Abstract
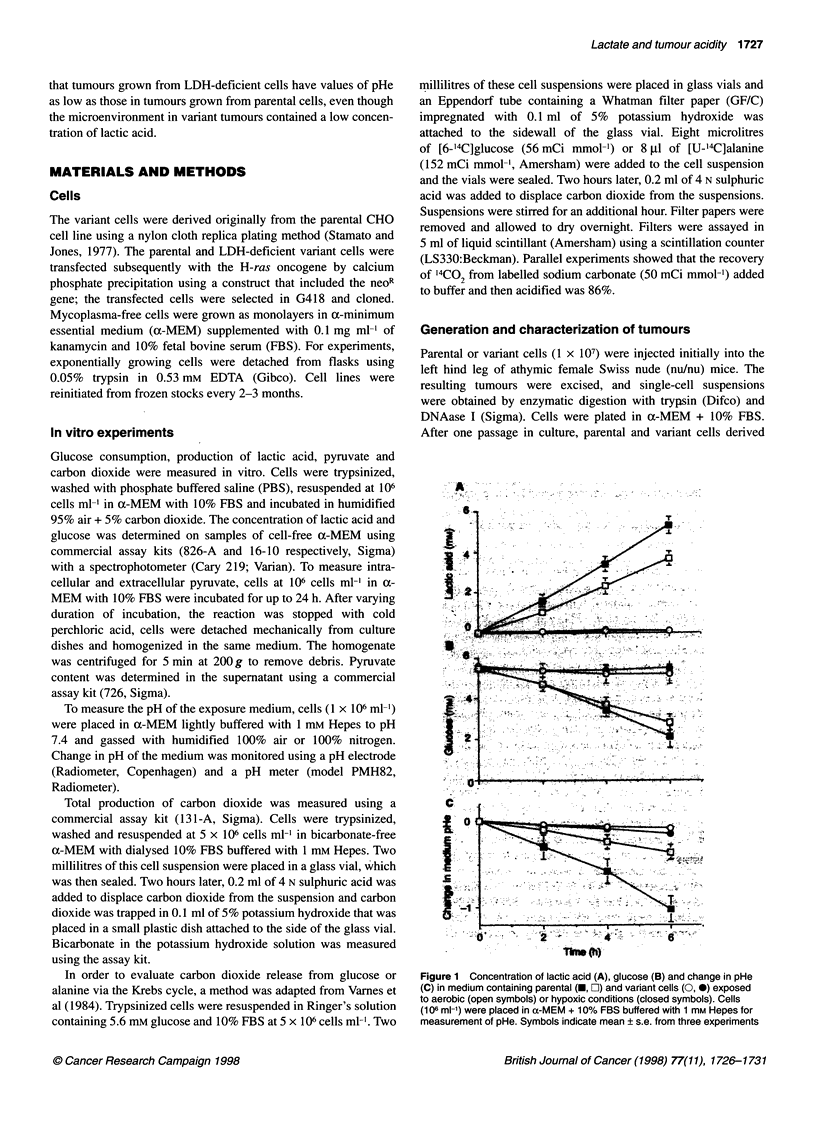
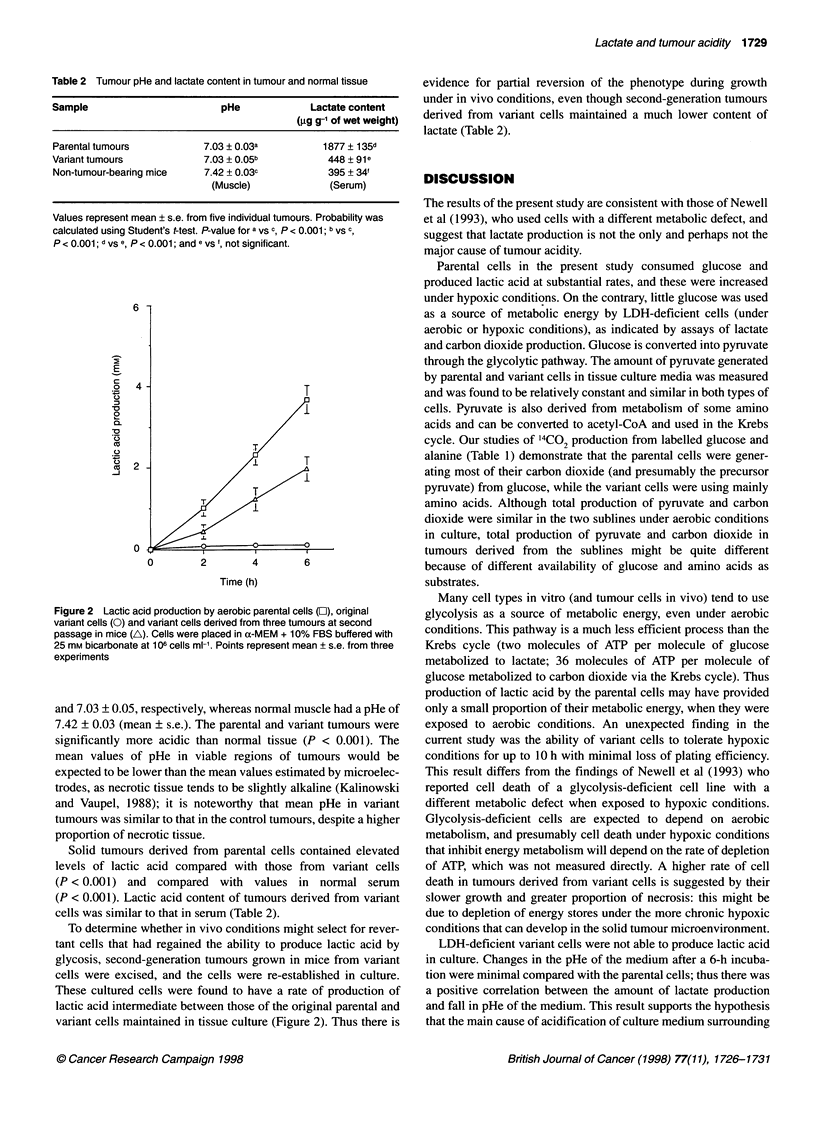
Solid tumours develop an acidic extracellular environment with high concentration of lactic acid, and lactic acid produced by glycolysis has been assumed to be the major cause of tumour acidity. Experiments using lactate dehydrogenase (LDH)-deficient ras-transfected Chinese hamster ovarian cells have been undertaken to address directly the hypothesis that lactic acid production is responsible for tumour acidification. The variant cells produce negligible quantities of lactic acid and consume minimal amounts of glucose compared with parental cells. Lactate-producing parental cells acidified lightly-buffered medium but variant cells did not. Tumours derived from parental and variant cells implanted into nude mice were found to have mean values of extracellular pH (pHe) of 7.03 +/- 0.03 and 7.03 +/- 0.05, respectively, both of which were significantly lower than that of normal muscle (pHe = 7.43 +/- 0.03; P < 0.001). Lactic acid concentration in variant tumours (450 +/- 90 microg g(-1) wet weight) was much lower than that in parental tumours (1880 +/- 140 microg/g(-1)) and similar to that in serum (400 +/- 35 microg/g(-1)). These data show discordance between mean levels of pHe and lactate content in tumours; the results support those of Newell et al (1993) and suggest that the production of lactic acid via glycolysis causes acidification of culture medium, but is not the only mechanism, and is probably not the major mechanism responsible for the development of an acidic environment within solid tumours.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fridman R., Kibbey M. C., Royce L. S., Zain M., Sweeney M., Jicha D. L., Yannelli J. R., Martin G. R., Kleinman H. K. Enhanced tumor growth of both primary and established human and murine tumor cells in athymic mice after coinjection with Matrigel. J Natl Cancer Inst. 1991 Jun 5;83(11):769–774. doi: 10.1093/jnci/83.11.769. [DOI] [PubMed] [Google Scholar]
- GULLINO P. M., CLARK S. H., GRANTHAM F. H. THE INTERSTITIAL FLUID OF SOLID TUMORS. Cancer Res. 1964 Jun;24:780–794. [PubMed] [Google Scholar]
- Helmlinger G., Yuan F., Dellian M., Jain R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997 Feb;3(2):177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- Jähde E., Rajewsky M. F. Tumor-selective modification of cellular microenvironment in vivo: effect of glucose infusion on the pH in normal and malignant rat tissues. Cancer Res. 1982 Apr;42(4):1505–1512. [PubMed] [Google Scholar]
- Kallinowski F., Tyler G., Mueller-Klieser W., Vaupel P. Growth-related changes of oxygen consumption rates of tumor cells grown in vitro and in vivo. J Cell Physiol. 1989 Jan;138(1):183–191. doi: 10.1002/jcp.1041380124. [DOI] [PubMed] [Google Scholar]
- Kallinowski F., Vaupel P. pH distributions in spontaneous and isotransplanted rat tumours. Br J Cancer. 1988 Sep;58(3):314–321. doi: 10.1038/bjc.1988.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Jain R. K. Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res. 1994 Nov 1;54(21):5670–5674. [PubMed] [Google Scholar]
- Mueller-Klieser W., Walenta S., Paschen W., Kallinowski F., Vaupel P. Metabolic imaging in microregions of tumors and normal tissues with bioluminescence and photon counting. J Natl Cancer Inst. 1988 Aug 3;80(11):842–848. doi: 10.1093/jnci/80.11.842. [DOI] [PubMed] [Google Scholar]
- Negendank W. Studies of human tumors by MRS: a review. NMR Biomed. 1992 Sep-Oct;5(5):303–324. doi: 10.1002/nbm.1940050518. [DOI] [PubMed] [Google Scholar]
- Newell K., Franchi A., Pouysségur J., Tannock I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1127–1131. doi: 10.1073/pnas.90.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins C. S., Stratford M. R., Dennis M. F., Stubbs M., Chaplin D. J. The relationship between extracellular lactate and tumour pH in a murine tumour model of ischaemia-reperfusion. Br J Cancer. 1997;75(3):319–323. doi: 10.1038/bjc.1997.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold H. S., van den Berg-Blok A. E., van den Berg A. P. Dose-effect relationships for glucose-induced tumour acidification and its erythrocyte flux. Eur J Cancer. 1991;27(9):1151–1154. doi: 10.1016/0277-5379(91)90314-4. [DOI] [PubMed] [Google Scholar]
- Stamato T. D., Jones C. Isolation of a lactic dehydrogenase-A-deficient CHO-K1 mutant by nylon cloth replica plating. Somatic Cell Genet. 1977 Nov;3(6):639–647. doi: 10.1007/BF01539071. [DOI] [PubMed] [Google Scholar]
- Tannock I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968 Jun;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistlethwaite A. J., Alexander G. A., Moylan D. J., 3rd, Leeper D. B. Modification of human tumor pH by elevation of blood glucose. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):603–610. doi: 10.1016/0360-3016(87)90078-2. [DOI] [PubMed] [Google Scholar]
- Varnes M. E., Tuttle S. W., Biaglow J. E. Nitroheterocycle metabolism in mammalian cells. Stimulation of the hexose monophosphate shunt. Biochem Pharmacol. 1984 May 15;33(10):1671–1677. doi: 10.1016/0006-2952(84)90290-9. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]


