuberculosis. Electrophoresis 21:3740-3756. [DOI] [PubMed] [Google Scholar]
- 22.Rosenkrands, I., P. B. Rasmussen, M. Carnio, S. Jacobsen, M. Theisen, and P. Andersen. 1998. Identification and characterization of a 29-kilodalton protein from Mycobacterium tuberculosis culture filtrate recognized by mouse memory effector cells. Infect. Immun. 66:2728-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenkrands, I., K. Weldingh, S. Jacobsen, C. V. Hansen, W. Florio, I. Gianetri, and P. Andersen. 2000. Mapping and identification of Mycobacterium tuberculosis proteins by two-dimensional gel electrophoresis, microsequencing, and immunodetection. Electrophoresis 21:935-948. [DOI] [PubMed] [Google Scholar]
- 24.Saier, M. H., Jr., J. J. Ye, S. Klinke, and E. Nino. 1996. Identification of an anaerobically induced phosphoenolpyruvate-dependent fructose-specific phosphotransferase system and evidence for the Embden-Meyerhof glycolytic pathway in the heterofermentative bacterium Lactobacillus brevis. J. Bacteriol. 178:314-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 27.Sudre, P., G. ten Dam, and A. Kochi. 1992. Tuberculosis: a global overview of the situation today. Bull. W. H. O. 70:149-159. [PMC free article] [PubMed] [Google Scholar]
- 28.Wayne, L. G. 1960. The bacteriology of resected tuberculous pulmonary lesions. II. Observations on bacilli which are sustainable but which cannot be cultured. Am. Rev. Respir. Dis. 82:370-377. [DOI] [PubMed] [Google Scholar]
- 29.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan, Y., D. D. Crane, and C. E. Barry III. 1996. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J. Bacteriol. 178:4484-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan, Y., D. D. Crane, R. M. Simpson, Y. Q. Zhu, M. J. Hickey, D. R. Sherman, and C. E. Barry III. 1998. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. USA 95:9578-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]