Abstract
Grapevines exhibit a wide spectrum of resistance to the powdery mildew fungus (PM), Erysiphe necator (Schw.) Burr., but little is known about the transcriptional basis of the defense to PM. Our microscopic observations showed that PM produced less hyphal growth and induced more brown-colored epidermal cells on leaves of PM-resistant Vitis aestivalis ‘Norton’ than on leaves of PM-susceptible Vitis vinifera ‘Cabernet sauvignon’. We found that endogenous salicylic acid levels were higher in V. aestivalis than in V. vinifera in the absence of the fungus and that salicylic acid levels increased in V. vinifera at 120 h postinoculation with PM. To test the hypothesis that gene expression differences would be apparent when V. aestivalis and V. vinifera were mounting a response to PM, we conducted a comprehensive Vitis GeneChip analysis. We examined the transcriptome at 0, 4, 8, 12, 24, and 48 h postinoculation with PM. We found only three PM-responsive transcripts in V. aestivalis and 625 in V. vinifera. There was a significant increase in the abundance of transcripts encoding ENHANCED DISEASE SUSCEPTIBILITY1, mitogen-activated protein kinase kinase, WRKY, PATHOGENESIS-RELATED1, PATHOGENESIS-RELATED10, and stilbene synthase in PM-infected V. vinifera, suggesting an induction of the basal defense response. The overall changes in the PM-responsive V. vinifera transcriptome also indicated a possible reprogramming of metabolism toward the increased synthesis of the secondary metabolites. These results suggested that resistance to PM in V. aestivalis was not associated with overall reprogramming of the transcriptome. However, PM induced defense-oriented transcriptional changes in V. vinifera.
Powdery mildew caused by an obligate biotrophic fungus, Erysiphe necator (synonym Uncinula necator [Schw.] Burr.), is an economically important disease of grapevines. The powdery mildew fungus (PM) infects green tissues of vines and causes significant losses in yield and reduction in berry quality. Most widely grown grape cultivars are highly susceptible to E. necator, because they are derived from Vitis vinifera, a species that was not exposed to this pathogen during its evolution (Mullins et al., 1992). In contrast, other grapevine species such as Vitis labrasca, Vitis rupestris (Doster and Schnathorst, 1985a), Vitis aestivalis (Giannakis et al., 1998), and Muscadinia rotundifolia (Olmo, 1971) co-evolved with E. necator on the North American continent and possess various levels of resistance to the pathogen (Mullins et al., 1992). Resistance to PM in grapevines is determined by either a single locus or quantitative trait loci (QTL). For example, resistance to PM in M. rotundifolia was traced back to Resistance against U. necator1 (Run1), a single dominant locus that contains two families of resistance gene analogs (Pauquet et al., 2001; Donald et al., 2002; Barker et al., 2005). The Run1-mediated resistance is believed to be mediated through a hypersensitive response (Donald et al., 2002). Resistance to PM in grapevines can also be linked with QTLs (Dalbo et al., 2000; Regner et al., 2003; Fischer et al., 2004). The constitutive expression of pathogenesis-related (PR) genes, such as genes encoding β-1,3-glucanases (PR-2) and chitinases (PR-3) in disease-resistant North American grapevine species (Robinson et al., 1997; Tattersall et al., 1997; Giannakis et al., 1998; Fung et al., 2007), may represent one type of QTL-mediated cumulative effects. In addition, physical barriers that block the penetration of the fungus may act as a passive defense mechanism (Doster and Schnathorst, 1985b; Heintz and Blaich, 1989; Ficke et al., 2004). Thus, it is apparent that the genetic basis of resistance to PM is complex in grapevine. While we have gained some understanding of the genetic basis of the grapevine's resistance to PM, little is known about the molecular processes and gene regulation during compatible and incompatible PM-grapevine interactions.
Upon contact with an epidermal cell, an E. necator conidiospore germinates to form a penetration peg that breaches the cuticle and the cell wall. Subsequently, an infection structure, termed a haustorium, is formed within the epidermal cell through which a dynamic exchange of signals and metabolites occurs between the pathogen and the host cell (Heintz and Blaich, 1990; Rumbolz et al., 2000). The process of penetration and haustorium formation in V. vinifera may be as short as 14 h under optimal conditions (Rumbolz et al., 2000). At the microscopic level, the germination rate of E. necator conidiospores does not differ on PM-susceptible and -resistant grapevines (Giannakis et al., 1998). During subsequent stages, however, susceptible and resistant grapevine cultivars differ significantly in their ability to limit the growth of hyphae and restrict the formation and development of PM colonies. Each stage of the infection process provides an interface for the grapevine cells to recognize E. necator-released molecules. In other PM-plant interactions, the entry of the penetration peg into an epidermal cell appears to be a defining point at which the outcome of the interaction between the fungus and the host cell is determined (Panstruga and Schulze-Lefert, 2002; Lipka et al., 2005). The prehaustorial and haustorial phases of the conidiospore-epidermal cell interaction also show distinct gene expression patterns in host cells (Caldo et al., 2004, 2006; O'Connell and Panstruga, 2006).
A plant's resistance to a pathogen can be executed at different levels and to differing degrees through reinforcing cell walls and mounting biochemical defenses (Glazebrook, 2005). A basal disease resistance is generally induced during the initial interaction between a host and a virulent pathogen (Chrisholm et al., 2006; Jones and Dangl, 2006) as a result of interactions among pathogen-associated molecular pattern-triggered immunity, effector-triggered susceptibility, and weak effector-triggered immunity (Jones and Dangl, 2006). It has been suggested that a biotrophic fungus may release effectors into the extrahaustorial matrix or inside the host cell. Some of these effectors can act as suppressors of basal defense, although the identity of these suppressors is not clear (Gregersen et al., 1997; Panstruga, 2003; Schulze-Lefert and Panstruga, 2003; Chrisholm et al., 2006). Thus, host susceptibility to a biotrophic fungus is in part a consequence of the pathogen's ability to circumvent the plant's basal defense responses (Panstruga, 2003; Schulze-Lefert and Panstruga, 2003). Transcriptome analysis of barley (Hordeum vulgare)-PM (Blumeria graminis f. sp. hordei) interactions demonstrated that the expression patterns of basal defense-related genes were similar in compatible and incompatible interactions during the first 16 h of the fungal infection. Expression of some of these genes declined to lower levels in the compatible interaction at 24 and 32 h postinoculation (hpi) with fungal conidiospores (Caldo et al., 2004, 2006). These results suggest that the fungus may interfere with signaling events involved in the host's basal defenses. The basal defense responses include changes in the redox state of cells (Huckelhoven and Kogel, 2003; Torres et al., 2006), phosphorylation of the mitogen-activated protein kinase (MAPK) components (Tena et al., 2001; Asai et al., 2002), MAPK-mediated activation of transcription factors such as WRKY proteins (Maleck et al., 2000; Xu et al., 2006), and the accumulation of PR proteins (van Loon et al., 2006). Salicylic acid (SA), jasmonic acid, and ethylene play vital roles in transmitting defense signals across pathways to initiate these responses (Broekaert et al., 2006). Genes encoding enzymes for secondary metabolism, particularly those involved in the biosynthesis of phenylpropanoids and oxylipins (La Camera et al., 2004), also are activated to increase production of defense-related compounds during basal defense (Scheideler et al., 2002).
Large-scale transcriptional profiling in response to pathogens has revealed novel aspects in compatible and incompatible interactions between plants and their pathogens (Mysore et al., 2002; Schenk et al., 2003; Tao et al., 2003; Caldo et al., 2004, 2006). Global transcriptional profiling in response to PM has not been reported in grapevines. In this study, we used the Vitis GeneChip to compare PM-responsive gene expression patterns in two grapevine genotypes to test the hypothesis that differential gene expression would be observed in response to PM in disease-resistant V. aestivalis ‘Norton’ and in disease-susceptible V. vinifera ‘Cabernet sauvignon’. Our hypothesis was confirmed; we found three PM-responsive transcripts in V. aestivalis as compared to 625 in V. vinifera. Among 625 PM-responsive transcripts in V. vinifera, proteins such as ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1), MAPKK, WRKY, PR1, PR10, and stilbene synthase are known to be associated with plant defense. In addition, we observed clear differences in the development of the fungus on the leaves of V. aestivalis and V. vinifera and in the SA content in the two grapevine genotypes.
RESULTS
Epidermal Cells of V. aestivalis and V. vinifera Respond Differently to Conidiospores
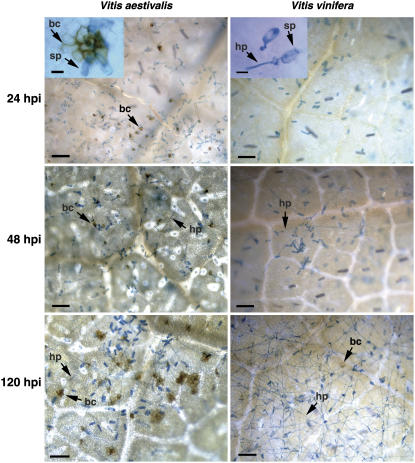
To compare the characteristics of PM-induced symptoms in the two grapevine genotypes, we conducted a microscopy study of conidiospore germination and hyphal development during a 6-d time period. Microscopic images of 24, 48, and 120 hpi are presented in Figure 1. Conidiospores produced appressoria and secondary hyphae on both V. vinifera and V. aestivalis leaves at 24 hpi. In V. aestivalis leaves, most epidermal cells invaded by the conidiospores exhibited brown coloration, which was visible after tissue was cleared of chlorophyll (Fig. 1). This browning appeared more intense in the region of the cell wall. Brown-colored cells were also observed beneath appressoria that developed from secondary hyphae on V. aestivalis leaves at 120 hpi. The infection led to the formation of colonies with dense secondary hyphae on V. vinifera leaves but only small colonies with sparse hyphae on V. aestivalis leaves by 120 hpi.
Figure 1.
Progression of PM on V. vinifera and V. aestivalis leaves. Shown are representative images taken at 24, 48, and 120 hpi with conidiospores. Spores and hyphae were stained with 0.05% aniline blue. Images were taken at 100× magnification under transmission light. Scale bar = 65 μm. Insets inside the top two photos are images of 600× magnification. Scale bar = 25 μm. bc, Brown cell; hp, secondary hyphae; sp, conidiospore.
SA Is Present at Elevated Levels in PM-Infected V. vinifera and in Mock-Inoculated V. aestivalis
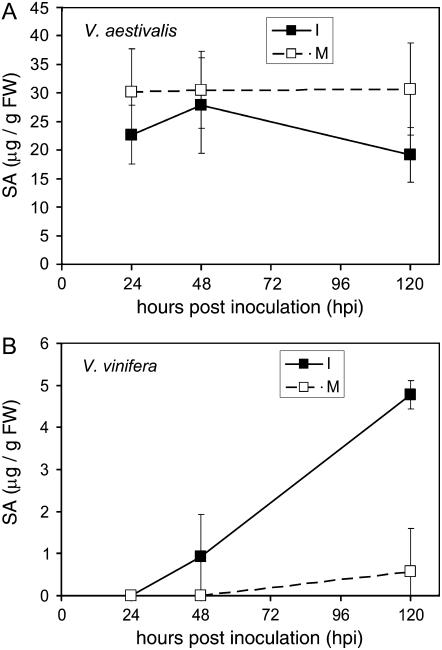
It is known that SA is a signal molecule in the induction of host defense responses, including hypersensitive response and systemic acquired resistance, and that the increase of endogenous SA levels is associated with the activation of PR gene expression (Shah, 2003). To assess if SA levels change during PM infection in V. aestivalis and V. vinifera, we measured the total SA content in PM-infected leaf tissue of V. aestivalis and V. vinifera in comparison with mock-inoculated samples by HPLC. Changes of SA content in PM-inoculated V. aestivalis at 24, 48, and 120 hpi were not statistically significant in comparison with mock-inoculated leaf tissue (Fig. 2A). In contrast, we found that SA levels increased in PM-infected leaf tissue of V. vinifera at 120 hpi, but there was no significant difference in SA levels between PM-inoculated and mock-inoculated samples at 24 and 48 hpi (Fig. 2B).
Figure 2.
Endogenous levels of total SA in V. aestivalis and V. vinifera. A, Accumulation of SA in the PM-infected V. aestivalis leaf tissue (I) in comparison with mock-inoculated samples (M) at 24, 48, and 120 hpi. B, Accumulation of SA in the PM-infected V. vinifera leaf tissue (I) in comparison with mock-inoculated samples (M) at 24, 48, and 120 hpi. Values are the average of three biological samples for each time point. Error bars represent sd, n = 3.
Our earlier results from the analysis of genotype-specific transcriptome changes demonstrated that representative PR genes including PR-2 and PR-3 were transcribed constitutively at higher levels in V. aestivalis than in V. vinifera (Fung et al., 2007). It is possible that higher expression of PR genes is a result of elevated SA levels in V. aestivalis. To test this possibility, we compared the levels of endogenous SA between the two grapevine genotypes under mock-inoculation conditions. We found that the endogenous SA content in V. aestivalis was significantly higher than in V. vinifera in the absence of the fungus (Fig. 2).
PM-Responsive Transcript Profiles Are Distinct in the Two Grapevine Genotypes
In a previous study, we found that transcriptome changes can be reliably measured in both V. aestivalis and V. vinifera by using the Vitis GeneChip (Fung et al., 2007). This finding allowed us to compare transcript abundance between the PM-inoculated and mock-inoculated plants in the two grapevine genotypes at 0, 4, 8, 12, 24, and 48 hpi. The data of three independent biological replicates were collected and analyzed. We conducted two independent F tests (one for each genotype) to determine whether the expression level of a transcript in the PM-inoculated plant was different from the mock-inoculated plant at any time point. We also conducted an additional F test with a model that incorporated data from both genotypes and included an effect of the genotypes to test the same null hypothesis. To account for heteroscedasticity of error variances between the two grapevine genotypes, the distribution of the residuals ɛijkm was assumed normal with error variance σ2N for residuals associated with V. aestivalis observations and σ2C for residuals associated with V. vinifera. If the null hypothesis was rejected, this indicated that the level of a transcript between PM-inoculated and mock-inoculated samples differed for at least one time point, and that the transcript was deemed to be PM-responsive for that genotype. To declare statistical significance and account for multiple tests, we used a false discovery rate (FDR) level of 0.05 approximated using the approach of Benjamini-Hochberg (Benjamini and Hochberg, 1995). We identified 626 transcripts on the Vitis GeneChip that were differentially expressed in V. vinifera. In contrast, only four transcripts were considered to be differentially expressed in V. aestivalis at a 0.05 FDR level; three of them were also found in the 626 PM-responsive transcripts of V. vinifera. In a serendipitous discovery, one of the PM-responsive transcripts (Affy ID 1615715_at) aligned with an EST of the fungus E. necator, and its predicted amino acid sequence is homologous to a hypothetical protein MG09900.4 from Magnaporthe grisea (e = 2e-15). This fungal transcript was consistently present in PM-inoculated V. aestivalis and V. vinifera but absent in the mock-inoculated samples across all six time points. This transcript fortuitously served as a control probe set confirming the presence and absence of conidia in the PM- and mock-inoculated samples, respectively. Thus, three and 625 plant-specific transcripts, respectively, were found. Gene names based on sequence homology to other plant species, UniGene ID, log-transformed expression value, fold-change, nominal P value, and FDR-corrected P value for the 625 PM-responsive transcripts in V. vinifera and the three PM-responsive transcripts in V. aestivalis are provided (Supplemental Table S1).
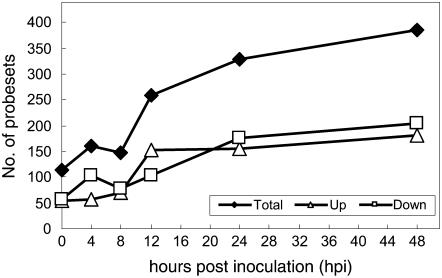
For the genes that were significantly different between PM- and mock-inoculated samples for at least one time point, we classified the differences at each time point as up-regulated, down-regulated, or the same based upon the nominal P value for the contrast between PM- and mock-inoculated samples at that time point and the direction of difference (Supplemental Table S1). We observed that the number of PM-responsive transcripts increased as PM developed in V. vinifera (Fig. 3). The total number of up- and down-regulated transcripts during the early infection stage (0–8 hpi) was around 100 to 150 and then increased to over 250 at 12 hpi and 350 at 48 hpi (Fig. 3). Further analysis of the 625 PM-responsive transcripts indicated that they represented 598 genes (510 UniGenes and 88 singletons) based on the UniGene assignment in the National Center for Biotechnology Information (NCBI). Twenty of the 510 UniGenes were represented by more than one probe set. In total, 240 genes (175 UniGenes and 65 singletons) were up-regulated and 345 genes (323 UniGenes and 22 singletons) were down-regulated in at least one time point, while four genes (UniGenes) were both up- and down-regulated during some of the time points. Expression of 12 of the genes (11 UniGenes and one singleton) was significant only for the initial test but not in the individual time-point test.
Figure 3.
Number of transcripts (probe sets) that are differentially expressed in response to PM inoculations relative to mock inoculations of V. vinifera at each of the six time points.
We hypothesized that a possible reason for the low number of PM-responsive transcripts in V. aestivalis was that many of the 625 PM-responsive transcripts were constitutively expressed at a higher or lower level in V. aestivalis than in V. vinifera even prior to PM infection. To test this hypothesis, we compared the transcript levels of the two genotypes for the 625 PM-responsive transcripts of V. vinifera. We first tested whether the two genotypes were different at any time point at an FDR of 0.05. The nominal P value for the contrast at an individual time point, together with the direction of the observed difference, was used to classify the difference between genotypes at that time point as higher, lower, or the same. We found that 508 out of 625 PM-responsive transcripts showed higher or lower expression in V. aestivalis in comparison with V. vinifera for at least one individual time point. Of these 508 transcripts, 83 transcripts were expressed at a higher level and 219 were expressed at a lower level in V. aestivalis in all six time points under the mock-inoculation condition. We also tested whether our findings were consistent with differential treatment response directly by testing the interaction of treatment and variety across all time points. Of the 625 transcripts identified, 533 showed evidence for an interaction between treatment and variety (FDR 0.20).
Representative Genes Are Verified by Quantitative PCR
We performed quantitative real-time PCR (qRT-PCR) assays on a subset of genes to verify differential expression measured in the microarray analysis. Thirteen genes were selected from the 598 PM-responsive genes in V. vinifera (FDR threshold P value < 0.05). Two of the three that were differentially regulated in V. aestivalis in response to PM infection were also analyzed by qRT-PCR. The degree of change in transcript abundance of each gene determined by the microarray and by the qRT-PCR assay was compared by using the difference in natural log values between PM- and mock-inoculated samples for each of the six time points (Supplemental Table S2). For 13 of the 15 genes, the results between the qRT-PCR and microarray were in agreement. The correlation between the microarray and qRT-PCR estimates was positive in all cases and significantly different from zero (Supplemental Table S2; Supplemental Fig. S1). The lowest observed correlation was 0.61 at 0 hpi and the highest was 0.90 at 24 and 48 hpi. For two of the 15 genes (1611550_at and 1611058_at), concordance at the 0 and 12 hpi time points was poor. It appeared that these two genes showed absolute differences in expression levels between the two platforms and, on this basis, were eliminated from comparisons for the remaining time points.
Expression Profiles of PM-Responsive Transcripts Are Distinct across the Six Time Points
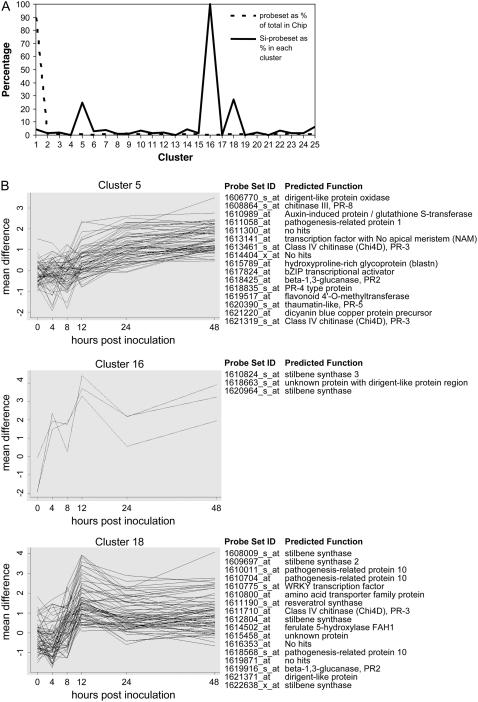
To acquire a global overview of the PM-responsive transcriptome, we performed a nonlinear cluster analysis on the difference between PM- and mock-inoculated samples of the 14,571 informative transcripts in V. vinifera that were detected in at least one sample (Qu and Xu, 2006). This approach clusters gene expression profiles based on the pattern of the mean differences between the expression values of PM- and mock-inoculated samples over the six time points. In total, 25 clusters were identified (Supplemental Fig. S2; Supplemental Table S3). We found that many of the genes in clusters 5, 16, and 18 were among the 625 statistically significant PM-responsive transcripts (25.4%, 100%, and 23.3%, respectively). Figure 4A shows the distribution of 625 PM-responsive transcripts in each cluster. Interestingly, these three clusters showed distinct expression patterns that seem to reflect a progression of PM infection (Fig. 4B). Furthermore, most PM-responsive transcripts from clusters 5, 16, and 18 were known to respond to plant pathogens (Fig. 4B). PM-responsive PR-1, PR-2, PR-3, PR-4, PR-5, and PR-8 belonged to cluster 5 together with a bZIP transcription factor and a dirigent-like protein oxidase. Cluster 18 contained three PR-10s and five stilbene synthase genes, a PR-2, a PR-3, a WRKY, and also a gene encoding a dirigent-like protein (Fig. 4B). Cluster 5 was characterized by a steady increase in transcript abundance starting from 12 hpi, while clusters 16 and 18 represented a pattern whose expression peaked at 12 hpi and then decreased at 24 hpi (Fig. 4B).
Figure 4.
Cluster analysis of the PM-responsive transcripts from V. vinifera. A, Percentage of entire probe sets on the whole Vitis GeneChip that was distributed in each cluster (dashed line) and proportion of the 625 significantly expressed transcripts (Si-probe set) in each of the 25 clusters (solid line). B, Distinct expression patterns of genes in clusters 5, 16, and 18. On the right is a list of genes among the 625 PM-responsive transcripts that are grouped together in that cluster.
Key Defense Genes Change in PM-Inoculated V. vinifera
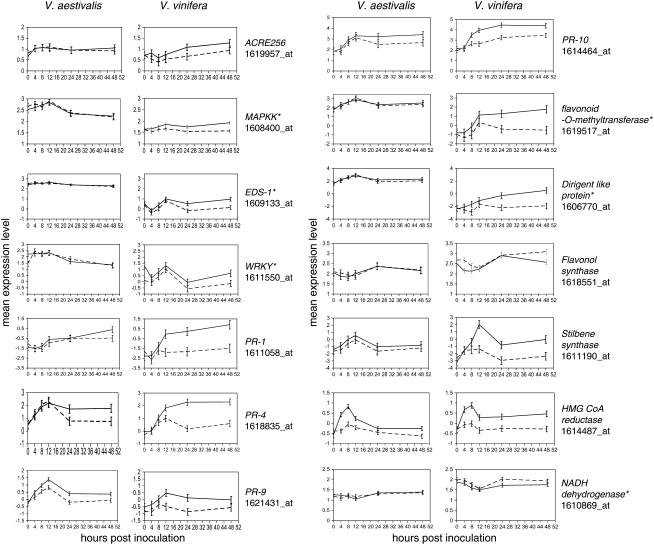
We found that the expression level of genes encoding PR-2 (β-1,3-glucanases), PR-3 (chitinases), and PR-5 (thaumatin-like protein) increased upon the PM infection across the course of the infection process (Supplemental Table S1), confirming previous reports that these genes are associated with grapevine defense against pathogens (Derckel et al., 1996; Busam et al., 1997; Salzman et al., 1998; Jacobs et al., 1999; Renault et al., 2000; Tattersall et al., 2001; Ferreira et al., 2004). We also identified many defense/PR genes that have not been well characterized in the interactions between PM and grapevine. These genes are potentially involved in defense signal perception and MAPK-mediated signal transduction, transcriptional regulation, phytoalexin and lignin biosynthesis, cell wall modification, and metabolism of reactive oxygen species (ROS; Table I). We found at least eight receptor-like kinase (RLK) genes (Table I). Three of them were homologous to the Avr9/Cf-9 rapidly elicited (ACRE) 256 gene in the tobacco (Nicotiana tabacum) plant. The expression profile of one RLK gene is presented in Figure 5. Two genes were homologous to Arabidopsis (Arabidopsis thaliana) AtMEK1 and AtMPK3 encoding a MAPKK and a MAPK, respectively (Table I). The MAPKK gene was induced as early as 12 hpi (Fig. 5). For the two EDS1 genes that were identified (Table I), one was induced at 24 and 48 hpi (Fig. 5), while the other was up-regulated at 4 and 48 hpi. Seven members of the WRKY family were up-regulated (Table I). The closest Arabidopsis orthologs of these WRKYs are AtWRKY11, 15, 33, 40, 53, and 75. The expression profile of the AtWRKY53 homolog representing an example of the WRKY gene expression pattern is shown in Figure 5. One PR-1 was induced in V. vinifera after 8 hpi (Fig. 5) and its closest Arabidopsis homolog At2g14610 is the only PR-1 that was induced by SA or pathogen infection (Uknes et al., 1992; van Loon et al., 2006). PR-1 is a marker gene that indicates the onset of local defense and systemic acquired resistance, although its precise enzymatic activity and its function have not been defined yet (van Loon et al., 2006). Five PR-10 genes were identified; four of them were up-regulated, while one was slightly repressed. One PR-10 was induced at 8 hpi and continued to increase from 12 to 48 hpi (Fig. 5). Thus, it is likely that the induction of PR-1 and PR-10 is indicative of the defense response during the grapevine-PM interaction as in other plant-microbe pathosystems (van Loon et al., 2006). We identified four different PR-9 genes involved in ROS metabolism that encode peroxidases; two of them were induced (Fig. 5), and the other two were repressed by PM in V. vinifera. Three NADH dehydrogenase genes were repressed in PM-inoculated V. vinifera leaves and also highly expressed in V. vinifera in comparison to V. aestivalis (Fig. 5). Three glutathione S-transferase (GST) genes were also induced by PM (Supplemental Table S1). Differential expression of these defense-related genes indicates the activation of defense pathways even in compatible interactions between grapevine and the fungus.
Table I.
Representative defense- and secondary metabolism-related transcripts that were differentially expressed at one of the six time points after V. vinifera was infected with PM
| Affymetrix Probe Set ID | Predicted Function (Organism) | AT Locus Tag (Gene ID) |
|---|---|---|
| RLK | ||
| 1619957_ata | ACRE 256 (tobacco)b | AT5G60900 (RLK1)c |
| 1615423_at | ACRE 256 (tobacco) | AT5G60900 (RLK1) |
| 1622142_at | ACRE 256 (tobacco) | AT5G60900 (RLK1) |
| 1620324_at | Kinase with Leu-rich repeat (Arabidopsis) | AT4G08850 |
| 1618208_s_at | Leu-rich repeat protein (Oryza sativa) | AT3G43740 |
| 1606597_at | Protein Ser/Thr kinase (Arabidopsis) | AT1G09970 |
| 1610386_at | Protein kinase (Medicago truncatula) | AT1G66980 |
| 1609263_at | Rust resistance kinase Lr10 (O. sativa) | AT1G58190 |
| MAPK pathway | ||
| 1608400_at | VaMAPKK (V. aestivalis) | AT4G26070 (MEK1) |
| 1606881_at | MAPK3 (Lycopersicon esculentum) | AT3G45640 (MPK3) |
| EDS1 | ||
| 1607262_at | EDS1-like protein (Nicotiana benthamiana) | AT3G48090 (EDS1) |
| 1609133_at | EDS1-like protein (L. esculentum) | AT3G48090 (EDS1) |
| WRKY transcription factors | ||
| 1609636_at | NtWRKY1 (tobacco) | AT2G38470 (WRKY33) |
| 1614806_s_at | VaWRKY4 (V. aestivalis) | AT1G80840 (WRKY40) |
| 1610064_at | Double WRKY type transfactor (Solanum tuberosum) | AT2G38470 (WRKY33) |
| 1611285_s_at | GmWRKY82 (Glycine max) | AT4G31550 (WRKY11) |
| 1611550_at | VaWRKY30 (V. aestivalis) | AT4G23810 (WRKY53) |
| 1612649_s_at | WRKY NtEIG-D48 (tobacco) | AT2G23320 (WRKY15) |
| 1610775_s_at | CaWRKY-b (Capsicum annuum) | AT5G13080 (WRKY75) |
| PR proteins | ||
| 1611058_at | PR protein1 precursor (V. vinifera) | AT2G14610 (PR1) |
| 1618568_s_at | PR-10 (V. vinifera) | |
| 1610704_at | PR-10 (V. vinifera) | AT1G24020 |
| 1614464_s_at | PR-10 (V. vinifera) | AT1G24020 |
| 1610011_s_at | PR-10 (Vitis pseudoreticulata) | AT1G24020 |
| 1613636_at | PR-10 (Vigna unguiculata) | AT5G45860 |
| 1618835_s_at | PR-4 (V. vinifera) | AT3G04720 |
| ROS metabolism | ||
| 1621431_at | Peroxidase, PR-9 (Asparagus officinalis) | AT5G05340 (peroxidase) |
| 1615967_at | Peroxidase, PR-9 (S. tuberosum) | AT5G67400 (peroxidae) |
| 1608586_at | Secretory peroxidase (Avicennia marina) | AT4G21960 (PRXR1) |
| 1618920_at | Class III peroxidase (Gossypium hirsutum) | AT4G25980 (peroxidase) |
| 1610243_at | GST T4 (L. esculentum) | AT2G29420 |
| 1611890_at | GST GST 14 (G. max) | AT3G09270 (ATGSTU8) |
| 1610869_at | NADH dehydrogenase (S. tuberosum) | AT5G08530 |
| 1613394_at | NADH-plastoquinone oxidoreductase subunit 4 (V. vinifera) | ATcG01050 (ndhD) |
| 1617935_at | NADH-plastoquinone oxidoreductase subunit 7 (V. vinifera) | ATcG01110 (ndhH) |
| 1608433_at | Phenol hydroxylase reductase (M. truncatula) | AT1G15140 |
| Lignin biosynthesis and cell wall modification | ||
| 1614502_at | Ferulate 5-hydroxylase (Camptotheca acuminate) | AT4G36220 (FAH1) |
| 1612124_at | Caffeic acid O-methyltransferase (V. vinifera) | AT5G54160 (ATOMT1) |
| 1606770_s_at | Dirigent-like protein oxidase (Sinopodophyllum hexandrum) | AT4G23690 |
| 1611671_at | Cellulose synthase 3 (Boehmeria nivea) | AT5G05170 (CESA3) |
| Phytoalexin and phenylpropanoid biosynthesis | ||
| 1619517_at | Flavonoid 4′-O-methyltransferase (Rosa hybrid cultivar) | AT4G35160 |
| 1609697_at | Stilbene synthase (V. pseudoreticulata) | AT5G13930 (ATCHS) |
| 1611190_s_at | Stilbene synthase (V. vinifera) | AT5G13930 (ATCHS) |
| 1615401_at | UDP-Glc glucosyltransferase (Catharanthus roseus) | AT2G29730 |
Probe sets in bold are discussed in detail in the text.
The best BLASTX homolog with E value below 1e−10.
The best-matched Arabidopsis (AT) homolog, if identified, whose function is used as reference for discussion.
Figure 5.
Comparative expression profiles of selected differentially expressed genes in the two grapevine genotypes. The natural log-transformed normalized expression values were plotted for each gene at six time points (0, 4, 8, 12, 24, and 48 hpi). SE was calculated based on three biological replicates. Solid line, PM-inoculated samples; dashed line, mock-inoculated samples. Left, V. aestivalis; right, V. vinifera. Asterisk indicates the genes that were significantly expressed at a higher or lower level (FDR-corrected P < 0.05) in V. aestivalis than in V. vinifera.
Primary Metabolism Is Affected in PM-Inoculated V. vinifera
We analyzed the expression patterns of genes that are involved in primary and secondary metabolism and presented the results in Supplemental Figure S3. Genes in tetrapyrrole synthesis, light harvesting, the Calvin cycle, and photorespiration were mostly down-regulated, suggesting the overall down-regulation of photosynthesis genes (Supplemental Fig. S3A). Genes for pyruvate metabolism involving the conversion of pyruvate to acetyl-CoA were down-regulated together with genes for glycolysis (Supplemental Fig. S3B). The redistribution of carbon reserves was evident based on the up-regulation of genes encoding invertase and α amylase, which function to convert Suc and starch into Fru and Glc (Supplemental Fig. S3B). In addition, the up-regulation of genes for Glc-6-P dehydrogenase and chorismate mutase suggested the involvement of shikimate pathway in the synthesis of flavonoid secondary metabolites (Supplemental Fig. S3B). Lipid metabolism was also strongly affected with the down-regulation of genes in the fatty acid synthesis pathway as well as the up-regulation of genes in the fatty acid degradation pathway (Supplemental Fig. S3C). These transcriptional changes indicated a possible remobilization of fatty acid carbon reserves back to the key precursor, acetyl-CoA, which was correlated with the up-regulation of isoprenoid biosynthesis during PM infection (Supplemental Fig. S3E). In addition, it was found that many of the PM-responsive genes involved in primary metabolism were expressed at a higher level in V. vinifera than in V. aestivalis under mock-inoculation conditions (Supplemental Fig. S3, A and B).
Major Secondary Metabolic Pathways Are Affected in PM-Inoculated V. vinifera
In combating pathogens, a range of secondary metabolites were synthesized, leading to antimicrobial compounds and to lignins, which reinforce cell walls (Nicholson and Hammerschmidt, 1992; Vermerris and Nicholson, 2006). Our data also suggested that the expression levels of genes that are part of the flavonoid synthesis branch within the phenylpropanoid metabolic grid steadily increased with the progression of the disease (Supplemental Fig. S3D). The up-regulation of stilbene synthase genes was the most noticeable PM-induced change, and the expression patterns of five PM-responsive stilbene synthase genes were similar (Figs. 4B and 5). Transcript abundance increased dramatically from 0 to 12 hpi, decreased from 12 to 24 dpi, and then increased again from 24 to 48 dpi (Figs. 4B and 5). One gene encoding caffeic acid O-methyltransferase (Table I), a key enzyme in the synthesis of monolignols coniferyl and sinapyl alcohols, was also up-regulated (Supplemental Fig. S3D). The dramatic up-regulation of genes for dirigent-like proteins, which were proposed to play a role in lignin synthesis (Davin and Lewis, 2000), was also observed (Figs. 4B and 5). Some of these genes, including flavonoid O-methyltransferase, caffeic acid O-methyltransferase, dirigent-like protein, and flavonoid 3-hydroxylase, were expressed at significantly higher levels in V. aestivalis under mock inoculation and, in some cases, also in PM-inoculated leaves (Fig. 5; Supplemental Fig. S3D).
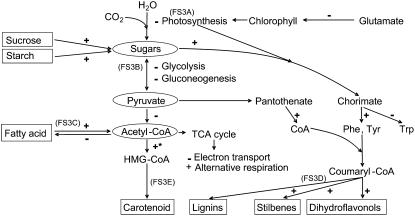
Within the isoprenoid biosynthetic pathway, there were two key genes up-regulated, hydroxymethylglutaryl-CoA (HMG CoA) synthase and HMG CoA reductase, that convert acetyl-CoA into HMG CoA and then to mevalonate, the precursor for isopentenyl pyrophosphate and the various terpene, carotenoid, or sterol compounds (Supplemental Fig. S3E). The HMG CoA reductase gene was also one of four genes that was significantly up-regulated in V. aestivalis during PM infection (Fig. 5). The up-regulation of these two genes suggested a tight coordination between the up-regulation of the isoprenoid pathway and the metabolism of acetyl-CoA via β-oxidation of fatty acid (Supplemental Fig. S3C). In particular, the transcript of HMG CoA synthase was expressed at a significantly higher level in V. aestivalis under both mock- and PM-inoculation conditions (Fig. 5; Supplemental Fig. S3E). An overview of the transcriptome changes in metabolic pathways in response to PM infection and possible consequences from mobilization of carbon reserves to secondary metabolites is presented in Figure 6.
Figure 6.
Overview of major metabolic pathways in response to PM in V. vinifera, as suggested by overall expression pattern of PM-responsive genes that are involved in primary and secondary biochemical pathways. Expression profile of each individual gene is presented in Supplemental Figure S3, A to E, indicated in parentheses as FS3A, B, C, D, and E for each major pathway. Pathways with transcripts that are up- or down-regulated are indicated with + or −. *, The HMG-CoA reductase gene that is also responsive to PM infection in V. aestivalis. Precursor metabolites are circled and end products are boxed.
DISCUSSION
PM-Induced Defense Response in V. aestivalis ‘Norton’
Based on previous findings in other plant-pathogen interactions (Mysore et al., 2002; Schenk et al., 2003; Tao et al., 2003), we hypothesized that a PM-induced transcriptional response would be detected in V. aestivalis ‘Norton’. We found many genes showing strong evidence for transcriptional change in the susceptible V. vinifera and a much weaker response in the disease-resistant V. aestivalis. We identified three differentially expressed genes in V. aestivalis and 625 in V. vinifera. Of these, 533 were statistically significantly different in their response to the fungus, indicating that the transcriptome of the resistant grape genotype responded weakly to PM. This weak transcriptome response in pathogen-affected tissues is consistent with recent findings in the tomato (Solanum lycopersicum)-PM interaction (Li et al., 2006) and also with the results that SA levels did not change significantly in PM-infected leaves of V. aestivalis (Fig. 2A). Analysis of the nonpathogen-challenged abundance of 625 PM-responsive transcripts between the two grape genotypes indicated that 83 were expressed at a higher level and 219 were expressed at a lower level in V. aestivalis relative to V. vinifera at all time points in the experiment. Therefore, a possible explanation for identifying only three PM-responsive transcripts in V. aestivalis is the constitutive high- or low-level expression of many defense genes in this cultivar. Transcripts that are present at constitutively high or low abundance in the absence of PM are expected to be only weakly modulated in response to PM attack. Another possible explanation is that the response in V. aestivalis was limited to only those relatively few epidermal cells that interacted with PM or because the Vitis GeneChip does not represent the entire grape genome.
Among the transcripts present at elevated levels in the mock-inoculated V. aestivalis leaves was EDS1 (Fig. 5), a key regulator of defense that is required for the pathogen-induced accumulation of SA (Parker et al., 1996; Wiermer et al., 2005; Bartsch et al., 2006). Other defense-related regulatory genes that were expressed at elevated levels in nonpathogen-challenged V. aestivalis were MAPKK and WRKY (Fig. 5). Several PR protein genes, such as PR-1, PR-2, PR-3, and PR-9, as well as other defense-related genes (e.g. a stilbene synthase, a flavonoid-O-methyltransferase, and a dirigent-like protein), also were transcribed at higher levels in nonpathogen-challenged V. aestivalis than in V. vinifera (Fig. 5; Fung et al., 2007). Is it possible that the constitutively elevated expression of these defense-related transcripts plays a role in the rapid epidermal cell response and the enhanced resistance to PM in V. aestivalis? Addressing this question will require additional experiments to provide evidence for the cause-effect relationship between expression levels of these genes and disease resistance.
In apple (Malus domestica), the association of elevated defense gene expression with resistance to the apple scab fungal pathogen (Venturia ineaqualis) was recently reported (Degenhardt et al., 2005). In the scab-resistant variety Remo genes encoding PR-10, PR-2, PR-3, Cys protease inhibitor, and metallothioneins were constitutively expressed at higher levels than in the scab-susceptible variety Elstar in the absence of the pathogen. Elevated defense gene expression is also known to be associated with pathogen resistance in the Arabidopsis lesions stimulating disease and constitutive expression of PR genes (cpr) mutants (Dietrich et al., 1994; Silva et al., 1999; Clarke et al., 2001). In these mutants, expression levels of defense genes can be tempered by reducing SA levels (Hunt et al., 1997; Silva et al., 1999). Furthermore, several of the Arabidopsis cpr mutants have constitutively elevated SA levels even in the absence of pathogen (Silva et al., 1999; Clarke et al., 2001). It is interesting to note that our HPLC assays showed that the SA content in the leaves of V. aestivalis was significantly higher than that in V. vinifera under the mock-inoculation condition (Fig. 2). At present, it is not known to what extent this SA content represents glycosylated and biologically active free SA in these grapevines. Nevertheless, the co-occurrence of disease resistance, constitutive high-level expression of defense genes, and elevated SA levels in V. aestivalis is intriguing and warrants further investigations.
PM-Induced Defense Response in V. vinifera ‘Cabernet sauvignon’
Accumulating evidence supports the hypothesis that in compatible interactions leading to susceptibility, obligate biotrophic pathogens inactivate host defense responses to sustain their interaction with living host cells (Bushnell and Rowell, 1981; Panstruga, 2003; Schulze-Lefert and Panstruga, 2003). In this study, the analysis of the total number of PM-responsive genes and their expression patterns in V. vinifera revealed that the most dynamic interactions between pathogen and host occurred after 12 hpi (Figs. 3 and 4B). This is consistent with observations that the majority of conidia penetrated host cells and began to form secondary hyphae, a sign of the formation of functional haustorium, by 24 hpi (Fig. 1). Induced expression of key defense genes and increased SA levels strongly suggested that defense responses were activated in the PM-infected V. vinifera leaf tissue (Supplemental Table S3; Figs. 2B, 4, and 5). We found that expression of a set of defense-related genes, including PR genes and secondary metabolite biosynthesis genes, reached maximum levels at 12 hpi and then declined (clusters 16 and 18, Fig. 4B). This phenomenon was also observed in the wheat (Triticum aestivum)-PM and barley-PM interactions (Caldo et al., 2004); the expression of genes involved in secondary metabolism first increased and then declined between 24 and 32 hpi (Caldo et al., 2004, 2006). In the wheat-PM compatible interactions, expression of PR genes (WIR1 and WIR2) increased within 1 d after fungal infection and then gradually dropped over a 6-d period (Waspi et al., 2001). The reduced expression level of these defense-related genes during the critical period in grapevine-, barley-, or wheat-PM compatible interactions suggests that the haustoria export fungal factors into host cells that could act as suppressors of defense-related gene expression (Panstruga, 2003; Schulze-Lefert and Panstruga, 2003; Caldo et al., 2004).
Changes of Metabolic Pathways in PM-Infected V. vinifera ‘Cabernet sauvignon’
The reprogramming of metabolic pathways is considered to be one of the defense strategies that plants utilize to generate antimicrobial compounds and signal molecules for restraining the growth of pathogens (La Camera et al., 2004). Our results suggest that the PM-susceptible V. vinifera reprograms primary metabolic pathways and activates secondary metabolism in response to PM infection (Fig. 6; Supplemental Fig. S3). The up-regulation of invertases (Supplemental Fig. S3B), genes that are involved in degradation of major carbon reserves into hexoses, could result in the reduction in net photosynthetic rate, as documented in other plant-biotrophic pathogen interactions (Scholes et al., 1994; Hahn and Mendgen, 2001; Walters and McRoberts, 2006). This metabolic shift seems to be coordinated with the up-regulations of genes involved in the synthesis of secondary metabolites (flavonoids and lignin) via the oxidative pentose phosphate pathway and shikimate pathway (Supplemental Fig. S3B; Scheideler et al., 2002). Similar regulation of genes in the last steps of shikimate pathway for the biosynthesis of phytoalexins and lignins was demonstrated in barley-PM interactions (Caldo et al., 2004). The demand for acetyl-CoA in isoprenoid synthesis also appears to be coordinated with genes of fatty acid degradation pathways when pyruvate metabolic genes are largely down-regulated (Supplemental Fig. S3B). It is possible that reliance on fatty acid degradation for supply of acetyl-CoA, together with the biosynthesis of Phe and Tyr via the shikimate pathway, is required to sustain the remaining anabolic pathway during PM infection. Because leaves are not generally considered major reserves of fatty acids, more studies are needed to determine if the metabolite flux through pathways via acetyl-CoA can help meet the demands of isoprenoid and other secondary metabolite biosynthesis.
Stilbene synthase, which is a key enzyme in the synthesis of trans-resveratrol and stilbene phytoalexins, is induced in response to fungal elicitors, the oomycete Plasmopara viticola, and the necrotrophic fungal pathogens Botrytis cinerea and Phomopsis viticola in V. vinifera (Melchior and Kindl, 1991; Bavaresco and Fregoni, 2001; Tassoni et al., 2005). In this study, multiple transcripts of stilbene synthase genes were induced in response to the biotrophic E. necator (Figs. 4B and 5), which is in agreement with the findings that high levels of trans-resveratrol accumulate in PM-infected berries (Piermattei et al., 1999). In contrast, the transcript abundance of flavonoid genes continuously increased over the course of infection (Fig. 5; Supplemental Fig. S3D), suggesting that they may be regulated in a different way from stilbene synthases. Genes in this group include ferulate-5-hydroxylase gene in monolignol synthesis and genes involved in flavonoid biosynthesis, such as flavonoid O-methyltransferase and UDP-Glc flavonoid 3-O-glucosyltransferase genes (Table I).
CONCLUSION
At the transcriptional level, disease-resistant V. aestivalis ‘Norton’ responded weakly to PM. In contrast, in disease-susceptible V. vinifera ‘Cabernet sauvignon’, genes encoding key defense components were significantly up-regulated in response to PM. Endogenous SA content was significantly higher in V. aestivalis ‘Norton’ than in V. vinifera ‘Cabernet sauvignon’. Although it is not clear if these genotype-specific differences in transcript abundance of defense-related genes and higher SA content contribute to PM resistance in V. aestivalis ‘Norton’, these new discoveries point toward future experiments that can uncover the mechanisms responsible for fungal resistance in V. aestivalis ‘Norton’ and for fungal susceptibility in V. vinifera ‘Cabernet sauvignon’.
MATERIALS AND METHODS
Full MIAME/Plant compliant descriptions of sources of the PM and grapevines, experiment design, plant samples, RNA extraction, array hybridization, and statistical analysis are included in the Supplemental Data.
Microscopic Observation
Fully expanded leaves at the third or fourth positions from the shoot tip were chosen for inoculation, the inoculated area of each leaf was marked with India ink, and the area was excised at 24-h intervals for light microscopic observation. Removal of chlorophyll and fixation of leaf tissue followed a previous protocol (Vanacker et al., 2000). Fungal spores and hyphae were stained by 0.05% aniline blue in a lacto-glycerol solution (1:1:1, lactic acid:glycerol:water; v/v). The growth conditions and inoculation procedure of leaves with conidiospores are the same as those described in the experiment design for the microarray (see MIAME/Plant in the Supplemental Data for details).
Measurement of SA
In the SA assays of Vitis aestivalis and Vitis vinifera with PM or mock inoculation, four leaves were randomly collected from each vine and pooled to form one biological replicate. In the comparative SA analysis of the two genotypes, five leaves were randomly chosen from each vine and pooled to form one biological replicate. Three independent biological replicates were sampled. Leaf samples were immediately frozen and ground in liquid nitrogen. One-half gram of leaf tissues was extracted for measuring acid-hydrolyzed SA by a procedure modified from previous protocols (Gaffney et al., 1993; Verberne et al., 2002). Vacuum-dried extracts were suspended in 300 μL of 20% methanol. Five microliters of samples was injected. SA was analyzed by the isocratic analysis on reversed phase columns (4.6 × 75 mm Zorbax SB-C18 3.5 μm and Zorbax High Pressure Reliance Cartridge Guard Columns, Agilent) with diode array detector on an Agilent HPLC 1100 Series instrument. Flow rate is 1.2 mL/min. Each sample was measured three times. Quantification of SA concentration was determined in a linear range of 1 to 100 ng/μL calibration curve for sodium salicylate.
Clustering of Expression Pattern
In each time point, the mean of the normalized expression level for the mock-inoculation treatment was subtracted from the mean of the normalized expression level for PM-inoculation treatment. The differences were analyzed using the method developed by Qu and Xu (2006). This method is based on fitting a polynomial model for expression over time for each gene and clustering genes with the same polynomial coefficients. For our analysis, we used polynomial models of order 4. The clustering strategy with the minimum Bayesian Information Criterion (i.e. the grouping that best fit the data) was selected. The profiles of the differences between treatments over time were grouped into 25 clusters. Most of the genes (13,078 of 14,571 genes) clustered in cluster 1, which is the cluster with a flat profile (no difference over time between the two treatments). Genes assigned to the remaining 24 clusters ranged from 3 (cluster 16) to 120 (cluster 23).
qRT-PCR
Total RNAs for qRT-PCR were from the same samples as used for GeneChip hybridization. Total RNA were treated with TURBO DNA-free DNase I (Ambion) and purified using RNeasy MinElute Cleanup kit (Qiagen). RNA quantity and quality were assessed by GeneQuant pro spectrophotometer (GE Healthcare) and by Agilent 2100 Bioanalyzer (Agilent). Two micrograms of DNase I-treated RNA from three biological replicate samples at each time point were pooled. Complementary DNA was synthesized from the RNA samples by MultiScribe reverse transcriptase with random hexamer oligonucleotide provided in the Taqman Reverse Transcription Reagent kit (Applied Biosystems). The amounts of transcripts of a selected gene in PM-inoculated versus mock-inoculated grapevine leaf tissues were compared by using actin as control. PCRs were performed in the MX3005P system (Stratagene) using SYBR Green. Reaction was set up following the protocol in the SYBR Green RT-PCR reagent kit (Applied Biosystems). Each reaction was run in triplicates in reaction volume of 20 μL. Cycling parameters were 95°C for 10 min, 50 cycles of 95°C for 15 s, and 60°C for 30 s. Data were analyzed in the MxPro-Mx3005P v3.00 QPCR software (Stratagene) according to the manufacturer's instructions. The differences in CT (threshold cycle) values for actin across all V. vinifera and V. aestivalis cDNA samples series were 1.73 and 1.2, respectively. PCR efficiency (E) was calculated from the exponential phase of each individual amplification plot and the equation (1 + E) = 10slope based on a previous method (Peirson et al., 2003). Expression level of genes of interest (GOI) was normalized to that of actin by subtracting the CT value of actin from the CT value of the GOI after the expression level of GOI and actin was adjusted with the mean E. Fold-change was calculated as the exponentiation of the difference in natural log values between PM-inoculated and mock-inoculated conditions.
Annotation of Affymetrix Probe Sets
For each Affymetrix probe set, component sequence with longer coding region and 3′ poly(A) tail was identified in the NCBI UniGene database. Then the closest homolog was identified through BLASTX searches (NCBI). The closest Arabidopsis (Arabidopsis thaliana) homolog was also identified by BLAST (NCBI) and cross checked with The Arabidopsis Information Resource.
Affymetrix data of this study have been deposited in the Gene Expression Omnibus. The accession number is GSE6404.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Relationship between the measurement of difference in the PM-inoculated versus mock-inoculated conditions in qRT-PCR and microarray of the 15 genes at six time points: 0, 4, 8, 12, 24, and 48 hpi.
Supplemental Figure S2. Profiles of the mean difference for the 25 clusters of 16,437 transcripts of V. vinifera after inoculation with the PM.
Supplemental Figure S3. Expression profiles of the PM-responsive genes in selected metabolic pathways.
Supplemental Table S1. Gene homologs, UniGene ID, log-transformed expression value, fold-change, nominal P value, FDR-corrected P value for 625 PM-responsive transcripts in V. vinifera, and three PM-responsive transcripts in V. aestivalis.
Supplemental Table S2. Pearson correlation between the measurement of difference in PM-inoculated versus mock-inoculated conditions in qRT-PCR and by microarray for the 15 PM-responsive genes in V. vinifera and V. aestivalis.
Supplemental Table S3. Affymetrix probe set ID in each of the 25 clusters of 16,437 transcripts of V. vinifera after inoculation with PM.
Supplementary Material
Acknowledgments
We thank Kari Huppert, Karen McPherson, and Nan Li for their outstanding technical support and Susanne Howard, Clayton Dennis, and Lisa Bono for their assistance with data management. We thank James Schoelz for insightful discussion and Walter Gassmann and Shauna Somerville for the critical reviewing of the manuscript. We also thank Don Baldwin of the University of Pennsylvania Microarray Core Facility for the excellent microarray processing services and Sunridge Nurseries for their generous gift of V. vinifera propagation material.
This work was supported by the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (grant nos. 2004–38901–02138 and 2006–38901–02138).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wenping Qiu (wenpingqiu@missouristate.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomerz-Gomerz L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415 977–983 [DOI] [PubMed] [Google Scholar]
- Barker CL, Donald T, Pauquet J, Ratnaparkhe MB, Bouquet A, Adam-Blondon AF, Thomas MR, Dry IB (2005) Genetic and physical mapping of the grapevine powdery mildew resistance gene, Run1, using a bacterial artificial chromosome library. Theor Appl Genet 111 370–377 [DOI] [PubMed] [Google Scholar]
- Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18 1038–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavaresco L, Fregoni C (2001) Physiological role and molecular aspects of grapevine stilbenic compounds. In KA Roubelakis-Angelakis, ed, Molecular Biology and Biotechnology of the Grapevine. Kluwer Academic Publisher, Boston, pp 183–202
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical approach to multiple testing. J R Stat Soc Ser B Stat Methodol 57 289–300 [Google Scholar]
- Broekaert WF, Delaure SL, De Bolle MFC, Cammue BPA (2006) The role of ethylene in host-pathogen interactions. Annu Rev Phytopathol 44 393–416 [DOI] [PubMed] [Google Scholar]
- Busam G, Kassemeyer H-H, Matern U (1997) Differential expression of chitinase in Vitis vinifera L. responding to systemic acquired resistance activators or fungal challenge. Plant Physiol 115 1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell WR, Rowell JB (1981) Suppressors of defense reactions: a model for roles in specificity. Phytopathology 71 1012–1014 [Google Scholar]
- Caldo RA, Nettleton D, Peng J, Wise RP (2006) Stage-specific suppression of basal defense discriminates barley plants containing fast- and delayed-acting Mla powdery mildew resistance alleles. Mol Plant Microbe Interact 19 939–947 [DOI] [PubMed] [Google Scholar]
- Caldo RA, Nettleton D, Wise RP (2004) Interaction-dependent gene expression in Mla-specified response to barley powdery mildew. Plant Cell 16 2514–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124 803–814 [DOI] [PubMed] [Google Scholar]
- Clarke JD, Aarts N, Feys BJ, Dong X, Parker JE (2001) Constitutive disease resistance requires EDS1 in the Arabidopsis mutants crp1 and cpr6 and is partially EDS1-dependent in cpr5. Plant J 26 409–420 [DOI] [PubMed] [Google Scholar]
- Dalbo MA, Weeden NF, Reisch BI (2000) QTL analysis of disease resistance in interspecific hybrid grapes. Acta Hortic 528 215–217 [Google Scholar]
- Davin LB, Lewis NG (2000) Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiol 123 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt J, Al-Masri AN, Kurkcuoglu S, Szankowski I, Gau AE (2005) Characterization by suppression subtractive hybridization of transcripts that are differently expressed in leaves of apple scab-resistant and susceptible cultivars of Malus domestica. Mol Genet Genomics 273 326–335 [DOI] [PubMed] [Google Scholar]
- Derckel JP, Legendre L, Audran JC, Haye B, Lambert B (1996) Chitinases of the grapevine (Vitis vinifera L.): five isoforms induced in leaves by salicylic acid are constitutively expressed in other tissues. Plant Sci 119 31–37 [Google Scholar]
- Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77 565–577 [DOI] [PubMed] [Google Scholar]
- Donald TM, Pellerone F, Adam-Blondon A-F, Bouquet A, Thomas MR, Dry IB (2002) Identification of resistance analogs linked to a powdery mildew resistance locus in grapevine. Theor Appl Genet 104 610–618 [DOI] [PubMed] [Google Scholar]
- Doster MA, Schnathorst WC (1985. a) Comparative susceptibility of various grapevine cultivars to the powdery mildew fungus Uncinula necator. Am J Enol Vitic 36 101–104 [Google Scholar]
- Doster MA, Schnathorst WC (1985. b) Effects of leaf maturity and cultivar resistance on development of the powdery mildew fungus on grapevines. Phytopathology 75 318–321 [Google Scholar]
- Ferreira RB, Monteiro SS, Picarra-Pereira MA, Teixeira AR (2004) Engineering grapevine for increased resistance to fungal pathogens without compromising wine stability. Trends Biotechnol 22 168–173 [DOI] [PubMed] [Google Scholar]
- Ficke A, Gadoury DM, Seem RC, Godfrey D, Dry IB (2004) Host barriers and responses to Uncinula necator in developing grape berries. Phytopathology 94 438–445 [DOI] [PubMed] [Google Scholar]
- Fischer BM, Salakhutdinov I, Akkurt M, Kortekamp A, Eibach R, Edwards KJ, Topfer R, Zyprian E (2004) Quantitative trait locus analysis of fungal disease resistance factors on a molecular map of grapevine. Theor Appl Genet 108 501–515 [DOI] [PubMed] [Google Scholar]
- Fung RWM, Qiu WP, Su YC, Schachtman D, Huppert K, Fekete C, Kovacs LG (2007) Gene expression variation in grapevine species Vitis vinifera L. and Vitis aestivalis Michx. Genet Resour Crop Evol 54 1541–1553 [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261 754–756 [DOI] [PubMed] [Google Scholar]
- Giannakis C, Bucheli CS, Skene KGM, Robinson SP, Scott NS (1998) Chitinase and β-1,3-glucanase in grapevine leaves. Aust J Grape Wine Res 4 14–22 [Google Scholar]
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205–227 [DOI] [PubMed] [Google Scholar]
- Gregersen PL, Thordal-Christensen H, Forster H, Collinge DB (1997) Differential gene transcript accumulation in barley leaf epidermis and mesophyll in response to attack by Blumeria graminis f. sp. hordei (syn. Erysiphe graminis f. sp. hordei). Physiol Mol Plant Pathol 51 85–97 [Google Scholar]
- Hahn M, Mendgen K (2001) Signal and nutrient exchange at biotrophic plant-fungus interfaces. Curr Opin Plant Biol 4 322–327 [DOI] [PubMed] [Google Scholar]
- Heintz C, Blaich R (1989) Structural characters of epidermal cell walls and resistance to powdery mildew of different grapevine cultivars. Vitis 28 153–160 [Google Scholar]
- Heintz C, Blaich R (1990) Ultrastructural and histochemical studies on interactions between Vitis vinifera L. and Uncinula necator (Schw.) Burr. New Phytol 115 107–117 [Google Scholar]
- Huckelhoven R, Kogel K-H (2003) Reactive oxygen intermediates in plant-microbe interactions: who is who in powdery mildew resistance? Planta 216 891–902 [DOI] [PubMed] [Google Scholar]
- Hunt MD, Delaney TP, Dietrich RA, Weymann KB, Dangl JL, Ryals JA (1997) Salicylate-independent lesion formation in Arabidopsis lsd mutants. Mol Plant Microbe Interact 10 531–536 [DOI] [PubMed] [Google Scholar]
- Jacobs AK, Dry IB, Robinson SP (1999) Induction of different pathogenesis-related cDNAs in grapevine infected with powdery mildew and treated with ethephon. Plant Pathol 48 325–336 [Google Scholar]
- Jones JDG, Dangl JL (2006) The plant immune system. Nature 444 323–329 [DOI] [PubMed] [Google Scholar]
- La Camera S, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, Legrand M, Heintz T (2004) Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipid pathways. Immunol Rev 198 267–284 [DOI] [PubMed] [Google Scholar]
- Li C, Bai Y, Jacobsen E, Visser R, Lindhout P, Bonnema G (2006) Tomato defense to the powdery mildew fungus: differences in expression of genes in susceptible, monogenic- and polygenic-resistance responses are mainly in timing. Plant Mol Biol 62 127–140 [DOI] [PubMed] [Google Scholar]
- Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosalh S, Scheel D, et al (2005) Pre- and post-invasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310 1180–1183 [DOI] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgen A, Schmid J, Lawton K, Dangl JL, Dietrich RA (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26 403–410 [DOI] [PubMed] [Google Scholar]
- Melchior F, Kindl H (1991) Coordinate and elicitor-induced expression of stilbene synthase and phenylalanine ammonia-lyase genes in Vitis cv. Optima. Arch Biochem Biophys 288 552–557 [DOI] [PubMed] [Google Scholar]
- Mullins MG, Bouquet A, Williams LE (1992) Biology of the Grapevine. Cambridge University Press, New York
- Mysore KS, Crasta OR, Tuori RP, Folkerts O, Swirsky PB, Martin GB (2002) Comprehensive transcript profiling of Pto- and Prf-mediated host defense response to infection by Pseudomonas syringae pv. tomato. Plant J 32 299–315 [DOI] [PubMed] [Google Scholar]
- Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30 369–389 [Google Scholar]
- O'Connell RJ, Panstruga R (2006) Tete a tete inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol 171 699–718 [DOI] [PubMed] [Google Scholar]
- Olmo H (1971) Vinifera x rotundifolia hybrids wine grapes. Am J Enol Vitic 22 87–91 [Google Scholar]
- Panstruga R (2003) Establishing compatibility between plants and obligate biotrophic pathogens. Curr Opin Plant Biol 6 320–326 [DOI] [PubMed] [Google Scholar]
- Panstruga R, Schulze-Lefert P (2002) Live and let live: insights into powdery mildew disease and resistance. Mol Plant Pathol 3 495–502 [DOI] [PubMed] [Google Scholar]
- Parker JE, Holub EB, Frost LN, Falk A, Gunn ND, Daniels MJ (1996) Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8 2033–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauquet J, Bouquet A, This P, Adam-Blondon A-F (2001) Establishment of a local map of AFLP markers around the powdery mildew resistance gene Run1 in grapevine and assessment of their usefulness for marker assisted selection. Theor Appl Genet 103 1201–1210 [Google Scholar]
- Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31 e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piermattei B, Piva A, Castellari M, Arfelli G, Amati A (1999) The phenolic composition of red grapes and wines as influenced by Oidium tuckeri development. Vitis 38 85–86 [Google Scholar]
- Qu Y, Xu S (2006) Quantitative trait associated microarray gene expression data analysis. Mol Biol Evol 23 1558–1573 [DOI] [PubMed] [Google Scholar]
- Regner F, Fardossi A, Eisenheld C, Steirschneider I, Haas M (2003) Genetic analysis of a segregating population derived by a cross of Welschriesling × Sirius. In E Hajdu, E Borbas, eds, Proceedings of the Eighth International Conference on Grape Genetics and Breeding, Vol 1. International Society for Horticultural Science, Leuven, Belgium, pp 141–148
- Renault AS, Deloire A, Letinois I, Kraeva E, Tesniere C, Ageorges A, Redon C, Brierne J (2000) β-1,3-glucanse gene expression in grapevine leaves as a response to infection with Botrytis cinerea. Am J Enol Vitic 51 81–87 [Google Scholar]
- Robinson SP, Jacobs AK, Dry IB (1997) A class IV chitinase is highly expressed in grape berries during ripening. Plant Physiol 114 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbolz J, Kassemeyer H-H, Steinmetz V, Deising HB, Mendgen K, Mathys D, Wirtz S, Guggerheim R (2000) Differentiation of infection structures of the powdery mildew fungus Uncinula necator and adhesion to the host cuticle. Can J Bot 78 409–421 [Google Scholar]
- Salzman RA, Tikhonova I, Bordelon BP, Hasegawa PM, Bressan RA (1998) Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defense response during fruit ripening in grape. Plant Physiol 117 465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheideler M, Schlaich NL, Fellenberg K, Beissbarth T, Hauser NC, Vingron M, Slusarenko AJ, Hoheisel JD (2002) Monitoring the switch from housekeeping to pathogen defense metabolism in Arabidopsis thaliana using cDNA arrays. J Biol Chem 277 10555–10561 [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Manners JM, Anderson JP, Simpson RS, Wilson IW, Somerville SC, Maclean DJ (2003) Systemic gene expression in Arabidopsis during an incompatible interaction with Alternaria brassicicola. Plant Physiol 132 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes JD, Lee PJ, Horton P, Lewis DH (1994) Invertase: understanding changes in the photosynthetic and carbohydrate metabolism of barley leaves infected with powdery mildew. New Phytol 126 213–222 [Google Scholar]
- Schulze-Lefert P, Panstruga R (2003) Establishment of biotrophy by parasitic fungi and reprogramming of host cells for disease resistance. Annu Rev Phytopathol 41 641–667 [DOI] [PubMed] [Google Scholar]
- Shah J (2003) The salicylic acid loop in plant defense. Curr Opin Plant Biol 6 365–371 [DOI] [PubMed] [Google Scholar]
- Silva H, Yoshioka K, Dooner HK, Klessig DF (1999) Characterization of a new Arabidopsis mutant exhibiting enhanced disease resistance. Mol Plant Microbe Interact 12 1053–1063 [DOI] [PubMed] [Google Scholar]
- Tao Y, Xie Z, Chen W, Glazebrook J, Chang H-S, Han B, Zhu T, Zou G, Katagiri F (2003) Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringe. Plant Cell 15 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassoni A, Fornale S, Franceschetti M, Musiani F, Michael AJ, Perry B, Bagni N (2005) Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol 166 895–905 [DOI] [PubMed] [Google Scholar]
- Tattersall DB, Pocock KF, Hayasaka Y, Adams K, van Heeswijk R, Waters EJ, Hoj RB (2001) Pathogenesis-related proteins: their accumulation in grapes during berry growth and their involvement in white wine heat instability: current knowledge and future perspectives in relation to winemaking practices. In KA Roubelakis-Angelakis, ed, Molecular Biology and Biotechnology of the Grapevine. Kluwer Academic Publisher, Boston, pp 183–201
- Tattersall DB, van Heeswijck R, Hoj RB (1997) Identification and characterization of a fruit-specific thaumatin-like protein that accumulates at high levels in conjunction with the onset of sugar accumulation and berry softening in grapes. Plant Physiol 114 759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena G, Asai T, Chiu W-L, Sheen J (2001) Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 4 392–400 [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J (1992) Acquired resistance in Arabidopsis. Plant Cell 4 645–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H, Carver TLW, Foyer C (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiol 123 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44 135–162 [DOI] [PubMed] [Google Scholar]
- Verberne MC, Brouwer N, Delbianco F, Linthorst HJM, Bol JF, Verpoorte R (2002) Method for the extraction of the volatile compound salicylic acid from tobacco leaf material. Phytochem Anal 13 45–50 [DOI] [PubMed] [Google Scholar]
- Vermerris W, Nicholson R (2006) The role of phenols in plant defense. In W Vermerris, R Nicholson, eds, Phenolic Compound Biochemistry. Springer, Dordrecht, The Netherlands, pp 211–234
- Walters DR, McRoberts N (2006) Plants and biotrophs: a pivotal role for cytokinins. Trends Plant Sci 11 581–586 [DOI] [PubMed] [Google Scholar]
- Waspi U, Schweizer P, Dudler R (2001) Syringolin reprograms wheat to undergo hypersensitive cell death in a compatible interaction with powdery mildew. Plant Cell 13 153–161 [PMC free article] [PubMed] [Google Scholar]
- Wiermer M, Feys BJ, Parker J (2005) Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol 8 383–389 [DOI] [PubMed] [Google Scholar]
- Xu X, Chen C, Fan B, Chen Z (2006) Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18 1310–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.