Abstract
Diminished muscular activity is associated with alterations of protein metabolism. The aim of this study was to evaluate the effect of short-term muscle inactivity on regulation of whole-body protein deposition during amino acid infusion to simulate an experimental postprandial state. We studied nine healthy young volunteers at the end of 14 day periods of strict bed rest and of controlled ambulation using a cross-over design. Subjects received a weight-maintaining diet containing 1 g protein kg−1 day−1. l[1-13C]leucine was used as a marker of whole-body protein kinetics in the postabsorptive state and during a 3 h infusion of an amino acid mixture (0.13 g amino acid (kg lean body mass)−1 h−1). In the postabsorptive state, bed rest decreased (P < 0.05) the rate of leucine disposal (Rd) to protein synthesis and tended to decrease leucine rate of appearance (Ra) from proteolysis, whereas the rate of leucine oxidation did not change significantly. Amino acid infusion increased leucine Rd to protein synthesis and oxidation and decreased leucine Ra from proteolysis in both the bed rest and ambulatory conditions. Changes from basal in leucine Rd to protein synthesis were lower (P < 0.05) during bed rest than those in the ambulatory period, whereas changes in leucine Ra from proteolysis and oxidation were not significantly different. During amino acid infusion, net leucine deposition into body protein was 8 ± 3% lower during bed rest than during the ambulatory phase. In conclusion, short-term bed rest leads to reduced stimulation of whole-body protein synthesis by amino acid administration. Results of this study were, in part, presented at the meeting, Experimental Biology, 2004, Washington DC.
In the physiological postabsorptive state, the balance between whole-body protein synthesis and degradation is negative. Such protein loss is immediately compensated in the postprandial state by a protein gain mediated by nutrient intake (Tessari et al. 1987). Thus, the efficiency of the mechanisms responsible for the regulation of protein synthesis and degradation in the postabsorptive and fed states appears to be crucial for maintaining lean body mass throughout the day, thereby avoiding protein wasting. Meal-induced protein gain is largely mediated by the postprandial rise in plasma amino acid concentrations that acutely stimulates muscle and whole-body protein synthesis (Rennie et al. 2002). This stimulatory effect of amino acid administration on muscle protein synthesis is enhanced by previous performance of physical exercise (Biolo et al. 1997).
Diminished muscular activity is associated with impairment of muscle function and loss of lean body mass (Biolo et al. 2003; di Prampero & Narici, 2003). However, assessment of protein kinetics in the postabsorptive state during experimental bed rest by stable isotopes of amino acids failed to detect significant protein loss at the muscle and whole-body levels (Shangraw et al. 1988; Ferrando et al. 1996; Lovejoy et al. 1999; Stein et al. 1999). Nonetheless, despite the importance of assessing the regulation of protein kinetics in the postprandial state, no study of the effects of amino acid/protein administration on protein kinetics during muscle unloading in humans has been published.
Within the frame of the ‘Short-Term Bed Rest Study–Integrated Physiology’ (STBR-IP) set up by the German Aerospace Institute (DLR) under the auspices of the European Space Agency we tested the hypothesis that a reduced stimulation of whole-body protein synthesis by amino acid administration represents a major mechanism for the bed rest-induced loss of lean body mass. Subjects were studied at the end of 14 day periods of bed rest or ambulatory conditions using a cross-over experimental design. This approach allowed strict control and monitoring of nutrient intake and levels of physical activity both during the bed rest and ambulatory periods.
Methods
Experimental design
Nine healthy, male, sedentary subjects (mean ± s.e.m.: age 24 ± 1 year; body mass index 23 ± 1 kg m−2) were recruited for a study investigating the effects of simulated microgravity on physiology. All subjects were physically active before admission to the Clinical Research Center at the DLR-German Aerospace Institute, Cologne, Germany. They gave their voluntary consent to participate in the study. Before enrolment, a thorough medical history was taken, and routine medical and laboratory analyses were used to exclude chronic diseases. None of the participants was on medication. The experiment was part of a comprehensive, international collaborative study, the Short-term Bed Rest – Integrated Physiology (STBR-IP) study, where specific examination periods were assigned to each study group. The experimental protocol was approved by the Ethical Committee of the ‘Ärztekammer Nordrhein’, Düsseldorf, Germany and conformed to the standards set by the Declaration of Helsinki (2002). The study protocol was divided into two phases. The first phase (July–August 2001) involved studying four subjects in ambulatory conditions and five subjects in bed rest conditions (6 deg head-down tilt) for a period of 14 days. Five months later, the second phase (February–March 2002) involved the subject group cross-over: the four patients previously studied in ambulatory conditions were studied in bed rest conditions (6 deg head-down tilt), and the five subjects studied during bed rest in the first phase were studied in ambulatory conditions. During the bed rest periods, all activities, including showering, were performed either in 6 deg head-down tilt bed rest or the horizontal position. During the ambulatory periods, the level of physical activity was defined and monitored. Each 14 day period was preceded by 9 days of adaptation in the DLR-Clinical Research Center. After both the bed rest and the ambulatory periods, the volunteers remained in the metabolic unit for an additional 3 days for post-bed rest testing. During the examination periods (adaptation, bed rest or ambulatory conditions and recovery) energy requirements were calculated for each individual according to the FAO/WHO equations (Lin et al. 2003). To maintain body weight, the energy content of the diets was reduced during the immobilization periods. Participants received a specifically prepared diet containing 1.4 or 1.1 times their basal metabolic rate during the ambulatory and bed rest periods, respectively. Ten per cent of the total kilocalories was added to account for dietary-induced thermogenesis (Aksnes et al. 1993). All subjects received 1 g protein (kg body weight)−1 (day)−1. The protein intake was identical each day. The fat content of the diet was planned to provide about 30% of the energy and included both saturated and polyunsaturated fatty acids. The remaining energy was supplied as carbohydrates. Daily intake of water, sodium, calcium and vitamin D was also defined and monitored during the two periods. For these nutrients, for which there was no experimental requirement, the German recommended dietary intake levels were used in this study. No caffeine, methylxanthine, or alcohol were allowed. Six meals were prepared daily, i.e. three main meals (breakfast, lunch, dinner) and three snacks. All food was weighed exactly for each participant, and participants were asked to consume the complete meal. The body composition of all subjects was measured by DEXA at the end of the adaptation period and at the beginning of the recovery period with a Hologic QDR-2000 (Waltham, MA, USA). Enhanced whole-body scans were analysed to calculate the lean tissue mass.
Infusion protocol
In the morning of the last day of the bed rest or ambulatory periods, after a 12 h overnight fast, a polyethylene catheter was inserted into an antecubital vein for infusion of all test substances. A second polyethylene catheter was inserted in a wrist vein of the opposite hand, which was heated to obtain arterialized venous blood. Blood and breath samples were taken before the start of isotope infusion to determine baseline natural enrichments of [1-13C]α-ketoisocaproic acid ([13C]KIC) in plasma and of 13CO2 in the expired air. Thereafter, a bolus injection (0.08 μmol kg−1) of [13C]sodium bicarbonate (Cambridge Isotope Laboratories, Andover, MA, USA) was intravenously administered followed by a primed (5.4 μmol kg−1) continuous (0.09 μmol kg−1 min−1) infusion of l[1-13C]leucine ([13C]leucine) (Cambridge Isotope Laboratories) which was continued throughout the 6 h study period (Wolfe, 1992; Biolo et al. 2002). After 160 min had been allowed for isotope equilibration, three blood and breath samples were obtained over 20 min to determine [13C]KIC enrichment and amino acid concentrations in the plasma and 13CO2 enrichment in the breath. Between 120 and 180 min, indirect calorimetry was performed to determine the rate of total CO2 using a ventilated hood system (MBM-200, Deltatrac, Datex, Finland), which is an open circuit computerized indirect calorimeter. Respiratory exchange measurements were recorded every minute. At 180 min, a primed (0.13 g (kg lean body mass (LBM))−1) constant (0.13 g (kg LBM)−1 h−1) intravenous infusion of an amino acid solution (Freamine III 8.5%, Clintec, Milano, Italy) was initiated and continued throughout the study for another 3 h. The priming dose was calibrated so as to rapidly attain steady-state plasma leucine concentrations (Wolfe, 1992; Biolo et al. 2002). The amino acid concentrations reported by the manufacturer (expressed as mg (100 ml)−1) were as follows: isoleucine 590; leucine 770; lysine 870; methionine 450; phenylalanine 480; threonine 340; tryptophan 130; valine 560; alanine 600, arginine 810; histidine 240; serine 500; cysteine 18; glycine 1190. These values of amino acid concentrations in the infused solution were confirmed by high-pressure liquid chromatography (Beckman Instruments Inc., San Ramon, CA, USA) (Biolo et al. 2002). The analytical method used (Biolo et al. 2002) did not allow the measurement of proline, cysteine and tryptophan concentrations. The infusion rates of the unlabelled leucine were 1.46 ± 0.04 and 1.49 ± 0.03 μmol min−1 kg−1 during the ambulatory and bed rest phases, respectively.
Analysis
Amino acid concentrations in plasma were determined by high-pressure liquid chromatography as previously described (Biolo et al. 2002). For determination of [13C]KIC isotopic enrichment (Biolo et al. 2002), plasma was deproteinated with ethanol, reacted with p-phenylendiamine (Sigma Chemical Co, St Louis, MO, USA) and subsequently with N,O-bis(trimethylsilyl)-trifluoroacetamide with 1% trimethylchlorosilane (Pierce Chemical, Rockford, IL, USA) to form the silylquinoxalinol derivative of KIC. Gas chromatography mass spectrometry (GCMS) analyses (Agilent – HP 5973 Mass Spectrometer, Albertville, MN, USA) were performed using electron impact ionization and monitoring m/z at 232 and 233. 13CO2 enrichments in the breath samples were determined by isotope ratio mass spectrometry (Delta S, Finnigan, MAT Bremen, Germany). Isotopic enrichments were expressed as tracer/tracee ratios (Wolfe, 1992; Biolo et al. 2002). The tracer/tracee ratio was calculated as the difference between isotopic abundance of sample and background (Wolfe, 1992; Biolo et al. 2002).
Calculations
Estimates of whole-body leucine kinetics were made at isotopic steady state, effectively attained at the end of each study period. Mean values of [13C]KIC and 13CO2 enrichments and of total CO2 production in each study period were used for data calculation. In the postabsorptive state, rate of appearance (Ra) of leucine is the result of amino acid release from proteolysis. Intracellular leucine Ra from proteolysis (μmol (kg min)−1) was calculated according to the reciprocal pool model (Wolfe, 1992; Biolo et al. 2002), i.e. by dividing the [13C]leucine infusion rate by the plasma [13C]KIC enrichment. KIC is the leucine deamination product and, during labelled leucine infusion, provides a direct estimation of the intracellular leucine enrichment (Wolfe, 1992). To calculate the rate of leucine oxidation, the rate of 13CO2 production in the expired air was determined by multiplying 13CO2 enrichment in the breath and total CO2 production (as determined by indirect calorimetry). This figure was divided by a correction factor of 0.74 in the postabsorptive state and of 0.84 during amino acid infusion, accounting for the incomplete recovery of CO2 in breath (Leijssen & Elia, 1996). We assumed that bed rest did not affect CO2 recovery in the expired air. Leucine oxidation was then calculated by dividing 13CO2 production by plasma [13C]KIC enrichment. The rate of leucine disposal (Rd) for protein synthesis in the postabsorptive state was calculated from the difference between the rate of appearance of leucine and its oxidation. During amino acid infusion the rate of total leucine Ra is the sum of leucine Ra from proteolysis plus the rate of leucine infusion. Thus, leucine Ra from proteolysis was calculated by subtracting the leucine infusion rate from the rate of total leucine Ra calculated according to the reciprocal pool model, as described above. During amino acid infusion, the rate of net leucine deposition into body protein was calculated from the difference between leucine Rd to protein synthesis and Ra from proteolysis.
Statistical analysis
All data were expressed as means ± s.e.m. Results in the four different experimental conditions (ambulatory postabsorptive state, ambulatory hyperaminoacidaemia, bed rest postabsorptive state, bed rest hyperaminoacidaemia) were compared by analysis of variance with a randomized block design followed by a Duncan test. Amino acid-mediated changes from the postabsorptive state in the ambulatory and bed rest conditions were compared using Student's paired t test. P values ≤ 0.05 were taken as indicating significant differences.
Results
Body weight did not change significantly during the bed rest (−0.30 ± 0.28 kg) or ambulatory (−0.14 ± 0.34 kg) periods. Mean values of lean body mass were not significantly different at the beginning (59.2 ± 1.2 kg) and end (58.9 ± 1.2 kg) of the bed rest period, nor did they change significantly during the ambulatory period: 58.9 ± 1.1 kg at the beginning and 59.0 ± 1.1 kg at the end of the period. Table 1 shows plasma amino acid concentrations at the end of the ambulatory and bed rest periods in the postabsorptive state and during amino acid infusions. In the postabsorptive state, plasma concentrations of the branched chain amino acids leucine, valine and isoleucine were greater during bed rest than in the ambulatory condition. In contrast, the alanine concentration was lower during bed rest. Intravenous infusion of the amino acid mixture resulted in variable increments in the plasma concentrations of all the infused amino acids. Increments from the postabsorptive values varied according to the individual amino acid infusion rates and pool sizes but they were similar in the ambulatory and bed rest conditions. The plasma concentrations of amino acids that were not included in the infused mixture did not change significantly, with the exception of asparagine, which decreased by about 12%, and glutamine, which increased by about 5%. Following the infusion, the plasma amino acid concentrations were similar in the ambulatory and bed rest conditions. The results of indirect calorimetry indicated that, in the postabsorptive state, the values for oxygen consumption were not significantly different in the bed rest and ambulatory phases (86 ± 1 and 88 ± 2 μmol min−1 (kg LBM)−1, respectively). Postabsorptive rates of CO2 excretion were also similar in the bed rest and ambulatory phases (73 ± 1 and 75 ± 2 μmol min−1 (kg LBM)−1, respectively). Intravenous infusion of the amino acid mixture resulted in similar increments in oxygen consumption and CO2 production in the bed rest and ambulatory phases. During hyperaminoacidaemia, the rates of oxygen consumption were 96 ± 3 and 97 ± 4 μmol min−1 (kg LBM)−1, in the bed rest and ambulatory phases, respectively, whereas the rates of CO2 production were 79 ± 2 and 83 ± 2 μmol min−1 (kg LBM)−1 in the bed rest and ambulatory phases, respectively.
Table 1.
Plasma amino acid concentrations at the end of the ambulatory and bed rest periods in the basal postabsorptive state and during amino acid (AA) infusion
| Ambulatory | Bed rest | |||
|---|---|---|---|---|
| Basal | AA | Basal | AA | |
| Aspartate | 4 ± 0.3 | 4 ± 0.2 | 4 ± 0.3 | 4 ± 0.2 |
| Glutamate | 54 ± 4 | 59 ± 5 | 52 ± 5 | 56 ± 5 |
| Asparagine | 50 ± 3 | 43 ± 2* | 50 ± 2 | 44 ± 2* |
| Serine | 107 ± 5 | 223 ± 4* | 111 ± 4 | 234 ± 5* |
| Glutamine | 636 ± 18 | 656 ± 20* | 607 ± 12 | 650 ± 11* |
| Histidine | 96 ± 4 | 143 ± 7* | 95 ± 4 | 146 ± 6* |
| Glycine | 213 ± 7 | 556 ± 20* | 220 ± 8 | 578 ± 19* |
| Threonine | 142 ± 6 | 230 ± 6* | 140 ± 5 | 230 ± 5* |
| Arginine | 83 ± 4 | 215 ± 14* | 76 ± 5 | 207 ± 11* |
| Alanine | 301 ± 21 | 423 ± 23* | 264 ± 18† | 414 ± 23* |
| Tyrosine | 60 ± 2 | 55 ± 2* | 60 ± 3 | 58 ± 2 |
| Methionine | 24 ± 1 | 117 ± 5* | 25 ± 1 | 115 ± 5* |
| Valine | 226 ± 9 | 476 ± 20* | 240 ± 10† | 510 ± 21* |
| Phenylalanine | 65 ± 2 | 147 ± 4* | 70 ± 2 | 152 ± 6* |
| Isoleucine | 58 ± 3 | 215 ± 11* | 64 ± 4† | 225 ± 10* |
| Leucine | 140 ± 5 | 335 ± 15* | 153 ± 6† | 356 ± 15* |
| Lysine | 209 ± 16 | 358 ± 27* | 204 ± 17 | 356 ± 23* |
| Branched chain amino acids | 424 ± 16 | 1026 ± 45* | 457 ± 20† | 1091 ± 45* |
| Total amino acids | 2511 ± 58 | 4302 ± 124* | 2480 ± 57 | 4387 ± 114* |
Units are μmol l−1.
P < 0.05 AA versus basal.
P < 0.05, bed rest versus ambulatory condition.
Plasma [13C]KIC tracer/tracee ratios (Fig. 1) and breath 13CO2 tracer/tracee ratios were at steady state in the basal postabsorptive period and at the end of amino acid infusion. Table 2 shows the results of intracellular whole-body protein kinetics as determined by the l[1–13C]leucine tracer data and the reciprocal pool model. In the basal postabsorptive state, intracellular Ra from proteolysis tended to be lower (P < 0.10) during bed rest than in the ambulatory condition. Leucine oxidation was similar in the bed rest and ambulatory periods. In contrast, non-oxidative leucine disposal, an index of whole-body protein synthesis, was 6 ± 2% lower during bed rest. Following amino acid infusion, leucine Ra from proteolysis was significantly suppressed during bed rest and in the ambulatory condition. Leucine oxidation significantly increased in both conditions. Leucine utilization for protein synthesis increased by 35 ± 2% in the ambulatory condition, whereas it increased only by 30 ± 2% (P < 0.05) during bed rest. During amino acid infusion, the rate of net protein deposition, i.e. protein synthesis minus protein degradation, was 8 ± 3% lower during bed rest than in the ambulatory condition (Fig. 2).
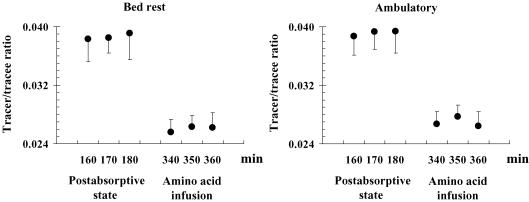
Figure 1. Plasma tracer/tracee ratios at steady-state.
Plasma [13C]KIC tracer/tracee ratios during primed-continuous l[1-13C]leucine infusion in the basal postabsorptive period (min 160, 170 and 180) and at the end of amino acid infusion (min 340, 350 and 360) in the bed rest and ambulatory phases.
Table 2.
Effects of amino acid infusion (AA) on whole-body intracellular protein kinetics at the end of the ambulatory and bed rest periods
| Leucine Ra from proteolysis | Leucine Rd to oxidation | Leucine Rd to protein synthesis | ||
|---|---|---|---|---|
| Ambulatory | Basal | 2.47 ± 0.05 | 0.23 ± 0.01 | 2.24 ± 0.05 |
| AA | 2.11 ± 0.07* | 0.54 ± 0.02* | 3.03 ± 0.08* | |
| Δ | −0.36 ± 0.05 | 0.31 ± 0.02 | 0.79 ± 0.05 | |
| Bed rest | Basal | 2.36 ± 0.05† | 0.25 ± 0.01 | 2.11 ± 0.04‡ |
| AA | 1.89 ± 0.06*‡ | 0.64 ± 0.04*† | 2.73 ± 0.07*‡ | |
| Δ | −0.47 ± 0.04 | 0.38 ± 0.04 | 0.63 ± 0.04‡ |
Units are μmol (kg LBM)−1 min−1. Ra, rate of appearance. Rd, rate of disposal Δ, delta changes from basal.
P < 0.05, AA versus basal.
P < 0.10, Bed rest versus ambulatory condition.
P < 0.05, Bed rest versus ambulatory condition.
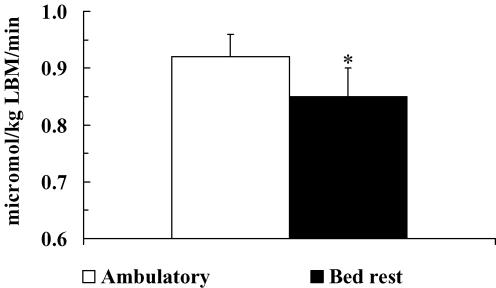
Figure 2. Leucine deposition into protein.
Rates of net leucine deposition (i.e. Rd to protein synthesis minus Ra from proteolysis) into body protein during amino acid infusion in ambulatory and bed rest conditions. *P < 0.05, bed rest versus ambulatory.
Discussion
We have assessed the response of whole-body protein kinetics to 14 days of bed rest in the postabsorptive state and during hyperaminoacidaemia in normal young volunteers using a cross-over experimental design. This approach allowed optimal control and monitoring of dietary intake and of levels of physical activity in both the ambulatory and the bed rest study phases. Subjects received a diet tailored for energy intake that maintained body weight at constant values in both study phases. Daily protein intake was fixed at 1 g (kg body weight−1). The results showed that an impaired amino acid-mediated stimulation of whole-body protein synthesis is a major catabolic mechanism for the effect of short-term bed rest on protein metabolism. Thus, the process of body protein synthesis become resistant to the anabolic stimulus of feeding during muscle inactivity, whereas protein balance is maintained in the fasted state, despite a reduction of whole-body protein turnover.
Our data in the postabsorptive state are in excellent agreement with previous human studies assessing changes in protein kinetics during bed rest using stable isotopes of amino acids. Ferrando et al. (1996) showed that after 14 days of bed rest postabsorptive values of muscle protein synthesis were decreased, while muscle protein balance did not significantly change. Also, at the whole-body level, short-term bed rest studies have demonstrated no change of protein balance in the postabsorptive state (Shangraw et al. 1988; Stuart et al. 1990; Lovejoy et al. 1999; Stein et al. 1999). We investigated for the first time the regulation of whole-body protein kinetics by hyperaminoacidaemia during bed rest. Amino acids were given intravenously for 3 h to simulate physiological postprandial conditions in the metabolic steady state. We have shown that, at the same level of hyperaminoacidaemia, net protein deposition was 8% lower during bed rest than in ambulatory conditions. This alteration was completely accounted for by less efficient stimulation of protein synthesis, while protein degradation was normally suppressed by amino acid infusion during bed rest.
Human studies clearly indicate that decreased protein synthesis is the main protein catabolic mechanism associated with muscle inactivity during bed rest (Ferrando et al. 1996) and in a microgravity environment (Stein et al. 1999). Stein et al. (1999) demonstrated that a 3 month space flight was associated with a 45% decrease in whole-body protein synthesis when compared with pre-flight rates. In contrast to protein synthesis, the rates of proteolysis appeared to be mostly unaffected or even slightly decreased by muscle unloading both at muscle and whole-body levels (Shangraw et al. 1988; Stuart et al. 1990; Ferrando et al. 1996; Lovejoy et al. 1999; Stein et al. 1999). Such decreased protein turnover associated with muscle unloading could contribute to the negative effects of immobility by delaying removal of defective proteins and impairing regulation of enzymatic systems and other metabolic processes. When acceleration of proteolysis was observed during short-term space flights (Stein & Schluter, 1997), this alteration was associated with the effect of stress mediators, e.g. cortisol (Ferrando et al. 1999).
We have recently shown that the protein anabolic effect of hyperaminoacidaemia was enhanced by previous performance of resistance exercise (Biolo et al. 1997). Taking this observation together with the results of the present study, we suggest that amino acid administration and, possibly, protein intake may interact with any level of physical activity by increasing stimulation of tissue protein synthesis in the case of exercise or by decreasing stimulation of tissue protein synthesis in the case of muscle inactivity, possibly at the level of skeletal muscle. The mechanisms of such an interaction between amino acid availability and levels of physical activity are unclear. They may include modulation of insulin sensitivity, peripheral blood flow and rates of amino acid delivery to tissues by exercise or muscle inactivity (Ferrando et al. 1996; Biolo et al. 1997).
In agreement with data obtained during short-term human space flight (Stein & Schluter, 1999), in our study we observed a selective increase in the plasma concentrations of the branched chain amino acids leucine, valine and isoleucine in the postabsorptive state during bed rest. Mechanisms leading to such an altered amino acid pattern following muscle unloading are unclear because the rate of leucine oxidation was not decreased during bed rest in our study. Nonetheless, due to the potential role of leucine in the regulation of tissue protein synthesis (Kimball & Jefferson, 2002), the bed rest-induced inhibition of protein synthesis appeared even greater when normalized for the prevailing value of plasma leucine concentration.
In the present study energy intake was carefully tailored to the resting energy expenditure of individual subjects and to the level of physical activity. Achievement of an energy balance throughout the experimental periods was demonstrated by the fact that the body weight and fat mass of the subjects did not change significantly during either the bed rest or the ambulatory periods. Despite the fact that energy balance was achieved both in the bed rest and the ambulatory phases, the difference in absolute energy content between the two groups (i.e. 30% of the basal metabolic rate) could have contributed to decreasing protein turnover rates during bed rest without affecting protein balance.
In agreement with the results of previous, diet-controlled, short-term bed rest studies (Ferrando et al. 1999; Lovejoy et al. 1999; Blanc et al. 2000), the whole-body lean mass of our subjects, as determined by DEXA, did not change significantly following 14 days of bed rest. Unfortunately, as a result of technical problems, we could not determine regional changes in lean body mass by DEXA. Thus, we cannot rule out the possibility that small changes in volume of selected muscles were not detected by our whole-body DEXA determinations, as previously shown in short-term bed rest using different techniques (Stuart et al. 1990). Nonetheless, on the basis of our leucine kinetic data we may predict a loss of lean body mass during the 14 days of bed rest of our study. We found that during amino acid infusion 57 ± 3 or 63 ± 2% of the administered leucine was incorporated into body protein during bed rest or the ambulatory phase, respectively. When these figures are applied to the level of daily protein intake adopted in our study (i.e. 1 g (kg body weight)−1 for both study phases) we may predict a negative difference between cumulative postprandial protein anabolism during the 14 days of bed rest and the ambulatory phase of approximately 67 ± 27 g of body protein, which was not compensated for in the postabsorptive state. Such a change is not easily detectable at the whole-body level using DEXA.
Daily protein intake was kept constant at the level of 1 g (kg body weight)−1 in both the bed rest and ambulatory phases. In a previous study, Stuart et al. showed that increasing the protein content in the diet from 0.6 to 1.0 g protein kg−1 day−1 prevented the nitrogen loss and the decrease in whole-body protein turnover associated with 7 days of bed rest at the low protein intake (Stuart et al. 1990). In the present study, 14 days of bed rest at a protein intake level of 1.0 g kg−1 day−1 depressed whole-body protein turnover in the postabsorptive state and impaired the anabolic effect of amino acid administration. Taken together, these results suggest the hypothesis that during short-term inactivity a greater than normal protein intake could be required to maintain normal protein turnover in the postabsorptive state and to achieve the same postprandial anabolic effect as are observed in subjects undertaking a normal level of physical activity.
The calculation of leucine oxidation requires a correction for the fact that not all labelled CO2 produced from [1-13C]leucine at the cellular level is excreted in the breath. Recovery in the breath of labelled CO2 increases during feeding and exercise proportionally to oxygen consumption (Wolfe, 1992; Leijssen & Elia, 1996). Labelled CO2 can be stored in bone or contribute to various metabolic pathways, including isotopic exchange in the trycarboxylic acid pool. The general experimental design of the STBR-IP did not allow us to determine the effect of bed rest and amino acid infusion on labelled CO2 recovery during labelled bicarbonate infusion on separate study days. Thus, we have assumed constant correction factors in the ambulatory and bed rest phases of 0.74 in the postabsorptive state and of 0.84 during amino acid infusion (Leijssen & Elia, 1996). Several lines of evidence indicate that bed rest should not have significantly affected the bicarbonate recovery factor. First, it has been shown that there is a direct relationship between metabolic rate and bicarbonate recovery in both physiological and pathological conditions (Wolfe, 1992). In our study, values of oxygen consumption in the postabsorptive state and during amino acid infusion were not significantly different in the ambulatory and bed rest phases and exhibited similar increments during hyperaminoacidaemia in both phases. We may predict therefore similar bicarbonate recovery in the two conditions. Second, values of CO2 production in the postabsorptive state and during amino acid infusion were also not significantly different in the ambulatory and bed rest phases, suggesting that the bicarbonate pool was similar in the two conditions. Third, evidence indicates that labelled CO2 recovery increases during exercise. In our study, assessment of leucine kinetics was performed with subjects lying in bed, even during the ambulatory phase. Nonetheless, in the case of changes in bicarbonate recovery in the bed rest phase, such changes would be expected to be in the opposite direction to changes during exercise. In the case of a decrease in bicarbonate recovery during bed rest, amino acid-mediated stimulation of whole-body leucine Rd to protein synthesis would have been even greater in the ambulatory phase than during bed rest. Finally, we have calculated the impact of a 15% increase in bicarbonate recovery during bed rest both in the postabsorptive state (i.e. from 0.74 to 0.87) and during hyperaminoacidaemia (i.e. from 0.84 to 0.97) on amino acid-mediated stimulation of whole-body leucine Rd to protein synthesis. Even assuming such large difference in bicarbonate kinetics between the two conditions, the amino acid-mediated stimulation of whole-body leucine Rd to protein synthesis would still have been 13 ± 5% greater (P = 0.04) in the ambulatory phase than during bed rest.
In conclusion, we have investigated the regulation of whole-body protein kinetics in the postabsorptive state and in an experimentally controlled postprandial state during short-term bed rest. Results indicated that, despite a reduction of whole-body protein turnover, protein balance was maintained in the fasted state. In contrast, a blunted amino acid-induced stimulation of protein synthesis appeared to be the main catabolic mechanism associated with short-term inactivity.
Acknowledgments
We thank the volunteers who gave time and effort to ensure the success of this project. We acknowledge the excellent technical assistance of Mrs Anna De Santis and Mrs Mariella Sturma. We also thank all scientific and technical staff of the Clinical Research Center at the DLR-German Aerospace Institute, Cologne, Germany. We acknowledge the invaluable support of Dr B. Elmann-Larsen (Life Science Unit, ESA-ESTEC). This study was supported by grants from the Italian Space Agency (ASI) and the Italian Ministry of Education, University and Research (MIUR) (COFIN 2001). The general experimental design was supported by grants from the European Space Agency (ESA) and from the German Space Agency (DLR).
References
- Aksnes AK, Brundin T, Hjeltnes N, Maehlum S, Wahren J. Meal-induced rise in resting energy expenditure in patients with complete cervical spinal cord lesions. Paraplegia. 1993;31:462–472. doi: 10.1038/sc.1993.75. [DOI] [PubMed] [Google Scholar]
- Biolo G, Ciocchi B, Bosutti A, Situlin R, Toigo G, Guarnieri G. Pentoxifylline acutely reduces protein catabolism in chronically uremic patients. Am J Kidney Dis. 2002;40:1162–1172. doi: 10.1053/ajkd.2002.36864. [DOI] [PubMed] [Google Scholar]
- Biolo G, Heer M, Narici M, Strollo F. Microgravity as a model of ageing. Curr Opin Clin Nutr Metab Care. 2003;6:31–40. doi: 10.1097/00075197-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Blanc S, Normand S, Pachiaudi C, Fortrat JO, Laville M, Gharib C. Fuel homeostasis during physical inactivity induced by bed rest. J Clin Endocrinol Metab. 2000;85:2223–2233. doi: 10.1210/jcem.85.6.6617. [DOI] [PubMed] [Google Scholar]
- di Prampero PE, Narici MV. Muscles in microgravity: from fibres to human motion. J Biomech. 2003;36:403–412. doi: 10.1016/s0021-9290(02)00418-9. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Stuart CA, Sheffield-Moore M, Wolfe RR. Inactivity amplifies the catabolic response of skeletal muscle to cortisol. J Clin Endocrinol Metab. 1999;84:3515–3521. doi: 10.1210/jcem.84.10.6046. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Control of protein synthesis by amino acid availability. Curr Opin Clin Nutr Metab Care. 2002;5:63–67. doi: 10.1097/00075197-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Leijssen DP, Elia M. Recovery of 13CO2 and 14CO2 in human bicarbonate studies: a critical review with original data. Clin Sci. 1996;91:665–677. doi: 10.1042/cs0910665. [DOI] [PubMed] [Google Scholar]
- Lin PH, Proschan MA, Bray GA, Fernandez CP, Hoben K, Most-Windhauser M, et al. DASH Collaborative Research Group. Estimation of energy requirements in a controlled feeding trial. Am J Clin Nutr. 2003;77:639–345. doi: 10.1093/ajcn/77.3.639. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Smith SR, Zachwieja JJ, Bray GA, Windhauser MM, Wickersham PJ, et al. Low-dose T(3) improves the bed rest model of simulated weightlessness in men and women. Am J Physiol. 1999;277:E370–E379. doi: 10.1152/ajpendo.1999.277.2.E370. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Bohe J, Wolfe RR. Latency, duration and dose–response relationships of amino acid effects on human muscle protein synthesis. J Nutr. 2002;132:3225S–327S. doi: 10.1093/jn/131.10.3225S. [DOI] [PubMed] [Google Scholar]
- Shangraw RE, Stuart CA, Prince MJ, Peters EJ, Wolfe RR. Insulin responsiveness of protein metabolism in vivo following bedrest in humans. Am J Physiol. 1988;255:E548–E558. doi: 10.1152/ajpendo.1988.255.4.E548. [DOI] [PubMed] [Google Scholar]
- Stein TP, Leskiw MJ, Schluter MD, Donaldson MR, Larina I. Protein kinetics during and after long-duration spaceflight on MIR. Am J Physiol. 1999;276:E1014–E1021. doi: 10.1152/ajpendo.1999.276.6.e1014. [DOI] [PubMed] [Google Scholar]
- Stein TP, Schluter MD. Human skeletal muscle protein breakdown during spaceflight. Am J Physiol. 1997;272:E688–E695. doi: 10.1152/ajpendo.1997.272.4.E688. [DOI] [PubMed] [Google Scholar]
- Stein TP, Schluter MD. Plasma amino acids during human spaceflight. Aviat Space Environ Med. 1999;70:250–255. [PubMed] [Google Scholar]
- Stein TP, Schluter MD, Leskiw MJ, Boden G. Attenuation of the protein wasting associated with bed rest by branched-chain amino acids. Nutrition. 1999;15:656–660. doi: 10.1016/s0899-9007(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Stuart CA, Shangraw RE, Peters EJ, Wolfe RR. Effect of dietary protein on bed-rest-related changes in whole-body-protein synthesis. Am J Clin Nutr. 1990;52:509–514. doi: 10.1093/ajcn/52.3.509. [DOI] [PubMed] [Google Scholar]
- Tessari P, Inchiostro S, Biolo G, Trevisan R, Fantin G, Marescotti MC, et al. Differential effects of hyperinsulinemia and hyperaminoacidemia on leucine-carbon metabolism in vivo. Evidence for distinct mechanisms in regulation of net amino acid deposition. J Clin Invest. 1987;79:1062–1069. doi: 10.1172/JCI112919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, Inc.; 1992. [Google Scholar]