Abstract
Hantaviruses, causing hemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS), are known to be sensitive to nitric oxide (NO) and to pretreatment with type I and II interferons (alpha interferon [IFN-α]/IFN-β and IFN-γ, respectively). Elevated serum levels of NO and IFN-γ have been observed in HFRS patients, but little is known regarding the systemic levels of other IFNs and the possible effects of hantaviruses on innate antiviral immune responses. In Puumala virus-infected HFRS patients (n = 18), we report that the levels of IFN-α and IFN-β are similar, whereas the level of IFN-λ (type III IFN) is significantly decreased, during acute (day of hospitalization) compared to the convalescent phase. The possible antiviral effects of IFN-λ on the prototypic hantavirus Hantaan virus (HTNV) replication was then investigated. Pretreatment of A549 cells with IFN-λ alone inhibited HTNV replication, and IFN-λ combined with IFN-γ induced additive antiviral effects. We then studied the effect of postinfection treatment with IFNs. Interestingly, an already-established HTNV infection was insensitive to subsequent IFN-α, -β, -γ, and -λ stimulation, and HTNV-infected cells produced less NO compared to noninfected cells when stimulated with IFN-γ and IL-1β. Furthermore, less phosphorylated STAT1 after IFN treatment was observed in the nuclei of infected cells than in those of noninfected cells. The results suggest that hantavirus can interfere with the activation of antiviral innate immune responses in patients and inhibit the antiviral effects of all IFNs. We believe that future studies addressing the mechanisms by which hantaviruses interfere with the activation and shaping of immune responses may bring more knowledge regarding HFRS and HCPS pathogenesis.
Hantaviruses, members of the Bunyaviridae family, are emerging pathogens that cause two zoonotic human diseases: hemorrhagic fever with renal syndrome (HFRS) in Europe and Asia and hantavirus cardiopulmonary syndrome (HCPS) on the American continent, with mortality up to 40% depending on the specific hantavirus (33, 41). Hantavirus infection per se is not cytopathogenic, and it has been suggested that HFRS and HCPS are caused rather by the immune responses raised during infection than by the virus itself (19, 39).
Type I interferons (IFNs) (consisting of IFN-β and of at least 13 different IFN-αs in humans) and type II IFN (IFN-γ) have long been known to have antiviral effects against numerous viruses (16, 31), including hantaviruses (38). Recently, type III IFNs (IFN-λ1, -λ2, and -λ3, also called interleukin-29 [IL-29], -28A, and -28B, respectively) were discovered and also shown to have antiviral capacities (23, 34).
Hantaviruses are sensitive to pretreatment of cells with nitric oxide (NO), IFN-α, -β, and -γ, and antiviral proteins such as the myxovirus resistance A protein (MxA) (11, 18, 22, 38). These observations suggest that innate immune responses play an important role in controlling hantavirus infection. It is not known whether or how infected cells recognize hantavirus replication or what signaling pathways might be used to activate innate immune responses. However, it was recently shown that pathogenic hantaviruses inhibit retinoic acid-inducible gene I (RIG-I)-mediated induction of type I IFNs in vitro (1), suggesting that hantaviruses can interfere with RIG-I-mediated IFN production.
The levels of IFN-γ and NO in serum are known to be elevated during hantavirus infections (9, 26), showing that at least parts of the antiviral immune defense are activated in HFRS and HCPS patients. IFN-γ is produced mainly by cells of the adaptive immune system, and we have shown that hantavirus infection per se does not induce production of NO (22), suggesting that the elevated NO production in patients is induced in cells stimulated by IFN-γ alone or in combination with other cytokines (5). If and to what extent type I and type III IFNs are produced by hantavirus-infected cells in patients has not been reported.
Numerous pathogenic viruses can inhibit the activation of type I IFN production and thereby evade innate immune responses (16). Furthermore, adenovirus and African swine fever virus interfere with inducible NO synthase (iNOS) production, thereby decreasing the amount of NO produced, showing that certain viruses can suppress also NO production (6, 15).
Hemorrhagic fever patients suffering from Ebola virus and Junin virus infections have elevated levels of IFN-α in serum (14, 25, 42). In vitro studies have shown that pathogenic hantaviruses induce a very weak type I IFN response in infected cells (1, 13, 28, 30). Based on these reports, we decided to analyze the levels of IFN-α, -β, and -λ in serum from patients with nephropathia epidemica, a form of HFRS caused by Puumala hantavirus (PUUV) (41). We further studied the pre- and postinfectious effect of IFN-α, -β, -γ, and -λ against hantavirus replication in vitro. We have previously shown that endogenously produced NO inhibits Hantaan hantavirus (HTNV) replication in Vero E6 cells (22), and here we studied whether the NO production in IFN-γ- and IL-1β-stimulated cells could be inhibited by an ongoing HTNV infection. The activation of the antiviral state in cells by IFNs involves phosphorylation of signal transducer and activator of transcription-1 (STAT1) upon binding of IFNs to their specific receptors (2, 16, 23, 31), and we therefore also studied whether an established HTNV infection could interfere with STAT1 phosphorylation in cells stimulated with IFNs.
MATERIALS AND METHODS
Patient samples.
Serum was collected from PUUV-infected patients hospitalized at the Department of Infectious Disease at Umeå University Hospital in Umeå, Sweden. Samples, obtained from 21 patients with typical clinical symptoms of acute nephropathia epidemica (serologically verified by enzyme-linked immunosorbent assay [ELISA] and/or an immunofluorescence test for PUUV-specific immunoglobulin M [IgM] and IgG) were stored at −70°C until further analyzed. Because 3 of a total of 21 patients were previously found to be positive for human anti-mouse antibodies (21), these patients were excluded from the study to avoid false positive results in the IFN ELISAs.
Acute samples were drawn at the time of hospitalization. The patients arrived at the hospital 2 to 12 days after the initial onset of fever. Convalescent-phase serum was drawn 3 months after recovery from the disease from 14 of the patients, 2 months after recovery from 1 patient, 1 month after recovery for 2 patients, and 10 days after recovery from 1 patient.
This project was approved by the Research Ethics Committee of Umeå University.
Cells and viruses.
The human lung epithelial cell line A549 (American Type Culture Collection [ATCC] CLL-185; ATCC, Manassas, VA) and the African green monkey kidney epithelial cell line Vero E6 (Vero C1008; ATCC) were used for the in vitro studies. The cells were grown in minimal essential medium supplemented with 5% fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml.
HTNV strain 76-118 was used in the present study. Propagation and titration of HTNV was performed on Vero E6 cells as previously described (22).
All experiments and assays were performed in a biosafety level 3 facility.
ELISAs for the detection of IFNs.
Commercial ELISAs (Mabtech [Nacka, Sweden] for IFN-α; Fujirebio [Tokyo, Japan] for IFN-β; and R&D Systems [Minneapolis, MN] for IFN-λ) were used to analyze the amounts of different IFNs in serum.
Tests of antiviral effects of IFNs.
Recombinant human IFN-α, -β, -γ, -λ1, and -λ2 were purchased from Peprotech (London, England).
To test whether pretreatment with IFN-λ had antiviral effects against HTNV, A549 cells were treated with IFN-α, -β, -γ, -λ1, or -λ2 at different concentrations for 24 h before infection. One hour after infection with HTNV, fresh medium with IFNs was added to the cells, and 29 h later the supernatants were titrated for the concentration of produced virus as previously described (22).
To test for synergistic effects, A549 cells were treated with IFNs alone or with different combinations of IFNs for 24 h before infection. Cells were stimulated with 10 ng of each IFN/ml. At 1 h after infection with HTNV fresh medium with IFNs was added to the cells, and 29 h later the supernatants were titrated for the concentration of produced virus.
To test whether an already-established infection was sensitive to IFN treatment, A549 cells were infected with HTNV, and 24 h later cells were stimulated with 10 ng of IFN-α, -β, -γ, -λ1, or -λ2/ml. At 24 h after initial treatment, fresh medium with IFNs was added to infected cells, and supernatants were sampled and titrated for the amount of replicating virus at 10, 24, and 48 h after initial stimulation.
Medium without IFN was used as a control in these experiments.
Cytokine induced NO production.
IFN-γ, together with IL-1β, induces production of iNOS and subsequent NO production in Vero E6 cells (22). Confluent, 2-week-old Vero E6 cells were infected with HTNV and 30 h later stimulated with 10 ng of IL-1β (Peprotech)/ml and 20 ng of IFN-γ/ml. At 24 h after induction, the supernatants were collected, and the amount of nitrite determined by a Griess assay.
Griess assay.
NO rapidly reacts with oxygen to form nitrite and nitrate, its two stable end products (36). The production of NO was measured indirectly in cell culture supernatants by determination of the level of nitrite using the Griess assay. Supernatant samples, and sodium nitrite as a standard, were mixed with equal volumes of Griess reagents (1% sulfanilamide and 0.1% naphtylethylenediamide in 5% phosphoric acid), and the optical density at 540 nm was measured by spectrophotometry. The nitrite standard was diluted in the same medium as used for the samples.
LDH assay.
A lactate dehydrogenase (LDH) assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay; Promega, Madison, WI) was used to measure cytotoxicity according to the manufacturer's protocol.
Detection of STAT1 phosphorylation by immunofluorescence.
Cells were grown on coverslips and infected. At 30 h after infection, cells were treated with IFN, followed by fixation and/or permeabilization in ice-cold methanol-acetone (1:1) and blocking in phosphate-buffered saline containing 10% horse serum, 1% bovine serum albumin, and 0.02% NaN3. To permeabilize the nuclei, cells were incubated in 0.5% Nonidet P-40 (Sigma). After a rinse in TBS, coverslips were incubated with antiphosphorylated (Tyr-701) STAT1 rabbit polyclonal antibody (Cell Signaling Technology, Beverly, MA) and convalescent human anti-PUUV serum. Alexa Fluor 549 goat anti-rabbit IgG (Invitrogen) and fluorescein isothiocyanate-conjugated goat anti-human IgG (Sigma) were used as secondary antibodies. Nuclei were stained using Hoechst 33258 (Sigma).
RESULTS
The level of IFN-λ in serum is decreased in PUUV-infected patients.
We analyzed the levels of IFN-α, -β, and -λ in serum from 18 PUUV-infected patients drawn during the acute phase (first day of hospitalization) and the convalescent phase of infection.
The levels of serum IFN-α (10.4 ± 12.0 ng/ml during the acute phase and 10.9 ± 12.7 ng/ml during the convalescent phase) and IFN-β (81.3 ± 179.7 IU/ml during the acute phase and 84.9 ± 179.6 IU/ml during the convalescent phase) did not differ significantly between the acute and the convalescent phases (Wilcoxon signed-rank test; P = 0.23 for IFN-α and P = 0.87 for IFN-β). However, the levels of serum IFN-λ (3.6 ± 5.5 ng/ml during the acute phase and 4.2 ± 6.5 ng/ml during the convalescent phase) was significantly lower during the acute phase than during the convalescent phase (P = 0.039 [Wilcoxon signed-rank test]).
Three of the patients were negative for IFN-α, and eight were negative for IFN-β, during the acute as well as the convalescent phase. One patient showed the same level of IFN-α during the acute and the convalescent phase. Five of the patients showed higher levels of IFN-α, five showed higher levels of IFN-β, and three showed higher levels of IFN-λ during the acute phase. Nine showed lower levels of IFN-α, five showed lower levels of IFN-β, and fifteen showed lower levels of IFN-λ during the acute than during the convalescent phase.
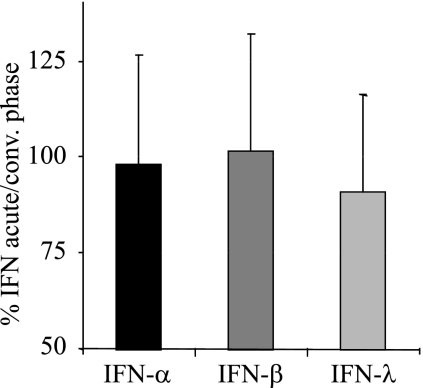
The percent IFN during the acute phase compared to the convalescent phase was calculated for all 18 patients. The mean percent IFN values for the individual patients observed during the acute phase compared to the convalescent phase were 97.9% ± 28.4% for IFN-α, 101.7% ± 30.2% for IFN-β, and 90.9% ± 25.3% for IFN-λ (Fig. 1), showing that the mean level of IFN-λ was 9.1% lower during the acute than during the convalescent phase (P < 0.05 for IFN-λ and P > 0.05 for IFN-α and IFN-β [chi-square test]).
FIG. 1.
The level of IFN-λ in serum is decreased during the acute phase of HFRS. The data shown are means plus the standard deviations (SD) of the percentages of IFN-α, IFN-β, and IFN-λ at acute phase compared to the convalescent phase for the individual patients.
IFN-λ has antiviral capacity against HTNV.
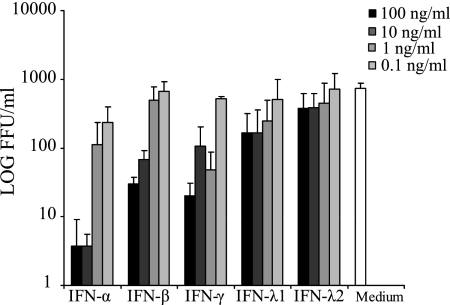
Stimulation of cells in vitro with IFN-α, -β, and -γ, followed by infection with hantaviruses, strongly inhibits hantavirus replication (38). To test whether the newly recognized type III IFNs also have antiviral capacities against hantaviruses and to be able to compare a possible effect with that of type I and type II IFNs, we incubated A549 cells in the presence of various concentrations of either IFN-α, -β, -γ, -λ1, or -λ2 for 24 h before infection with HTNV.
As expected, a dose-dependent inhibition of HTNV was observed for IFN-α, -β, and -γ (P < 0.001 for IFN-α, IFN-β, and IFN-γ [one-way analysis of variance]) (Fig. 2). Both 10 and 100 ng of IFN-λ/ml decreased the amount of progeny virus, with ca. 75 and 50% for IFN-λ1 and IFN-λ2, respectively, whereas lower levels of IFN-λ had less-pronounced effects, showing that type III IFN inhibits HTNV in a dose-dependent manner and that higher concentrations of IFN-λ than 10 ng/ml do not add to the antiviral effect (P < 0.001 for IFN-λ1, P = 0.102 for IFN-λ2 [one-way analysis of variance]) (Fig. 2).
FIG. 2.
IFN-λ inhibits HTNV replication in A549 cells. Titers of HTNV in supernatants sampled 30 h postinfection of A549 cells incubated in medium with or without various concentrations of IFN-α, -β, -γ, λ1, or -λ2. Cells were treated with IFNs for 24 h before infection with HTNV. The data represent the means plus the SD of three individual experiments.
Additive effect of IFN-λ and IFN-γ against HTNV.
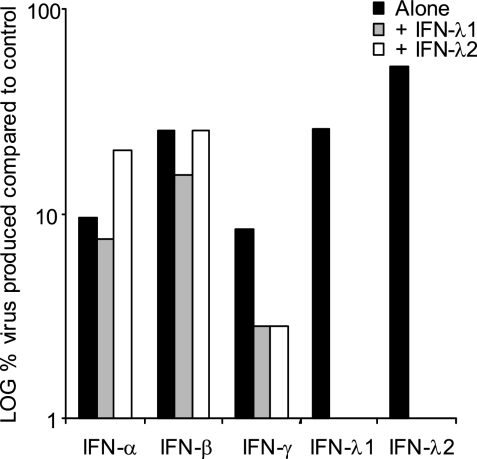
To test whether IFN-λ could enhance the antiviral effects of other IFNs, cells were pretreated with different IFNs alone and in combination before infection with HTNV.
An additive effect was observed when IFN-λ1 was combined with IFN-γ (Fig. 3). We did not observe a clear effect of IFN-λ1 on the antiviral effect of IFN-α or -β (Fig. 3), suggesting that IFN-λ does not enhance the antiviral effect of type I IFNs. Similar results were observed for IFN-λ2.
FIG. 3.
IFN-λ combined with IFN-γ has additive antiviral effect on HTNV replication. Cells were treated with IFN-α, -β, or -γ alone and with different combinations of IFN-α, -β, or -γ, -λ1, and -λ2 (10 ng of each IFN/ml) for 24 h and were then infected with HTNV. Supernatants were collected 30 h later and titrated. The data represent the medians of three individual experiment.
Established HTNV infection is insensitive to IFN treatment.
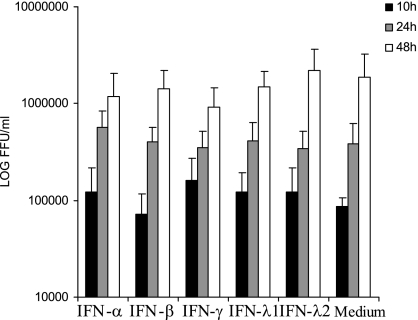
To test whether IFN-α, -β, -γ, and -λ had an antiviral effect against an established hantavirus infection, we infected A549 cells for 24 h with HTNV and then started treatment with 10 ng of IFN-α, -β, -γ, -λ1, or -λ2/ml. No significant inhibition of HTNV replication, as evidenced by the amount of progeny virus detected in supernatant, was observed for any of the IFN treatments compared to the medium control at 10, 24, or 48 h after the initiation of IFN treatment (P = 0.63, 0.68, and 0.44 for 10 h, 24 h, and 48 h after infection, respectively [Kruskal-Wallis test]) (Fig. 4), showing that established hantavirus infection is insensitive to type I, II, and III IFNs.
FIG. 4.
An established HTNV-infection is insensitive to IFN treatment. The titers of HTNV in supernatants sampled 10, 24, and 48 h after treatment with IFN-α, -β, -γ, -λ1, or -λ2 were determined. A549 cells were infected with HTNV for 24 h before treatment with 10 ng of the different IFNs/ml. The data represent means plus the SD of three individual experiments.
HTNV infection inhibits IFN-γ- and IL-1β-induced NO production.
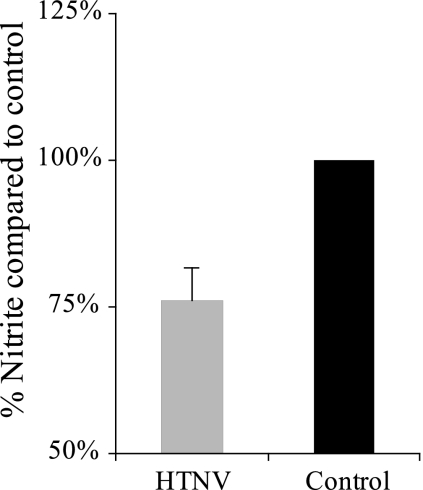
To test whether HTNV has an impact on NO production, Vero E6 cells were first infected with HTNV for 30 h and then stimulated with IFN-γ, together with IL-1β. At 24 h later, the amount of nitrite in the supernatant was measured. Approximately 25% less nitrite was detected in supernatants from infected cells than from noninfected cells, showing that hantavirus can inhibit NO production (t test, P < 0.001) (Fig. 5). No cytopathic effect was observed on the infected cells, and no differences in LDH content was observed in supernatants from infected cells compared to controls (data not shown), showing that the decrease in NO production was not due to increased cell death.
FIG. 5.
Established HTNV infection inhibits IFN-γ- and IL-1β-induced NO production. The concentration of nitrite was measured in supernatants from cells stimulated with IFN-γ and IL-1β. Vero E6 cells were infected with HTNV for 30 h before stimulation or left uninfected as a control. The data represent means plus the SD of three individual experiments.
HTNV interferes with the phosphorylation of STAT1 after stimulation with IFN-γ.
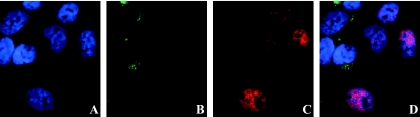
A549 cells infected with HTNV for 30 h were stimulated with 100 ng of IFN-γ/ml for 30 min, fixed, and stained for STAT1 phosphorylation and hantavirus proteins. We observed less phosphorylated STAT1 in the nuclei of infected cells than in those of noninfected neighboring cells (Fig. 6), suggesting that the decreased level of NO observed in IFN-γ- or IL-1β-stimulated cells is due to decreased signaling via STAT1.
FIG. 6.
Established HTNV infection inhibits IFN-γ-induced STAT1 phosphorylation. A549 cells were infected with HTNV and stimulated 30 h later with 100 ng of IFN-γ/ml for 30 min, fixed, and stained for DNA (A), HTNV (B), phosphorylated STAT1 (C), and merged (D). Infected cells (positive for HTNV antigen) show less nuclear phosphorylated STAT1 than do noninfected cells.
To quantify the amount of cells with phosphorylated STAT1, we infected Vero E6 cells with HTNV. At 30 h after infection the cells were stimulated with 20 ng of IFN-γ/ml for 20 min, fixed, and stained for phosphorylated STAT1, nuclei, and hantavirus proteins. Of the infected cells (n = 500), 40.4% were positive for nuclear phosphorylated STAT1, whereas 97.1% of the noninfected cells (n = 70) were positive for nuclear phosphorylated STAT1, showing that HTNV infection significantly decreased the level of STAT1 phosphorylation (P < 0.001 [Fisher exact test]).
DISCUSSION
Here we report that (i) the levels of serum IFN-α and IFN-β are not elevated and the level of serum IFN-λ is decreased in HFRS-patients; (ii) IFN-λ has antiviral capacity against subsequent hantavirus replication; (iii) an established HTNV infection is insensitive to treatment with IFN-α, -β, -γ, and -λ, and IFN-γ-induced NO production is reduced in HTNV-infected cells; and (iv) STAT1 phosphorylation after IFN treatment is inhibited in hantavirus-infected cells.
Whereas IFN-γ is produced by activated T cells and NK cells, the primary producers of IFN-α, -β, and -λ are virus-infected cells. We did not observe systemically elevated levels of IFN-α, -β, or -λ in PUUV-infected patients, indicating that infected cells in HFRS patients do not produce increased levels of type I or III IFNs. Low levels of circulating type I and III IFNs most probably enhance the spread of hantaviruses early in human hantavirus infection. Interestingly, a correlation between the level of viral RNA in plasma and the severity of disease has been reported for HCPS patients; the higher the viral load the higher the risk for severe disease (43), suggesting that the lack of elevated IFN production in HFRS patients observed here might contribute to disease.
This is the first time any member within the Bunyaviridae family has been shown to be sensitive to IFN-λ in vitro. As reported earlier for encephalomyocarditis virus (3), we also observed a stronger antiviral effect against HTNV replication by IFN-λ1 than by IFN-λ2. Not much is known regarding the possible synergistic effects of IFN-λ with other IFNs. The finding that IFN-λ has an additive antiviral effect on HTNV replication when combined with IFN-γ, but not with IFN-α or IFN-β, is of interest since IFN-γ is known to be elevated in serum during hantavirus infection (26). In line with our results, Ank et al. recently showed that there is no synergistic effect against EMCV or herpes simplex virus type 2 when IFN-α is combined with IFN-λ in vitro (3). Interestingly, these authors also detected elevated levels of IFN-γ in serum from herpes simplex virus type 2-infected mice pretreated with IFN-λ (3), suggesting that the antiviral role of IFN-λ might include stimulation of the immune system.
The prime _target of hantaviruses are endothelial cells, however, currently it is not known whether endothelial cells are responsive to IFN-λ. We therefore used A549 cells to study the effect of IFN-λ against HTNV, since this cell line has earlier been shown to elicit an antiviral state upon IFN-λ treatment (3).
The activation of the antiviral state in cells by IFN-α and IFN-β involves the phosphorylation of STATs. After the binding of IFN-α/β to the common IFN type I receptor, the Janus kinases JAK-1 and TYK-2 phosphorylate STAT1 and STAT2. Heterodimers of phosphorylated STAT1/STAT2 then recruit IFN regulatory factor 9, forming the IFN-stimulated gene factor 3 complex, which translocates into the nucleus and binds to IFN-stimulated response elements in the promoter region of IFN-stimulated genes encoding antiviral proteins such as MxA, protein kinase R, and 2′-5′-oligoadenylate synthetase (16, 31). In contrast, signaling via the IFN-γ receptor, after binding of IFN-γ, is dependent on JAK-1 and JAK-2 phosphorylation of STAT1 alone. Homodimers of phosphorylated STAT1 then enter the nuclei and bind to gamma-activated sequences in the promoter region of IFN-γ-inducible genes (31), e.g., the gene encoding iNOS (7). IFN-λ binds to the IL-28R/IL-10R complex, where the IL-28 receptor part is specific for IFN-λ (23, 34). The antiviral effects of type III IFNs are, like those of type I IFNs, believed to involve signaling via ISGF3, suggesting that most of the antiviral activity of IFN-λ is dependent on phosphorylation of STAT1 and STAT2 (2).
Spiropoulou et al. recently showed that Andes hantavirus and Prospect Hill hantavirus can inhibit STAT1 and STAT2 phosphorylation in Vero E6 cells after treatment with IFN-β, showing for the first time that hantavirus can interfere with innate antiviral IFN responses (35). Interestingly, this capacity was shown to depend on the viral envelope glycoprotein Gn and, although it is currently not known how Gn inhibits phosphorylation of STAT, the cytoplasmic tail of Gn contains a conserved and functional ITAM motif (12) that might be involved. Our findings that a previously established HTNV infection is insensitive to all antiviral IFNs and that STAT1 phosphorylation is inhibited in HTNV-infected cells after stimulation with IFN-γ suggest that the capacity to interfere with STAT phosphorylation, and thereby activation of genes encoding antiviral proteins is a general feature for many, if not all, hantaviruses.
How infected cells sense hantavirus infection is not exactly known. Consistent with our results from hantavirus-infected patients, only low levels of IFN production have been reported from in vitro studies of hantavirus-infected cells (13, 24, 30, 35). HTNV infection of human umbilical vein endothelial cells has been shown to induce a low level of IFN-β mRNA, but no increased levels of IFN-α or IFN-λ mRNA have been observed (13, 24). Andes hantavirus was recently shown to induce a weak IFN-β production in human pulmonary microvascular endothelial cells compared to the nonpathogenic Prospect Hill hantavirus (35). Furthermore, it was recently shown that early after inoculation, UV-inactivated hantavirus induced stronger IFN responses than replicating virus, suggesting that cellular recognition of hantaviruses is not dependent on viral replication and that a replicating virus can inhibit antiviral responses or escape recognition (30). NY-1 hantavirus can inhibit the production of IFNs via the inhibition of RIG-I and TBK-1-directed signaling (1). However, whether or not RIG-I recognizes hantavirus RNA remains to be shown, and it is currently unclear whether other mechanisms, such as Toll-like-receptor-mediated signaling, are involved in detecting hantaviruses.
It was recently reported that an established hantavirus infection in vitro is less sensitive to subsequent IFN-α treatment than to pretreatment (1). Our findings that established hantavirus infection is insensitive not only to type I IFN but also to type II and -III IFNs and that systemically elevated levels of IFN-α, -β, or -λ are lacking in patients indicate that the IFN responses are inhibited and to a large degree unable to stop the infection during HFRS or HCPS. Being able to inhibit innate immune responses, especially the production and/or function of antiviral IFNs, is clearly useful for a virus (16). How hantaviruses are cleared from infected patients is not known, but the observed strong and hantavirus-specific cytotoxic-T-cell (CTL) responses (20), together with the insensitivity of infected cells to IFNs reported here, strongly suggest that virus clearance is due to the hantavirus-specific CTL and/or NK cell responses rather than to the innate IFN responses.
The mechanisms behind hantavirus pathogenesis are not known. Hantaviruses primarily infect endothelial cells lining the vascular system, and increased permeability followed by capillary leakage is behind most of the pathogenesis (41). Understanding why this occurs would help in developing specific treatments and prophylaxis against HFRS and HCPS. Since hantaviruses per se are not cytopathogenic, it has been suggested that the increased capillary permeability is caused by the immune responses directed against the infection (19, 39). We recently showed that there is a correlation between the levels of serum LDH with that of extracellular perforin and the endothelial cell apoptosis marker caspase-cleaved cytokeratin-18 during acute PUUV infection, indicating that endothelial cell damage in patients is caused by cytotoxic immune cells and that apoptosis is involved (21). During hantavirus infections, a remarkably strong activation of hantavirus-specific T-cell responses occur (20, 40). Up to 44% of all CD8+ T cells have been shown to be specific for SNV epitopes in HCPS patients (20). CTLs do not require type I IFNs for expansion and function (37), and our results suggest that the CTL response taking place in HCPS and HFRS patients is independent of IFNs.
Early stages of diseases caused by certain viruses, such as the Ebola virus, influenza virus, measles virus, severe acute respiratory syndrome coronavirus, and West Nile virus, are characterized by a transient lymphopenia (8, 10, 27, 29, 32). In contrast, increased levels of lymphocytes are observed during hantavirus infections (41). It has been shown that type I IFNs cause lymphopenia, suggesting that systemically increased levels of IFN-α and IFN-β are needed for this process to occur (17). Lymphopenia allows for the generation of a more diverse adaptive T-cell response, by reducing immunodomination (4). The lack of type I IFN responses can therefore play a role in the development and function of hantavirus specific CD4+ and CD8+ T cells, and the observed lack of lymphopenia might in turn explain the high concentration of CD8+ T cells specific for single epitopes detected in hantavirus-infected patients (20).
In conclusion, we observed significantly decreased levels of IFN-λ in serum during HFRS, and we show here that IFN-λ has antiviral effects against hantaviruses. This is, to the best of our knowledge, the first time any form of IFN has been found at decreased levels in circulation during an acute virus infection in humans. The results further show that an established hantavirus infection resists treatment with all antiviral IFNs, thereby preventing IFN-induced clearance of virus from infected cells. We also show that cells infected with hantavirus produce less NO when stimulated with IFN-γ and that STAT1 phosphorylation after IFN-γ treatment is inhibited. Future studies on the mechanisms behind activation, inhibition, and the shaping of innate and adaptive immune responses during hantavirus infection may explain some of the mechanisms behind pathogenesis during HFRS and HCPS.
Acknowledgments
This project was supported by grants from the Swedish Society of Medicine, Stiftelsen Goljes Minne, Magn. Bergvalls Stiftelse, Lars Hiertas Stiftelse, the County Councils of Northern Sweden and the medical faculty of Umeå University, and the Swedish Medical Research Council (projects 12177 and 12642).
This publication has been partially funded under the EU 6th Framework Program (GOCE-CT-2003-010284 EDEN) and is officially catalogued by the EDEN Steering Committee as EDEN0034.
The content of this publication does not represent the official position of the European Commission and is entirely under the responsibility of the authors.
Footnotes
Published ahead of print on 23 May 2007.
REFERENCES
- 1.Alff, P. J., I. N. Gavrilovskaya, E. Gorbunova, K. Endriss, Y. Chong, E. Geimonen, N. Sen, N. C. Reich, and E. R. Mackow. 2006. The pathogenic N.Y.-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. J. Virol. 80:9676-9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ank, N., H. West, and S. R. Paludan. 2006. IFN-λ: novel antiviral cytokines. J. Interferon Cytokine Res. 26:373-379. [DOI] [PubMed] [Google Scholar]
- 3.Ank, N., H. West, C. Bartholdy, K. Eriksson, A. R. Thomsen, and S. R. Paludan. 2006. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 80:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahl, K., S.-K. Kim, C. Calgagno, D. Ghersi, R. Puzone, F. Celada. L. K. Selin, and R. M. Welsh. 2006. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 176:4284-4295. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan, C., N. Röllinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 6.Cao, W., C. Bao, and C. J. Lowenstein. 2003. Inducible nitric oxide synthase expression inhibition by adenovirus E1A. Proc. Natl. Acad. Sci. USA 100:7773-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C.-W., Y.-H. Chang, C.-J. Tsi, and W.-W. Lin. 2003. Inhibition of IFN-γ-mediated inducible nitric oxide synthase induction by the peroxisome proliferator-activated receptor γ agonist, 15-deoxy-Δ12,14-prostaglandin J2, involves inhibition of the upstream Janus kinase/ signaling pathway. J. Immunol. 171:979-988. [DOI] [PubMed] [Google Scholar]
- 8.Cunha, B. A., V. Minnaganti, D. H. Johnson, and N. C. Klein. 2003. Profound and prolonged lymphocytopenia with West Nile encephalitis. Clin. Infect. Dis. 31:1116-1117. [DOI] [PubMed] [Google Scholar]
- 9.Davis, I. C., A. J. Zajac, K. B. Nolte, J. Botten, B. Hjelle, and S. Matalon. 2002. Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome. J. Virol. 76:8347-8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher-Hoch, S. P., G. S. Platt, G. H. Neild, T. Southee, A. Baskerville, R. T. Raymond, G. Lloyd, and D. I. Simpson. 1985. Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola). J. Infect. Dis. 152:887-894. [DOI] [PubMed] [Google Scholar]
- 11.Frese, M., G. Kochs, H. Feldmann, C. Hertkorn, and O. Haller. 1996. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J. Virol. 70:915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geimonen, E., R. LaMonica, K. Springer, Y. Farooqui, I. N. Gavrilovskaya, and E. R. Mackow. 2003. Hantavirus pulmonary syndrome-associated hantaviruses contain conserved and functional ITAM signaling elements. J. Virol. 77:1638-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geimonen, E., S. Neff, T. Raymond, S. S. Kocer, I. N. Gavrilovskaya, and E. R. Mackow. 2002. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. USA 99:13837-13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geisbert, T. W., and P. B. Jahrlin. 2004. Exotic emerging viral diseases: progress and challenges. Nat. Med. 12:S110-S121. [DOI] [PubMed] [Google Scholar]
- 15.Granja, A. G., P. Sabina, M. L. Salas, M. Fresno, and Y. Revilla. 2006. Regulation of inducible nitric oxide synthase expression by viral A238L-mediated inhibition of p65/RelA acetylation and p300 transactivation. J. Virol. 80:10487-10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller, O., G. Kocks, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamphuis, E., T. Junt, Z. Waibler, R. Forster, and U. Kalinke. 2006. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 108:3253-3261. [DOI] [PubMed] [Google Scholar]
- 18.Kanerva, M., K. Melen, A. Vaheri, and I. Julkunen. 1996. Inhibition of Puumala and Tula hantaviruses in Vero cells by MxA protein. Virology 224:55-62. [DOI] [PubMed] [Google Scholar]
- 19.Khaiboullina, S. F., and S. C. St Jeor. 2002. Hantavirus immunology. Viral Immunol. 15:609-625. [DOI] [PubMed] [Google Scholar]
- 20.Kilpatrick, E. D., M. Terajima, F. T. Koster, M. D. Catalina, J. Cruz, and F. A. Ennis. 2004. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J. Immunol. 172:3297-3304. [DOI] [PubMed] [Google Scholar]
- 21.Klingström, J., J. Hardestam, M. Stoltz, B. Zuber, Å. Lundkvist, S. Linder, and C. Ahlm. 2006. Loss of cell membrane integrity in Puumala hantavirus-infected patients correlates with levels of epithelial cell apoptosis and perforin. J. Virol. 80:8279-8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klingström, J., S. Åkerström. J. Hardestam, M. Stoltz, M. Simon, K. I. Falk, A. Mirazimi, M. Rottenberg, and Å. Lundkvist. 2006. Nitric oxide and peroxynitrite have different antiviral effects against hantavirus replication and free mature virions. Eur. J. Immunol. 36:2649-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotenko, S., V. G. Gallagher, V. V. Baurin, A. Lewis-Antes, M. Shen, N. K. Shah, J. A. Langer, F. Sheikh, H. Dickensheets, and R. P. Donnelly. 2003. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69-77. [DOI] [PubMed] [Google Scholar]
- 24.Kraus, A. A., M. J. Raftery, T. Giese, T. R. Ulrich, R. Zawatzky, S. Hippenstiel, N. Suttorp, D. H. Kruger, and G. Schönrich. 2004. Differential antiviral response of endothelial cells after infection with pathogenic and nonpathogenic hantaviruses. J. Virol. 78:6143-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levis, S. C., M. C. Saavedra, C. Ceccoli, E. Falcoff, M. R. Feuillade, D. A. Enria, J. I. Maiztegui, and R. Falcoff. 1984. Endogenous interferon in Argentine hemorrhagic fever. J. Infect. Dis. 149:428-433. [DOI] [PubMed] [Google Scholar]
- 26.Linderholm, M., C. Ahlm, B. Settergren, A. Waage, and A. Tärnvik. 1996. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 173:38-43. [DOI] [PubMed] [Google Scholar]
- 27.Nahmias, A. J., D. Griffith, C. Salsbury, and K. Yoshida. 1967. Thymic aplasia with lymphopenia, plasma cells, and normal immunoglobulins. Relation to measles virus infection. JAMA 201:729-734. [PubMed] [Google Scholar]
- 28.Nam, J. H., K. A. Hwang, C. H. Yu, T. H. Kang, J. Y. Shin, W. Y. Choi, I. B. Kim, Y. R. Joo, H. W. Cho, and K. Y. Park. 2003. Expression of interferon inducible genes following Hantaan virus infection as a mechanism of resistance in A549 cells. Virus Genes 26:31-38. [DOI] [PubMed] [Google Scholar]
- 29.Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, and K. Y. Yuen. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescott, J., C. Ye, G. Sen, and B. Hjelle. 2005. Induction of innate immune response genes by Sin Nombre hantavirus does not require viral replication. J. Virol. 79:15007-15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheinberg, M., N. R. Blacklow, A. L. Goldstein, T. A. Parrino, F. B. Rose, and E. S. Cathcart. 1976. Influenza: response of T-cell lymphopenia to thymosin. N. Engl. J. Med. 294:1208-1211. [DOI] [PubMed] [Google Scholar]
- 33.Schmaljohn, C. S., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheppard, P., W. Kindsvogel, W. Xu, K. Henderson, S. Schlutsmeyer, T. E. Whitmore, R. Kuestner, U. Garrigues, C. Birks, J. Roraback, C. Ostrander, D. Dong, J. Shin, S. Presnell, B. Fox, B. Haldeman, E. Cooper, D. Taft, T. Gilbert, F. J. Grant, M. Tackett, W. Krivan, G. McKnight, C. Clegg, D. Foster, and K. M. Klucher. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63-68. [DOI] [PubMed] [Google Scholar]
- 35.Spiropoulou, C. F., C. G. Albarino, T. G. Ksiazek, and P. E. Rollin. 2007. Andes and Prospect Hill hantaviruses differ in early induction of interferon although both can downregulate interferon signaling. J. Virol. 81:2769-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamler, J. S., D. J. Singel, and J. Loscalzo. 1992. Biochemistry of nitric oxide and its redox-activated forms. Science 258:1896-1902. [DOI] [PubMed] [Google Scholar]
- 37.Stetson, D. B., and R. Medzhitov. 2006. Type I interferon in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 38.Tamura, M., H. Asada, K. Kondo, M. Takahashi, and K. Yamanishi. 1987. Effects of human and murine interferons against hemorrhagic fever with renal syndrome (HFRS) virus (Hantaan virus). Antiviral Res. 8:171-178. [DOI] [PubMed] [Google Scholar]
- 39.Terajima, M., O. Vapalahti, H. L. Van Epps, A. Vaheri, and F. A. Ennis. Immune responses to Puumala virus infection and the pathogenesis of nephropathia epidemica. Microbes Infect. 6:238-245. [DOI] [PubMed]
- 40.Van Epps, H. L., M. Terajima, J. Mustonen, T. P. Arstila, E. A. Corey, A. Vaheri, and F. A. Ennis. 2002. Long-lived memory T lymphocyte responses after hantavirus infection. J. Exp. Med. 196:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vapalahti, O., J. Mustonen, Å. Lundkvist, H. Henttonen, A. Plyusnin, and A. Vaheri. 2003. Hantavirus infections in Europe. Lancet. Infect. Dis. 3:653-661. [DOI] [PubMed] [Google Scholar]
- 42.Villinger, F., P. E. Rollin, S. S. Brar, N. F. Chikkala, J. Winter, J. B. Sundstrom, S. R. Zaki, R. Swanepoel, A. A. Ansari, and C. J. Peters. 1999. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J. Infect. Dis. 179:S188-S191. [DOI] [PubMed] [Google Scholar]
- 43.Xiao, R., S. Yang, F. Koster, C. Ye, C. Stidley, and B. Hjelle. 2006. Sin Nombre viral RNA load in patients with hantavirus cardiopulmonary syndrome. J. Infect. Dis. 194:1403-1409. [DOI] [PubMed] [Google Scholar]