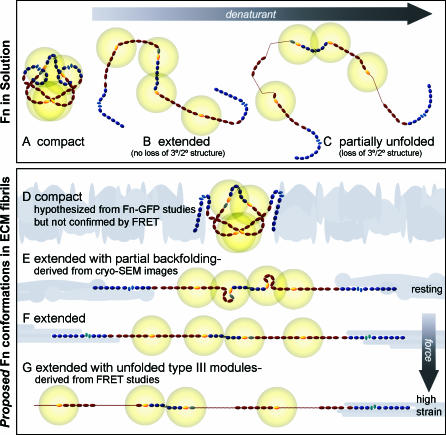
Figure 1. Schematic Sketch of Putative Fn Conformations in Solution and within ECM Fibrils.
Fn consists of tandem repeats of type I (dark blue ovals), II (narrow, light blue ellipses), and III modules (dark red ovals). Average end-to-end lengths of each module type are drawn to scale using lengths of 2.5 nm for Fn type I [51], 0.7 nm for Fn type II [52], and 3.2 nm for FnIII modules [53]. Two free cysteines are present on each monomer within FnIII7 and III15 (yellow). Energy transfer between donors and acceptors bound to free cysteines are limited to within approximately double the Förster radius (∼12 nm), denoted by gold circles around III7 and III15. Fn in solution assumes a compact conformation stabilized through ionic interactions between dimer arms (A) [7]. Denaturant destabilizes these ionic interactions, leading to an extended conformation (B), and higher denaturant concentrations lead to loss of tertiary/secondary structure of FnIII modules (C). The quaternary model for fibril elongation [11,12] predicts a compact conformation with crossover of opposing arms in the absence of tension (D). In contrast, high-resolution cryo-scanning electron microscopic images of Fn fibrils [39,40], taken together with our FRET studies, suggest that fully relaxed fibers do not contain the compact quaternary structure, but are composed of Fn in an extended conformation with partial backfolding of its arms upon themselves (nodules; E). Cell-generated tensile forces first extend Fn fibrils (F) and finally unfold FnIII modules (G).