Abstract
Considering the physiological Ca2+ dynamics within the ER (endoplasmic reticulum), it remains unclear how efficient protein folding is maintained in living cells. Thus, utilizing the strictly folding-dependent activity and secretion of LPL (lipoprotein lipase), we evaluated the impact of ER Ca2+ content and mitochondrial contribution to Ca2+-dependent protein folding. Exhaustive ER Ca2+ depletion by inhibition of sarcoplasmic/endoplasmic reticulum Ca2+-ATPases caused strong, but reversible, reduction of cell-associated and released activity of constitutive and adenovirus-encoded human LPL in CHO-K1 (Chinese-hamster ovary K1) and endothelial cells respectively, which was not due to decline of mRNA or intracellular protein levels. In contrast, stimulation with the IP3 (inositol 1,4,5-trisphosphate)-generating agonist histamine only moderately and transiently affected LPL maturation in endothelial cells that paralleled a basically preserved ER Ca2+ content. However, in the absence of extracellular Ca2+ or upon prevention of transmitochondrial Ca2+ flux, LPL maturation discontinued upon histamine stimulation. Collectively, these data indicate that Ca2+-dependent protein folding in the ER is predominantly controlled by intraluminal Ca2+ and is largely maintained during physiological cell stimulation owing to efficient ER Ca2+ refilling. Since Ca2+ entry and mitochondrial Ca2+ homoeostasis are crucial for continuous Ca2+-dependent protein maturation in the ER, their pathological alterations may result in dysfunctional protein folding.
Keywords: Ca2+-dependent protein folding, endoplasmic reticulum, intraluminal Ca2+, lipoprotein lipase, mitochondrion
Abbreviations: BHQ, 2,5-di-(t-butyl)-1,4-hydroquinone; CHO-K1, Chinese-hamster ovary K1; ER, endoplasmic reticulum; fura 2/AM, fura 2 acetoxymethyl ester; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IP3, inositol 1,4,5-trisphosphate; LPL, lipoprotein lipase; MOI, multiplicity of infection; RT, reverse transcriptase; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
INTRODUCTION
The ER (endoplasmic reticulum) constitutes the main releasable Ca2+ store in most cell types and actively contributes to cellular Ca2+ homoeostasis via the release and re-uptake of Ca2+ in the course of precisely controlled signalling cascades [1] and extensive communication with mitochondria and adjacent ion channels of the plasma membrane [2,3].
Moreover, by its oxidizing milieu, resident molecular chaperones and folding catalysts, the lumen of the ER provides an optimized environment for the synthesis and post-translational processing of most secretory and membrane proteins [4]. Among the most important molecular chaperones of the ER, calreticulin and its transmembrane homologue calnexin provide a highly efficient quality-control system for immature glycoproteins [5].
Initially characterized as Ca2+-binding protein of the sarcoplasmic reticulum [6], conformation [7], chaperone interactions [8] and thus activity of calreticulin have been shown to be directly affected by [Ca2+]ER (free ER Ca2+ concentration). A decrease in ambient [Ca2+] renders the calreticulin cycle and associated folding catalysts, i.e. protein disulphide-isomerase and ERp57, inactive [9]. However, these principles of Ca2+-dependent protein folding have been assessed mainly on recombinant proteins [7,8,10] or by artificially applying excessive ER stress [11–13], while a detailed analysis of its kinetics and correlation with physiological Ca2+ fluctuations are lacking. While there are studies that address this issue [14], it still remains questionable whether the ER protein-folding machinery is influenced by spatial and temporal dynamics of [Ca2+]ER in intact cells and, if so, how efficient protein folding is maintained under physiological cell stimulation. Moreover, since recent studies revealed a crucial role of mitochondria in the process of rescuing the Ca2+ content of the ER during stimulation with IP3 (inositol 1,4,5-trisphosphate)-elevating agonists [15], the importance of mitochondrial for Ca2+-dependent protein maturation in the ER awaits to be assessed.
Notably, protein misfolding is considered to be a cause and/or consequence of numerous diseases [16,17], including diabetes mellitus [18]. The latter is known to be associated with mitochondrial dysfunction that becomes obvious by increased free radical production [19] and altered mitochondrial Ca2+ homoeostasis [20]. However, since the link between mitochondrial Ca2+ homoeostasis and ER protein folding has not been investigated so far, the consequences of pathological alterations in mitochondrial Ca2+ function on ER protein maturation cannot be estimated.
In order to elucidate the feasible impact of [Ca2+]ER dynamics and the contribution of mitochondrial Ca2+ homoeostasis on protein folding, the expression, secretion and enzyme activity of LPL (lipoprotein lipase) as a measure for the efficiency of protein processing machinery of the ER was investigated. LPL, the major lipolytic enzyme for the hydrolysis of circulating lipoproteins, is a secretory N-linked glycoprotein, which contains two conserved disulphide bridges, and interacts with the Ca2+-dependent protein-folding machinery of the ER during its maturation [11,13,21]. As only the correctly folded protein is active and secreted [11,21], LPL activity is dependent on proper maturation in the ER and it was therefore assumed to be a suitable sensor for ER chaperone-dependent folding processes.
MATERIALS AND METHODS
Materials
Cell culture media and substitutes, specific primers, DNA standards, Moloney murine leukaemia virus RT (reverse transcriptase) and first-strand buffer were obtained from Invitrogen Life Technologies. Foetal calf serum was from PAA Laboratories. Cell culture plasticware was purchased from Bertoni. The RNeasy Mini kit was from Qiagen. RQ1 RNase-free DNase I and TransFast™ were obtained from Promega. 3H-labelled and non-radioactive triolein, BHQ [2,5-di-(t-butyl)-1,4-hydroquinone], histamine and BSA fraction V were from Sigma–Aldrich. Fura 2/AM (fura 2 acetoxymethyl ester) was from Molecular Probes. RNAguard and random hexameric primers were purchased from Amersham Biosciences. CGP 37157 was purchased from Tocris Cookson. Heparin was from Boehringer Ingelheim. Hot Fire DNA polymerase I was from Solis BioDyne. Luminol reagent was obtained from Santa Cruz Biotechnology, and Ultima Gold scintillation cocktail was from PerkinElmer. X-ray films were from Siemens. Nitrocellulose, protein standards, dNTPs and all other chemicals were purchased from Roth.
Cell culture and adenovirus infection
CHO-K1 (Chinese-hamster ovary K1) cells were cultured on six-well dishes in Ham's F12 containing 10% foetal calf serum. Upon reaching confluence, cells were incubated with 1% (w/v) BSA in serum-free Dulbecco's minimum essential medium for 24 h before experiments. The human umbilical-vein-derived endothelial cell line EA.hy926 [22] at passages higher than 44 was grown on six-well dishes in culture medium containing 10% foetal calf serum and 1% HAT (5 mM hypoxanthine, 20 μM aminopterine and 0.8 mM thymidine). Subconfluent cells were infected with 100 MOI (multiplicity of infection) of adenovirus encoding human LPL [23] in culture medium containing 2% foetal calf serum which was replaced for regular medium after approx. 12 h.
RT-PCR
RT-PCR was performed as described previously [24]. Amplification was carried out with initial denaturation at 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C and annealing at 55 °C for 30 s each and elongation at 72 °C for 60 s. The specific primers used for human LPL were 5′-GCATTGCAGGAAGTCTGACC-3′ and 5′-GATGTTCTCACTCTCGGCCA-3′ yielding a 632 bp product. As a control, a 537 bp fragment of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was amplified using the primers 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. PCR products were separated on 2% TAE (Tris/acetate/EDTA)–agarose gels.
Western blot analysis
Western blots were carried out following our protocol published previously [25]. Briefly, 50 μg of total protein or 200 μl of vacuum-concentrated supernatant was mixed with sample buffer yielding a final concentration of 4% (v/v) glycerol, 1% (w/v) SDS, 0.03% (w/v) Bromophenol Blue and 0.6% (v/v) 2-mercaptoethanol in 12.6 mM Tris/HCl, pH 6.8, and was boiled at 95 °C for 10 min before separation on SDS/12.5% polyacrylamide gels. After electrotransfer of the proteins on to nitrocellulose membranes, non-specific binding sites were blocked with 5% (w/v) non-fat instant milk powder in PBS, and the membranes were probed at 4 °C overnight with rabbit polyclonal antibody against recombinant human LPL diluted 1:1000 in 5% (w/v) non-fat instant milk powder in PBS containing 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4·2H2O, 1.5 mM KH2PO4, pH 7.4. Subsequently, membranes were washed with PBS containing 0.05% (v/v) Tween 20, incubated for 1 h with horse-radish-peroxidase-conjugated secondary antibody and detected by enhanced chemiluminescence after additional washing steps.
Determination of LPL enzyme activity
Following incubation with the respective substances in culture medium containing 0.5 μg/ml heparin, supernatants were collected on ice, and the cells were lysed by sonication in chilled 50 mM NH3/NH4Cl solution, pH 8.1. The protein concentration of cell lysates was determined using the established method of Lowry [26]. LPL activity was determined by the hydrolysis of 3H-labelled triacylglycerols as described previously [27]. [3H]Triolein (1 μCi) and 920 μg of non-radioactive triolein per sample were evaporated under a stream of nitrogen and mixed with 20 μl of 1 M Tris/HCl, pH 8.6, 20 μl of 1% (v/v) Triton X-100 and 80 μl of water by extensive sonication in ice-cooled water. Following addition of 40 μl of heat-inactivated (50 °C for 1 h) human serum containing apoCII as activator and 40 μl of 10% (w/v) BSA, 100 μl of cell lysate or supernatant was mixed with 200 μl of the resultant chilled substrate and were incubated for 30 min at 37 °C with continuous shaking. The reaction was stopped by adding 3.25 ml of an organic solvent mixture consisting of methanol/chloroform/n-heptane (1.41:1.25:1, by vol.). By addition of 1 ml of 0.1 M K2CO3/H3BO3, pH 10.5, non-esterified (‘free’) fatty acids became extractable from a two-phase system by vigorous shaking for 20 s and subsequent centrifugation at 2700 g for 20 min. A 1 ml sample of the upper phase was mixed with 8 ml of Ultima Gold scintillation cocktail and measured in a β-counter (Beckmann). LPL activity was expressed as amount of non-esterified fatty acids, hydrolysed per minute by lipolytic enzyme contained within or in the supernatant of 1 mg of total cellular protein as described previously [27]. This assay was not directly affected by BHQ, histamine or CGP 37187.
Determination of [Ca2+]cyto (cytosolic free Ca2+ concentration)
[Ca2+]cyto was measured as described previously [28]. Briefly, endothelial cells grown on glass coverslips or culture dishes of 3 cm diameter were loaded for 45 min at room temperature (22 °C) in the dark in loading buffer (2 mM CaCl2, 135 mM NaCl, 1 mM MgCl2, 5 mM KCl, 10 mM Hepes, 2.6 mM NaHCO3, 0.44 mM KH2PO4, 10 mM D-glucose, 0.1% vitamins, 0.2% essential amino acids, 1% penicillin/streptomycin and 1% fungizone, pH 7.4) containing 2 μM fura 2/AM. Before experiments, cells were washed twice with loading buffer and were equilibrated for a further 15 min in experimental buffer (145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2 and 10 mM Hepes, pH 7.4) in the dark. Cells were illuminated alternately at excitation wavelengths of 340 and 380 nm (340HT15 and 380HT15; Omega Optical), and emission was monitored at 510 nm (510WB40; Omega Optical). [Ca2+]cyto was expressed as (F340/F380)/F0.
Measurement of [Ca2+]ER
Intraluminal free Ca2+ concentration was monitored as described previously [3,29]. In brief, at a confluence of approx. 80%, endothelial cells grown on glass coverslips were transiently transfected with 2 μg of purified pcDNA3 encoding the improved versions of ER-_targeted D1ER probe [30] using TransFast™ transfection reagent according to the manufacturer's protocol. At between 36 and 48 h after transfection, cells were used for experiment. D1ER was excited at 440 nm (440AF21; Omega Optical) and emission was collected simultaneously at 535 and 480 nm using an optical beam splitter (Dual-View MicroImager™, Optical Insights, Visitron Systems). Changes of [Ca2+]ER were expressed as (F535/F480)/F0. Each data point represents the mean±S.E.M. for three to four cells over a measurement period of 3 min.
Statistics
Results are presented as means±S.E.M. Error bars are shown for every data point presented. If no bar is visible, the S.E.M. was smaller than the symbol size. ANOVA and Scheffe's post hoc F test were used for evaluation of the statistical significance. P<0.05 was considered to be significant.
RESULTS
To elucidate the general mechanisms of the regulation of LPL maturation by ER Ca2+ content, we initially investigated the maturation and secretion of constitutive LPL in CHO-K1 cells [31] under control conditions and upon exhaustive Ca2+ depletion of the ER.
Inhibition of SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) yields efficient Ca2+ depletion of the ER
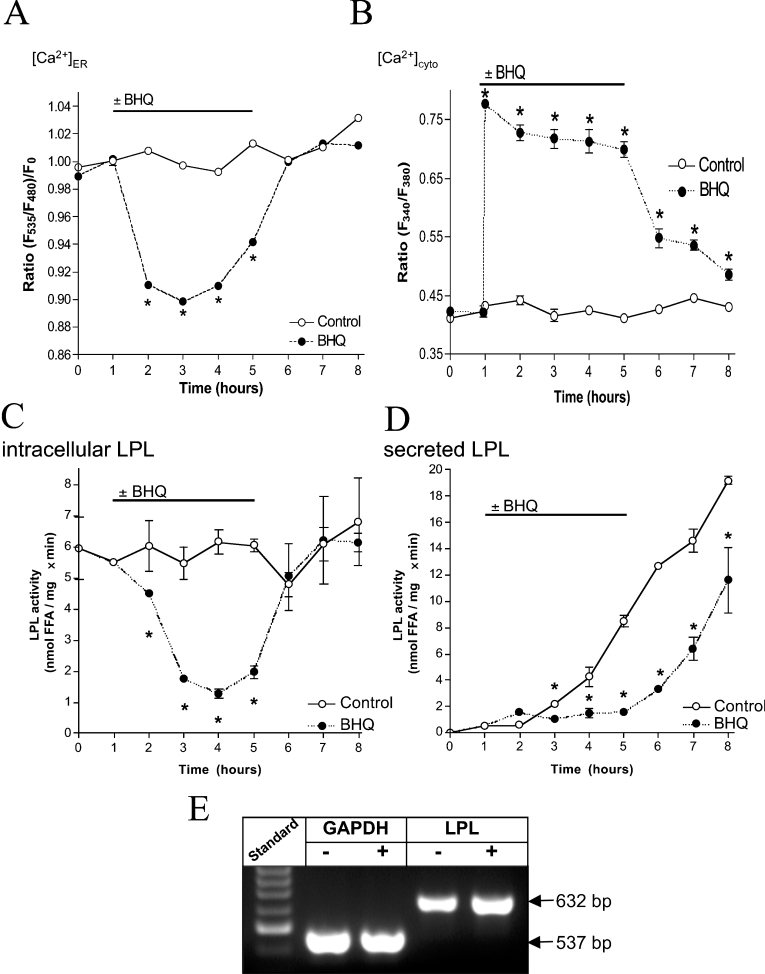
As a consequence of an inhibition of SERCA with 15 μM BHQ, the ER was emptied of Ca2+ (Figure 1A) [3,15], despite the continuous presence of extracellular Ca2+. This effect was fully reversible upon removal of BHQ. Concomitantly, [Ca2+]cyto increased and remained elevated as long as BHQ was present (Figure 1B).
Figure 1. Inhibition of SERCA is a proper tool for an exhaustive ER Ca2+ depletion and consequently attenuates activity and secretion, but not transcription of endogenous LPL.
Cells were transiently transfected with D1ER or loaded with fura 2/AM in order to monitor Ca2+ signals in the ER (A) and cytoplasm (B) respectively. Cells were kept in the continuous presence of 2 mM extracellular Ca2+ under control conditions (○) or were stimulated with 15 μM BHQ as indicated (●). Results are means±S.E.M. for three (A) or eight (B) experiments. *P<0.05 compared with control. (C and D) Serum-starved CHO-K1 cells were propagated in culture medium under control condition (○) or with application of 15 μM BHQ as indicated (●). LPL activity in cell lysates (C) and supernatants (D) was measured in samples taken every 1 h as described in the Materials and methods section. One representative experiment out of three is shown. *P<0.05 compared with control. (E) Using RT-PCR, the constitutive expression of GAPDH and LPL in untreated CHO-K1 cells (−) and after 3 h of exposure to 15 μM BHQ (+) were validated.
SERCA inhibition attenuates activity and secretion, but not expression of endogenous LPL
To induce endogenous expression of LPL, CHO-K1 cells were propagated in serum-free medium for 24 h before experiments. Ca2+ depletion of the ER with 15 μM BHQ resulted in gradual decline of LPL activity in cell lysates (Figure 1C). Subsequent removal of BHQ yielded complete recovery of enzyme activity within 1 h (Figure 1C). LPL activity released into the supernatant of CHO-K1 cells rose constantly (Figure 1D). Application of 15 μM BHQ retained extracellular LPL activity at a low level, while, immediately after washout of BHQ, the secretion of active LPL increased with identical kinetics as in control (slope of 3.0±0.7 compared with 3.0±0.2 nmol·mg−1·min−1·h−1 without BHQ pre-treatment) (Figure 1D). Using RT-PCR, the mRNA levels of LPL and GAPDH were found to be unaffected by 3 h of treatment with BHQ (Figure 1E).
These data suggest that endogenous transcription of LPL in wild-type CHO cells is not altered by depletion of [Ca2+]ER, whereas its enzymatic activity and secretion are reversibly reduced.
Owing to the lack of a suitable antibody against LPL of hamster origin, possible alterations on the protein level could not be verified. Consequently, in order to correlate ER Ca2+ content further with protein transcription and expression, as well as maturation and secretion, human LPL was overexpressed in endothelial cells utilizing adenovirus [23].
Depletion of [Ca2+]ER attenuates activity and secretion of adenovirus-encoded LPL
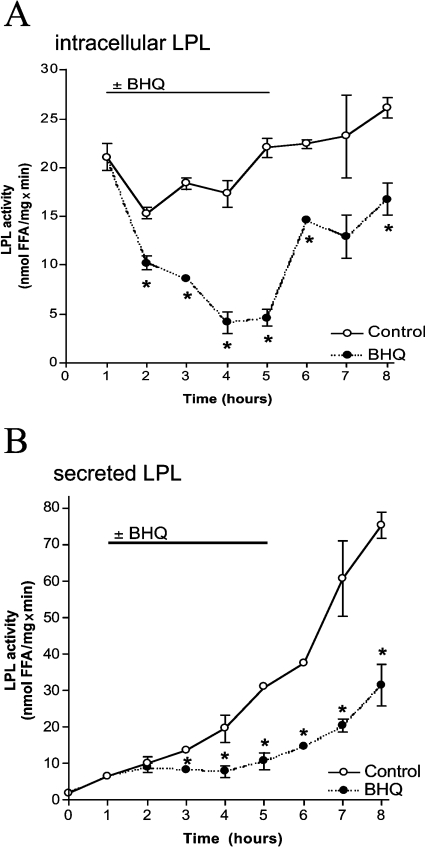
In endothelial cells, adenovirus-mediated expression of human LPL yielded a steady enzyme activity in cell lysates and continuous LPL secretion into the medium (Figure 2). In the presence of 15 μM BHQ, which achieved exhaustive ER Ca2+ depletion similar to that shown in CHO-K1 cells [3], LPL activity in cell lysates decreased and partially recovered upon removal of BHQ (Figure 2A).
Figure 2. ER Ca2+ depletion reduces activity and secretion of adenovirus-encoded LPL in endothelial cells.
Cells were infected with adenovirus encoding human LPL (100 MOI) and incubated in serum-free culture medium without any further treatment (○) or with 15 μM BHQ as indicated (●). Every 1 h, samples were taken, and LPL activity in cell lysates (A) and aliquots of the supernatant (B) was acquired as described in the Materials and methods section. One representative experiment out of three is shown. Results are means±S.E.M. for all experiments. *P<0.05 compared with control.
In agreement with the results obtained on the constitutive LPL in CHO-K1 cells, activity of adenovirus-encoded human LPL increased gradually in the medium of untreated endothelial cells with a slope of 6.3±0.7 nmol·mg−1·min−1·h−1 in the first 3 h. Addition of 15 μM BHQ arrested the enzyme activity at low level. In consequence of BHQ washout, in the first 3 h, LPL activity in the supernatant started to rise with a slope comparable with that initially seen in untreated cells (6.8±1.2 nmol ·mg−1·min−1·h−1) (Figure 2B).
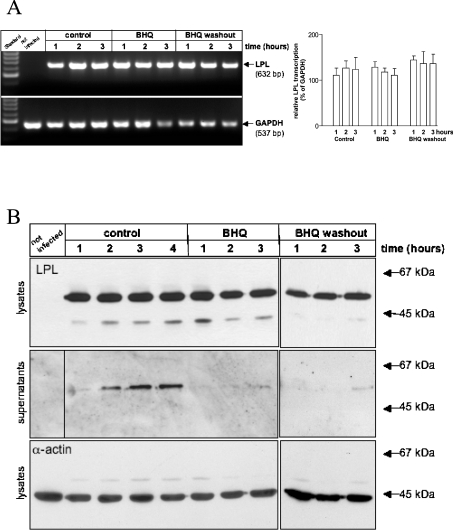
Adenovirus-mediated expression of LPL is unaffected by depletion of [Ca2+]ER
As shown in Figure 3(A), mRNA levels of human LPL in relation to GAPDH remained unchanged in endothelial cells by up to 4 h of BHQ treatment and during the subsequent washout period. Moreover, the content of LPL protein within equal amounts of cell lysate was unaffected by BHQ (Figure 3B, top panel). However, the additional band of lower molecular mass that was detectable with the antibody increased slightly within the first 1 h after BHQ application and disappeared during the washout period. In the supernatant of adenovirus-infected endothelial cells, LPL protein content paralleled the raise in enzymatic activity found in the medium. Consecutively, BHQ abolished LPL secretion and LPL protein in the medium did not reach a detectable level up to 3 h after removal of the SERCA inhibitor (Figure 3B, middle panel). In comparison, the expression level of α-actin remained unaffected under each condition (Figure 3B, bottom panel).
Figure 3. Adenovirus-mediated expression of LPL is not altered by BHQ treatment.
Using RT-PCR (A), the adenovirus-mediated transcription of LPL (left-hand panel) was assessed in endothelial cells and normalized for endogenous GAPDH (right-hand panel) after 1, 2 and 3 h in the absence (control), presence (BHQ) or following washout (BHQ washout) of 15 μM BHQ. Results are means±S.E.M. for three independent experiments. *P<0.05 compared with control. For Western Blot analysis (B), identical amounts of protein of cell lysates (top panel) and identical volumes of the corresponding supernatants (bottom panel) were separated on SDS/12.5% polyacrypamide gels and blotted on to nitrocellulose. Lanes show the expression of LPL and α-actin in adenovirus-infected endothelial cells after 1, 2, 3 and 4 h in the absence (control) or after 1, 2 and 3 h in presence (BHQ) or following washout (BHQ washout) of 15 μM BHQ. Positions of standard proteins are marked, with sizes in kDa.
These data indicate that strong depletion of the ER by BHQ diminished the activity and secretion of adenovirus-encoded human LPL in endothelial cells in a partially reversible manner, while mRNA levels and intracellular protein content remained unaffected by BHQ treatment.
Histamine causes moderate ER Ca2+ depletion despite strong elevation of cytoplasmic Ca2+
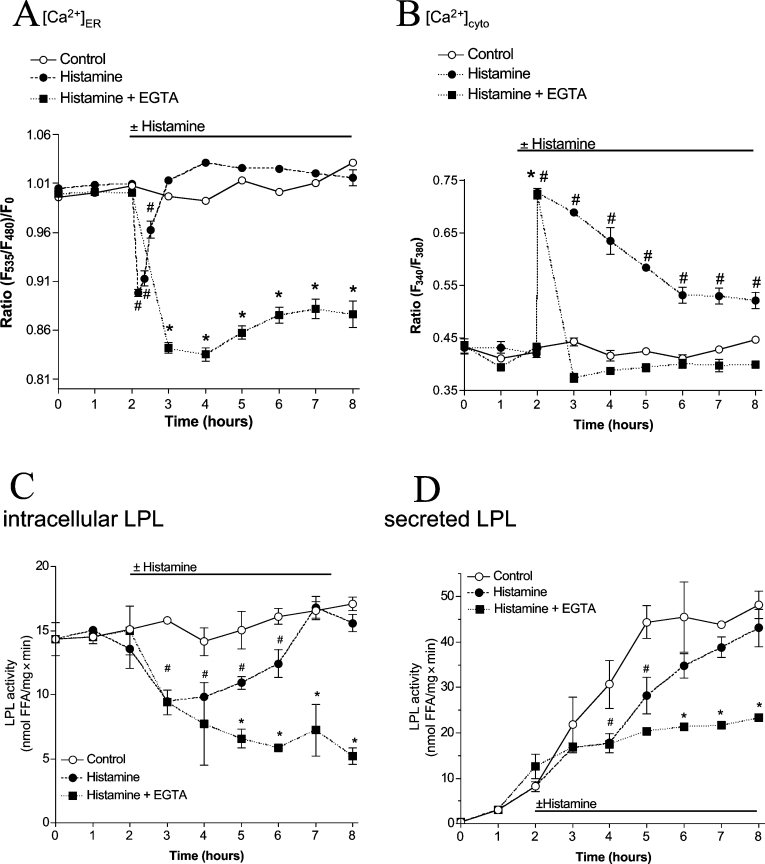
In order to analyse the impact of agonist-triggered ER Ca2+ depletion on LPL maturation and secretion, the effects of the IP3-generating autacoid histamine on [Ca2+]ER and the activity of adeno-virus-encoded human LPL were investigated in endothelial cells.
Stimulation of endothelial cells expressing LPL with 100 μM histamine in the presence of extracellular Ca2+ resulted in immediate and reversible reduction of [Ca2+]ER (Figure 4A). This effect was comparable with that achieved with BHQ, but represented a transient phenomenon as ER Ca2+ content fully recovered within 1 h, even in the presence of the agonist. This histamine-initiated ER Ca2+ depletion was accompanied by a rapid initial cytosolic Ca2+ elevation and a subsequent elevated Ca2+ plateau until washout of the agonist (Figure 4B).
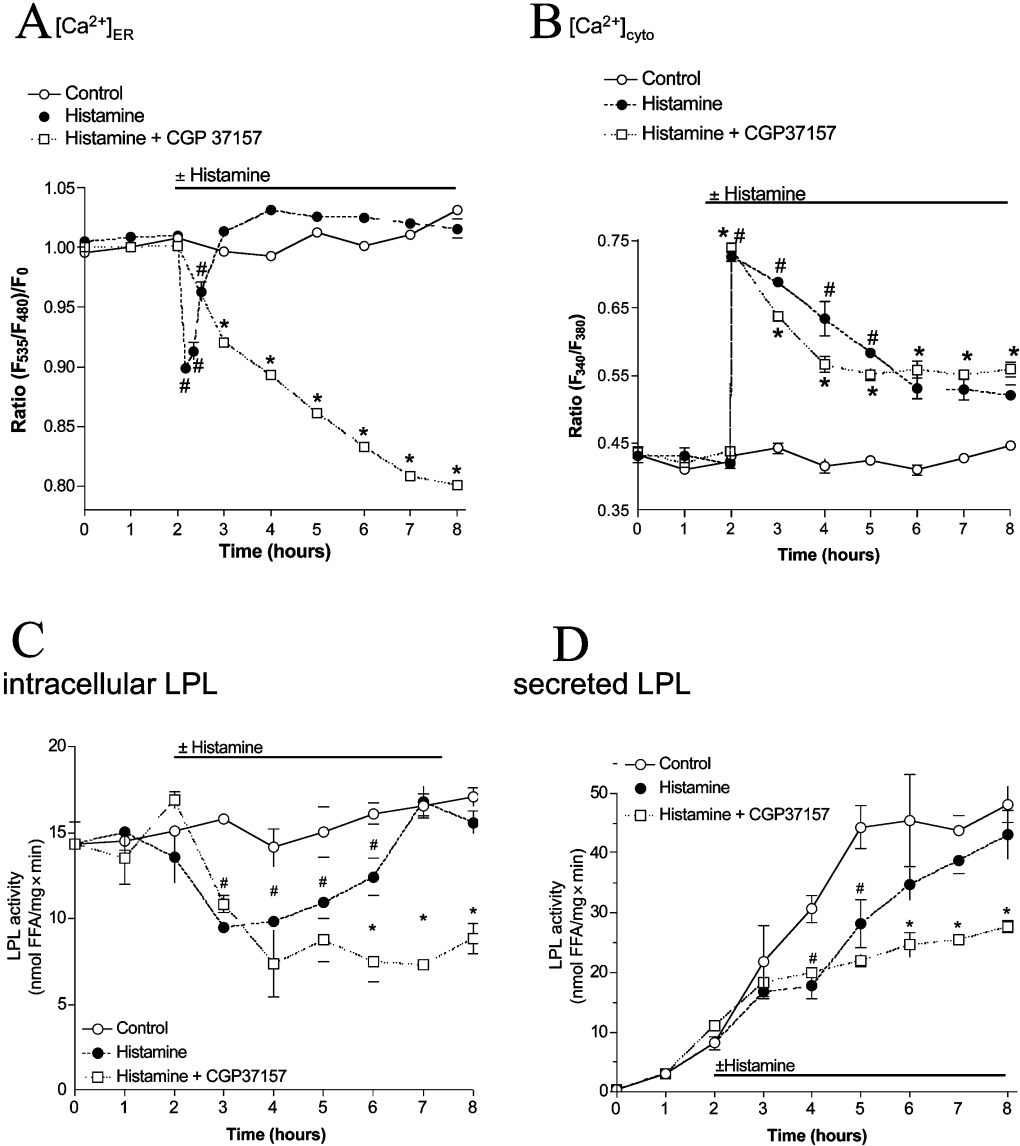
Figure 4. Under physiological conditions, LPL maturation is largely preserved by strong ER Ca2+ refilling due to influx.
Stimulation of endothelial cells with histamine yields temporary ER Ca2+ depletion despite large elevation in the cytoplasmic Ca2+ concentration and transiently lowers activity and secretion of LPL, whereas removal of extracellular Ca2+ yields pronounced Ca2+ depletion and attenuation of LPL maturation. Ca2+ signals in the ER (A) and cytoplasm (B) of endothelial cells were monitored using D1ER and fura 2 respectively. The respective Ca2+ concentration was monitored under control conditions (○) or upon stimulation with 100 μM histamine in the presence of 2 mM extracellular Ca2+ (●) or 1 mM EGTA (i.e. nominal Ca2+-free buffer; ■) as indicated. Results are means±S.E.M. for three (A), eight (B, ○) or ten (B, ● and ■) experiments. *P<0.05 compared with control. (C and D) Adenovirus-infected endothelial cells were incubated in serum-free culture medium without (○) or with addition of 100 μM histamine (●) or stimulated with histamine in nominal Ca2+-free solution (i.e. serum-free culture medium containing 2.5 mM EGTA; ■). Every 1 h, LPL activity in cell lysates (C) and aliquots of the supernatant (D) was validated as described in the Materials and methods section. *P<0.05 compared with control (n=6).
Agonist-induced ER Ca2+ reduction transiently attenuates activity and secretion of human LPL
Upon application of 100 μM histamine, intracellular LPL activity was reduced after 1 h and subsequently re-established slowly to reach complete recovery after 4 h, despite the presence of the agonist (Figure 4C). In the supernatant, histamine decreased LPL activity in the medium for 2 h, while further progression of LPL secretion was only slightly diminished (Figure 4D).
However, in the absence of extracellular Ca2+ (i.e. EGTA-containing solution), histamine (100 μM) yielded complete ER Ca2+ depletion (Figure 4A) that was accompanied by a transient cytosolic Ca2+ elevation (Figure 4B). Under these conditions, intracellular LPL activity upon histamine did not recover and decreased continuously over time (Figure 4C). Consistently, in the absence of extracellular Ca2+, secretion of LPL was prevented permanently by stimulation with histamine (Figure 4D). In agreement with our findings that point to a tight correlation between ER Ca2+ content and LPL folding, EGTA (i.e. without histamine stimulation), which has been shown previously to result in a slow ER depletion [32] and yielded approx. 50% ER depletion within 4 h (results not shown), gradually diminished intracellular activity and secretion of LPL over time (Figure 5).
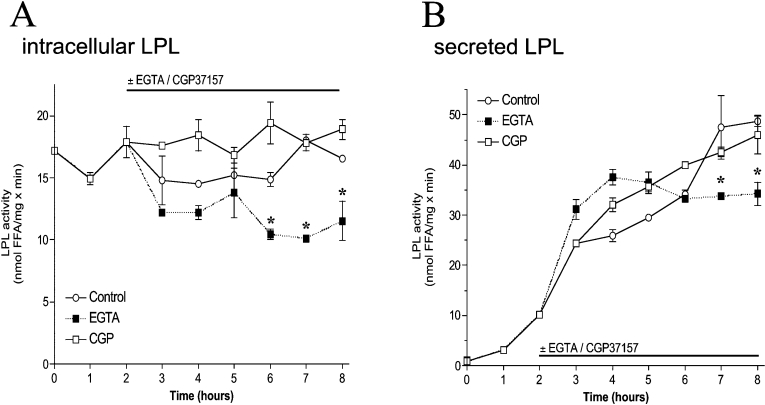
Figure 5. Activity and secretion of LPL is marginally attenuated by removal of extracellular Ca2+ but is not affected by CGP 37157.
Adenovirus-infected endothelial cells were incubated in serum-free culture medium without (○) or with (□) 20 μM CGP 37187 or 2.5 mM EGTA (■). LPL activity was measured every 1 h in cell lysates (A) and aliquots of the supernatant (B). Results are means±S.E.M. for three to six experiments. #P<0.05 compared with control (n=6); *P<0.05 compared with histamine (n=3–6).
Thus, in contrast with BHQ that yielded exhaustive and long-lasting ER Ca2+ depletion and, consequently, prevented post-translational processing of LPL, cell stimulation with an IP3-generating agonist under physiological conditions (i.e. in the presence of extracellular Ca2+) only weakly and transiently (within 4 h) attenuated the maturation and secretion of LPL, most likely due to the moderate ER Ca2+ depletion under these conditions.
To investigate the contribution of mitochondrial Ca2+ flux to the maintenance of the Ca2+-dependent protein folding machinery of the ER during cell stimulation with the physiologically relevant agonist histamine, mitochondrial Ca2+ extrusion was prevented by CGP 37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger, that represents the main mechanism of Ca2+ extrusion from mitochondria in endothelial cells [3,33].
Inhibition of mitochondrial Ca2+ flux prevents LPL maturation and secretion
CGP 37157 has been demonstrated to inhibit mitochondrial Ca2+ flux and, thus, ER Ca2+ refilling upon stimulation in endothelial cells very efficiently [3]. As shown in Figure 6(A), in the presence of 20 μM CGP 37157, histamine-induced ER Ca2+ depletion was more pronounced than in the control, while [Ca2+]cyto remained elevated (Figure 6B).
Figure 6. During agonist stimulation, maintenance of LPL maturation critically depends on transmitochondrial flux.
The inhibition of transmitochondrial Ca2+ flux yields pronounced Ca2+ depletion of the ER, prevents its refilling and decreases activity and secretion of LPL during physiological cell stimulation. Ca2+ signals in the ER (A) and cytoplasm (B) of endothelial cells were monitored using D1ER and fura 2 respectively. As indicated, cells were either kept under control conditions (○; A, n=3; B, n=8) or stimulated with 100 μM histamine in Ca2+-containing buffer without (●; A, n=3; B, n=8) or with 20 μM CGP 37157 (□; A, n=4; B, n=9) and the respective Ca2+ concentration was monitored as described in the Materials and methods section. Results are means±S.E.M. for three (A, ○ and ●), four (A, □), eight (B, ○ and ●) or nine (B, □) experiments. *P<0.05 compared with control. (C and D) Adenovirus-infected endothelial cells were incubated in serum-free culture medium (○) or stimulated with 100 μM histamine without (●) or with (□) 20 μM CGP 37187. LPL activity was measured every 1 h in cell lysates (C) and aliquots of the supernatant (D). #P<0.05 compared with control (n=6); Results are means±S.E.M. for three to six experiments. *P<0.05 compared with histamine (n=3–6).
In the presence of CGP 37157, cytosolic LPL activity was largely decreased by stimulation with histamine and did not recover with time (Figure 6C). Accordingly, LPL secretion was prevented upon histamine stimulation in the presence of CGP 37157 (Figure 6D). In contrast, application of CGP 37157 without any further treatment did not affect ER Ca2+ content within 4 h (results not shown) and also had no effect on intracellular or secreted LPL activity (Figure 5).
These data suggest, that besides extracellular Ca2+, mitochondrial Ca2+ transfer essentially contributes to Ca2+-dependent maturation of LPL in the ER and its subsequent secretion during cell stimulation with an IP3-generating agonist.
DISCUSSION
In virtually all non-excitable cells, the ER serves as the main releasable Ca2+ store and actively contributes to cellular Ca2+ homoeostasis and post-translational protein processing [1]. Notably, the activity of some of the most important ER-resident chaperones, such as calreticulin, calnexin and BiP (immunoglobulin heavy-chain-binding protein), was recognized to be sensitive to ambient Ca2+ [9]. However, it remains still unclear whether and, if so, how eminent Ca2+ fluctuations faced by the ER under physiological conditions due to regular cell stimulation [2,3,15] influence ER chaperone activity [8]. Additionally, since mitochondria are of pivotal importance to direct entering Ca2+ towards the ER for its refilling [15], the contribution of such mitochondrial Ca2+ transfer to Ca2+-dependent protein maturation in the ER needs to be elucidated. Therefore the efficiency of Ca2+-dependent protein maturation in the ER was determined utilizing LPL, the activity and secretion of which strictly depends on proper folding by Ca2+-sensitive chaperones and folding catalysts in the ER [11,13,21]. Consequently, LPL expression was correlated with enzyme activity as a measure of correctly folded LPL.
Initially, the activity of endogenous LPL in serum-starved CHO-K1 cells [31] was assessed. Under resting conditions, intracellular LPL activity remained constant over the whole experiment, while, in the supernatant, a linear accumulation of LPL activity occurred. These findings are in agreement with the general concept of a continuous LPL synthesis, maturation and its subsequent secretion [31]. However, if the ER was exhaustively depleted from Ca2+ by the SERCA inhibitor BHQ [3], intracellular LPL activity disappeared and recovered fully 1 h after BHQ washout. The decline of cellular LPL activity due to BHQ was associated with retention of LPL secretion that was completely restored 2 h after BHQ removal. Since the changes in LPL activity and secretion were not accompanied by alterations in the mRNA level, these data suggest that ER Ca2+ depletion prevents protein maturation and thus its subsequent secretion.
However, at this point, it was conceivable that ER Ca2+ depletion harms protein translation, resulting in attenuation of active LPL protein, despite unchanged mRNA levels. Unfortunately, potential effects of a reduction of ER Ca2+ content on LPL protein levels could not be verified because of the lack of a suitable antibody against hamster LPL. In order to provide information on the protein content for the correlation of LPL expression, maturation and secretion with ER Ca2+ concentration, we used adenovirus-mediated overexpression of human LPL [23], against which a polyclonal antibody was available. Furthermore, any modulation of the gene expression of constitutive LPL by Ca2+-sensitive transcription factors could be excluded owing to the stable LPL transcription driven by the virus promotor. In addition, to avoid contamination by constitutively expressed LPL, cells lacking endogenous LPL [34,35] were infected. Accordingly, because of our extensive experience on the Ca2+ homoeostasis in the human umbilical vein derived endothelial cell line EA.hy926 [2,3,15,29,36], these cells were chosen as a suitable model system.
In agreement with previous reports [3,15], SERCA inhibition by BHQ efficiently emptied the ER and permanently elevated cytosolic free Ca2+ concentration also in endothelial cells that were infected with adenovirus-encoding human LPL (results not shown). Concurrently, activity of ectopically expressed human LPL in cell lysates decreased upon BHQ addition, which was in line with a blunted LPL accumulation in the medium. However, in contrast with the complete recovery of LPL activity in CHO-K1 cells upon BHQ washout, in infected endothelial cells, LPL activity only partially recovered, despite total refilling of ER Ca2+ stores [3,15]. The slower onset of LPL appearance in the supernatant under these conditions might be either related to the delay in intracellular LPL recovery after BHQ or to the change in the cell type used.
Expression of functional recombinant proteins, especially at high concentrations as achieved by viral infection, requires an efficient and undisturbed operation of the protein processing machinery of the ER [37], which can easily be overloaded [38]. Thus it is reasonable to speculate that delay of LPL recovery following washout of BHQ is due to attenuation of LPL synthesis and/or maturation by ER stress [39]. Nevertheless, these data are in line with that obtained in CHO-K1 cells and point to a strong correlation between LPL maturation/secretion and ER Ca2+ content. Importantly, the effect of ER Ca2+ depletion on the activity of LPL was not due to alterations in mRNA levels and intracellular protein content. Albeit, there was no intracellular accumulation of LPL despite prevented secretion, pointing to mobilization of ER-associated degradation under these conditions [40]. Consistently, efficient degradation of LPL by ER-associated degradation mechanisms has been shown for maturation-defective variants of that protein in the context of inherited primary lipase deficiency [41] and cld/cld mice [42].
Thus the maturation and secretion of both ectopically over-expressed and endogenous LPL proved to be sensitive to depletion of [Ca2+]ER.
Nevertheless, it remains unclear whether the ER protein-folding machinery is also susceptible to fluctuations in ER Ca2+ content in response to physiological stimuli. Therefore the impact of the physiological mediator histamine on LPL maturation and secretion was elucidated in endothelial cells expressing adenovirus-encoded human LPL. Remarkably, even stimulation with supramaximal concentrations of this IP3-generating agonist (i.e. 100 μM histamine [36]), was found to initiate limited ER Ca2+ depletion due to counteracting refilling processes as described previously [3,15]. In line with these reports, the reduction of ER Ca2+ content as well as cytosolic Ca2+ elevations in response to histamine were comparable with that achieved by BHQ. Moreover, ER Ca2+ content recovered within 1 h, even in the presence of the agonist. In agreement with such transient ER Ca2+ depletion and the subsequent ER Ca2+ refilling in the presence of histamine, secretion of adenovirus-encoded LPL was delayed after 2 h of histamine stimulation, but recovered with the same slope as in resting cells thereafter. In contrast, the recovery of the intracellular activity of LPL in histamine-stimulated cells was much slower, and full recovery was found after 5 h of histamine stimulation. These data indicate that, upon ER Ca2+ refilling, the recovery of intracellular LPL maturation is masked by the rapid secretion of newly folding protein. Overall, these data point to a strict dependency between LPL maturation, secretion and [Ca2+]ER. However, our data, which indicate that LPL maturation and secretion were prevented if the cells were stimulated with histamine in the absence of extracellular Ca2+ (i.e. conditions where no ER Ca2+ refilling occurs [3]), suggest that, for the maintenance of Ca2+-dependent protein maturation in the ER, Ca2+ entry is required to preserve ER Ca2+ content during physiological cell stimulation. Notably, attenuation of Ca2+ entry pathways has been frequently observed under pathological conditions (e.g. diabetes mellitus [43]), and thus pathologically altered Ca2+ channel activity might contribute to protein misfolding in diseases.
Similar to the removal of extracellular Ca2+, inhibition of transmitochondrial Ca2+ flux by CGP 37157 [3,15] prevented Ca2+-dependent protein maturation in the ER. These findings are in line with our previous report that mitochondrial Ca2+ flux is a prerequisite for ER Ca2+ refilling during agonist stimulation [15] and points to the maintenance of the Ca2+-dependent protein folding machinery of the ER in a physiological environment as a new, so far unknown, function of mitochondria. In view of these findings and the increasing evidence of mitochondrial dysfunction in various metabolic diseases (e.g. diabetes mellitus [20,44]), it is tempting to speculate that disease-associated alterations in mitochondrial (Ca2+) function may subsequently affect Ca2+-dependent protein maturation in the ER and thus facilitate cellular dysfunction by incorrectly folded or incompletely assembled proteins.
In conclusion, these data suggest that the protein-folding machinery of the ER is weakly affected by agonist-mediated [Ca2+]ER fluctuations, while displaying strong sensitivity to pronounced and/or long-lasting attenuation of [Ca2+]ER. Thus, under physiological conditions, ER protein maturation is largely preserved, regardless of cell stimulation with IP3-generating agonists due to strong ER Ca2+-refilling processes that depend on Ca2+ entry and transmitochondrial flux and efficiently counteract ER Ca2+ depletion. Based on the strong evidence that cellular/mitochondrial Ca2+ homoeostasis represents an early _target of cell dysfunction in numerous diseases (e.g. diabetes mellitus [45–47] or atherosclerosis [48]), the impact of the reported alterations in intracellular Ca2+ handling on protein maturation in the ER needs to be explored in more detail.
Acknowledgments
We thank Dr C. J. S. Edgell (Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, NC, U.S.A.) for the EA.hy926 cells and Anna Schreilechner (Institute of Molecular Biology and Biochemistry, Medical University Graz), Michaela Tritscher (Institute of Molecular Biosciences, Karl-Franzens University Graz) and Renate Schreiber (Institute of Molecular Biosciences, Karl-Franzens University Graz) for their excellent technical assistance. D1ER was a gift from Dr R. Y. Tsien (Department of Pharmacology and Department of Chemistry and Biochemistry, University of California, San Diego, San Diego, CA, U.S.A.). This work was supported by the Austrian Science Funds (SFB 714, P16860-B9 to W. F. G.; SFB 713 to R. Z.) and the Franz-Lanyar-Stiftung at the Medical University of Graz. The Department of Molecular Biology and Biochemistry is a member of the Institutes of Basic Medical Sciences (IBMS) at the Medical University of Graz and was supported by the infrastructure program (UGP4) of the Austrian Ministry of Education, Science and Culture.
References
- 1.Berridge M. J. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 2.Malli R., Frieden M., Osibow K., Graier W. F. Mitochondria efficiently buffer subplasmalemmal Ca2+ elevation during agonist stimulation. J. Biol. Chem. 2003;278:10807–10815. doi: 10.1074/jbc.M212971200. [DOI] [PubMed] [Google Scholar]
- 3.Malli R., Frieden M., Osibow K., Zoratti C., Mayer M., Demaurex N., Graier W. F. Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J. Biol. Chem. 2003;278:44769–44779. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- 4.Kleizen B., Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 2004;16:343–349. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Ellgaard L., Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 6.Fliegel L., Burns K., MacLennan D. H., Reithmeier R. A., Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1989;264:21522–21528. [PubMed] [Google Scholar]
- 7.Corbett E. F., Michalak K. M., Oikawa K., Johnson S., Campbell I. D., Eggleton P., Kay C., Michalak M. The conformation of calreticulin is influenced by the endoplasmic reticulum luminal environment. J. Biol. Chem. 2000;275:27177–27185. doi: 10.1074/jbc.M002049200. [DOI] [PubMed] [Google Scholar]
- 8.Corbett E. F., Oikawa K., Francois P., Tessier D. C., Kay C., Bergeron J. J., Thomas D. Y., Krause K. H., Michalak M. Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J. Biol. Chem. 1999;274:6203–6211. doi: 10.1074/jbc.274.10.6203. [DOI] [PubMed] [Google Scholar]
- 9.Corbett E. F., Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem. Sci. 2000;25:307–311. doi: 10.1016/s0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor M., Srinivas H., Kandiah E., Gemma E., Ellgaard L., Oscarson S., Helenius A., Surolia A. Interactions of substrate with calreticulin, an endoplasmic reticulum chaperone. J. Biol. Chem. 2003;278:6194–6200. doi: 10.1074/jbc.M209132200. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Zeev O., Mao H. Z., Doolittle M. H. Maturation of lipoprotein lipase in the endoplasmic reticulum: concurrent formation of functional dimers and inactive aggregates. J. Biol. Chem. 2002;277:10727–10738. doi: 10.1074/jbc.M108128200. [DOI] [PubMed] [Google Scholar]
- 12.Jethmalani S. M., Henle K. J. Calreticulin associates with stress proteins: implications for chaperone function during heat stress. J. Cell. Biochem. 1998;69:30–43. doi: 10.1002/(sici)1097-4644(19980401)69:1<30::aid-jcb4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Wu G., Tate C. G., Lookene A., Olivecrona G. Calreticulin promotes folding/dimerization of human lipoprotein lipase expressed in insect cells (Sf21) J. Biol. Chem. 2003;278:29344–29351. doi: 10.1074/jbc.M300455200. [DOI] [PubMed] [Google Scholar]
- 14.Neve B. P., Verhoeven A. J., Kalkman I., Jansen H. Maturation and secretion of rat hepatic lipase is inhibited by α1B-adrenergic stimulation through changes in Ca2+ homoeostasis: thapsigargin and EGTA both mimic the effect of adrenaline. Biochem. J. 1998;330:701–706. doi: 10.1042/bj3300701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malli R., Frieden M., Trenker M., Graier W. F. The role of mitochondria for Ca2+ refilling of the ER. J. Biol. Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- 16.Dobson C. M. The structural basis of protein folding and its links with human disease. Philos. Trans. R. Soc. London Ser. B. 2001;356:133–145. doi: 10.1098/rstb.2000.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwich A. Protein aggregation in disease: a role for folding intermediates forming specific multimeric interactions. J. Clin. Invest. 2002;110:1221–1232. doi: 10.1172/JCI16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araki E., Oyadomari S., Mori M. Endoplasmic reticulum stress and diabetes mellitus. Intern. Med. 2003;42:7–14. doi: 10.2169/internalmedicine.42.7. [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi S. I., Edelstein D., Du X. L., Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001;50:1491–1494. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- 20.Paltauf-Doburzynska J., Malli R., Graier W. F. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J. Cardiovasc. Pharmacol. 2004;44:424–437. doi: 10.1097/01.fjc.0000139449.64337.1b. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Zeev O., Doolittle M. H., Davis R. C., Elovson J., Schotz M. C. Maturation of lipoprotein lipase: expression of full catalytic activity requires glucose trimming but not translocation to the cis-Golgi compartment. J. Biol. Chem. 1992;267:6219–6227. [PubMed] [Google Scholar]
- 22.Edgell C. J., McDonald C. C., Graham J. B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. U.S.A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauss J. G., Frank S., Kratky D., Hammerle G., Hrzenjak A., Knipping G., von Eckardstein A., Kostner G. M., Zechner R. Adenovirus-mediated rescue of lipoprotein lipase-deficient mice: lipolysis of triglyceride-rich lipoproteins is essential for high density lipoprotein maturation in mice. J. Biol. Chem. 2001;276:36083–36090. doi: 10.1074/jbc.M104430200. [DOI] [PubMed] [Google Scholar]
- 24.Zoratti C., Kipmen-Korgun D., Osibow K., Malli R., Graier W. F. Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 2003;140:1351–1362. doi: 10.1038/sj.bjp.0705529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaeffer G., Levak-Frank S., Spitaler M. M., Fleischhacker E., Esenabhalu V. E., Wagner A. H., Hecker M., Graier W. F. Intercellular signalling within vascular cells under high D-glucose involves free radical-triggered tyrosine kinase activation. Diabetologia. 2003;46:773–783. doi: 10.1007/s00125-003-1091-y. [DOI] [PubMed] [Google Scholar]
- 26.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Zechner R. Rapid and simple isolation procedure for lipoprotein lipase from human milk. Biochim. Biophys. Acta. 1990;1044:20–25. doi: 10.1016/0005-2760(90)90213-h. [DOI] [PubMed] [Google Scholar]
- 28.Graier W. F., Paltauf-Doburzynska J., Hill B. J., Fleischhacker E., Hoebel B. G., Kostner G. M., Sturek M. Submaximal stimulation of porcine endothelial cells causes focal Ca2+ elevation beneath the cell membrane. J. Physiol. 1998;506:109–125. doi: 10.1111/j.1469-7793.1998.109bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frieden M., Malli R., Samardzija M., Demaurex N., Graier W. F. Subplasmalemmal endoplasmic reticulum controls KCa channel activity upon stimulation with a moderate histamine concentration in a human umbilical vein endothelial cell line. J. Physiol. 2002;540:73–84. doi: 10.1113/jphysiol.2002.017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer A. E., Jin C., Reece J. C., Tsien R. Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. U.S.A. 2003;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berryman D. E., Bensadoun A. Heparan sulfate proteoglycans are primarily responsible for the maintenance of enzyme activity, binding, and degradation of lipoprotein lipase in Chinese hamster ovary cells. J. Biol. Chem. 1995;270:24525–24531. doi: 10.1074/jbc.270.41.24525. [DOI] [PubMed] [Google Scholar]
- 32.Paltauf-Doburzynska J., Frieden M., Graier W. F. Mechanisms of Ca2+ store depletion in single endothelial cells in a Ca2+-free environment. Cell Calcium. 1999;25:345–353. doi: 10.1054/ceca.1999.0038. [DOI] [PubMed] [Google Scholar]
- 33.Sedova M., Blatter L. A. Intracellular sodium modulates mitochondrial calcium signaling in vascular endothelial cells. J. Biol. Chem. 2000;275:35402–35407. doi: 10.1074/jbc.M006058200. [DOI] [PubMed] [Google Scholar]
- 34.Camps L., Reina M., Llobera M., Bengtsson-Olivecrona G., Olivecrona T., Vilaro S. Lipoprotein lipase in lungs, spleen, and liver: synthesis and distribution. J. Lipid Res. 1991;32:1877–1888. [PubMed] [Google Scholar]
- 35.Camps L., Reina M., Llobera M., Vilaro S., Olivecrona T. Lipoprotein lipase: cellular origin and functional distribution. Am. J. Physiol. 1990;258:C673–C681. doi: 10.1152/ajpcell.1990.258.4.C673. [DOI] [PubMed] [Google Scholar]
- 36.Paltauf-Doburzynska J., Frieden M., Spitaler M., Graier W. F. Histamine-induced Ca2+ oscillations in a human endothelial cell line depend on transmembrane ion flux, ryanodine receptors and endoplasmic reticulum Ca2+-ATPase. J. Physiol. 2000;524:701–713. doi: 10.1111/j.1469-7793.2000.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cudna R. E., Dickson A. J. Endoplasmic reticulum signaling as a determinant of recombinant protein expression. Biotechnol. Bioeng. 2003;81:56–65. doi: 10.1002/bit.10445. [DOI] [PubMed] [Google Scholar]
- 38.Rutkowski D. T., Kaufman R. J. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Verkhratsky A., Petersen O. H. The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur. J. Pharmacol. 2002;447:141–154. doi: 10.1016/s0014-2999(02)01838-1. [DOI] [PubMed] [Google Scholar]
- 40.Ahner A., Brodsky J. L. Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 2004;14:474–478. doi: 10.1016/j.tcb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Kim P. S., Arvan P. Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: disorders of protein trafficking and the role of ER molecular chaperones. Endocr. Rev. 1998;19:173–202. doi: 10.1210/edrv.19.2.0327. [DOI] [PubMed] [Google Scholar]
- 42.Boedeker J. C., Doolittle M. H., White A. L. Differential effect of combined lipase deficiency (cld/cld) on human hepatic lipase and lipoprotein lipase secretion. J. Lipid Res. 2001;42:1858–1864. [PubMed] [Google Scholar]
- 43.Pieper G. M., Dondlinger L. Glucose elevations alter bradykinin-stimulated intracellular calcium accumulation in cultured endothelial cells. Cardiovasc. Res. 1997;34:169–178. doi: 10.1016/s0008-6363(97)00007-2. [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa T., Edelstein D., Du X. L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M. A., Beebe D., Oates P. J., Hammes H. P., et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature (London) 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 45.Graier W. F., Simecek S., Kukovetz W. R., Kostner G. M. High D-glucose-induced changes in endothelial Ca2+/EDRF signaling are due to generation of superoxide anions. Diabetes. 1996;45:1386–1395. doi: 10.2337/diab.45.10.1386. [DOI] [PubMed] [Google Scholar]
- 46.Graier W. F., Wascher T. C., Lackner L., Toplak H., Krejs G. J., Kukovetz W. R. Exposure to elevated D-glucose concentrations modulates vascular endothelial cell vasodilatory response. Diabetes. 1993;42:1497–1505. doi: 10.2337/diab.42.10.1497. [DOI] [PubMed] [Google Scholar]
- 47.Fleischhacker E., Esenabhalu V. E., Spitaler M., Holzmann S., Skrabal F., Koidl B., Kostner G. M., Graier W. F. Human diabetes is associated with hyperreactivity of vascular smooth muscle cells due to altered subcellular Ca2+ distribution. Diabetes. 1999;48:1323–1330. doi: 10.2337/diabetes.48.6.1323. [DOI] [PubMed] [Google Scholar]
- 48.Fleischhacker E., Esenabhalu V. E., Holzmann S., Skrabal F., Koidl B., Kostner G. M., Graier W. F. In human hypercholesterolemia increased reactivity of vascular smooth muscle cells is due to altered subcellular Ca2+ distribution. Atherosclerosis. 2000;149:33–42. doi: 10.1016/s0021-9150(99)00290-7. [DOI] [PubMed] [Google Scholar]