Abstract
Deleted in Malignant Brain Tumours 1 (DMBT1) is a secreted scavenger receptor cysteine-rich protein that binds and aggregates various bacteria and viruses in vitro. Studies in adults have shown that DMBT1 is expressed mainly by mucosal epithelia and glands, in particular within the respiratory tract, and plays a role in innate immune defence. We hypothesized that respiratory DMBT1 levels may be influenced by various developmental and clinical factors such as maturity, age and bacterial infection. DMBT1 levels were studied in 205 tracheal aspirate samples of 82 ventilated preterm and full-term infants by enzyme-linked immunosorbent assay. Possible effects of various clinical parameters were tested by multiple regression analysis. DMBT1 levels increased significantly with lung maturity (P < 0·0001 for both gestational and postnatal age) and in small-for-gestational-age infants (P = 0·0179). An increase of respiratory DMBT1 levels was detected in neonatal infections (P < 0·0001). These results were supported by Western blotting. Immunohistochemical analyses of archived newborn lung sections (n = 17) demonstrated high concentrations of DMBT1 in lungs of neonates with bacterial infections. Our data show that preterm infants are able to up-regulate DMBT1 in infection as an unspecific immune reaction.
Keywords: DMBT1, gestational age, gp-340, innate immunity, prematurity
Introduction
The newborn infant is particularly dependent upon innate immunity, because the adaptive immune response develops after confrontation with microorganisms. Decreased plasma concentrations of cytokines [1], complement factors [2] and mannose binding lectin [3] are examples of immature innate immunity.
Recently, Deleted in Malignant Brain Tumours 1 (DMBT1), also known as glycoprotein-340 (gp340) or salivary agglutinin (SAG), has been shown to play a role in local innate immune defence [4]. DMBT1 belongs to the scavenger receptor cysteine-rich (SRCR) group B protein family and was described first as a putative tumour-suppressor gene in medulloblastoma and glioblastoma multiforme [5]. Then, glycoprotein-340 (DMBT1gp340) and salivary agglutinin (DMBT1SAG) were detected as alternative splicing forms of DMBT1 [6]. DMBT1 is expressed mainly by mucosal epithelia and glands, in particular in the respiratory tract [4,5,7]. In adults, research interest in DMBT1 was stimulated by the finding of its possible role in tumour suppression [7,8].
In the adult respiratory tract, DMBT1 is expressed by alveolar type II cells, epithelial cells and associated glands [6,7]. The participation of DMBT1 in pathogen defence includes binding of a broad range of bacteri, including various species and strains of streptococci [9–12], interaction with surfactant proteins A and D [6,9,13] and secretory IgA [14], activation of the complement system [15] and stimulation of alveolar macrophages [13]. DMBT1 inhibits infection by HIV type 1 and influenza A viruses in vitro [16,17].
So far, there has been no literature available about DMBT1 expression in premature or full-term neonates. Because of its potential role in innate immunity, we hypothesized that respiratory DMBT1 levels may be influenced by various developmental and clinical factors such as maturity, age and bacterial infection.
Materials and methods
Infants and tracheal aspirates
The study group comprised 82 mechanically ventilated newborn infants (40 males, 42 females) treated at the Perinatal Center of the University of Heidelberg. The study was approved by the Ethics Committee and the parents agreed by informed consent. Gestational age is defined as time elapsed between the first day of the last menstrual period and the day of delivery. The mean values of the infants' gestational age derived from the maternal history and were confirmed clinically. The total study population had an averaged gestational age of 28·5 ± 4·4 weeks (range: 23–40 weeks; 23–25 weeks: 29; 26–29 weeks: 32; 30–36 weeks: 16; 37–40 weeks: 5). Twenty-two of the 82 infants were small-for-gestational-age (SGA) at birth, defined as birth weight below the 10th percentile (Bavarian growth charts).
A total of 205 tracheal aspirates were collected from 82 infants. The first sample was taken during the first 3 days from 40 infants and between days 4 and 120 from the other 42 infants. Subsequent samples were obtained every 2–4 days and stored immediately at −20°C. Sterile isotonic sodium chloride (0·9%; 0·5–1·0 ml) was instilled into the endotracheal tube and the trachea was suctioned once or twice after three breaths of manual or mechanical ventilation. The total protein concentration of the tracheal aspirates was measured by using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA).
Production and purification of human recombinant DMBT1 (hrDMBT1)
Human rDMBT1 was produced and purified in the laboratory of the Institute of Molecular Biology and Cell Culture Technology, University of Applied Sciences Mannheim, as described elsewhere [18]. Briefly, the hrDMBT1 producer cell line T3 was induced with 10 μg/ml tetracycline for 48 h. After collecting the hrDMBT1-containing supernatant, the protein was isolated by mixing the supernatant with a suspension of Streptococcus gordonii. Phosphate-buffered saline (PBS) containing 10 mM ethylenediamine tetraacetic acid (EDTA) was used to elute bound hrDMBT1. The eluted protein was concentrated and applied to a size-exclusion chromatography column.
Monoclonal and polyclonal antibodies used for enzyme-linked immunosorbent assay (ELISA), Western blot and immunohistochemistry
The DMBT1-specific monoclonal antibody Hyb213-06 used for ELISA measurements and Western blotting was raised against DMBT1gp340 isolated from broncheoalveolar lavage, as described by Holmskov [19]. The specificity of the antibody was confirmed by Western blotting [20]. The monoclonal antibody anti-DMBT1h12 was generated against a synthetic peptide (RTTDYASLIPSEVPLC) corresponding to amino acids 26–40 of DMBT1 [4]. The polyclonal anti-serum (anti-DMBT1p84) was made against the recombinant DMBT1 and its specificity was confirmed by a comparison of immunhistochemical sections with RNA in situ hybridization (data not shown).
Determination of the DMBT1 concentration in tracheal aspirates by ELISA
As concentration standard we used purified hrDMBT1. Microtiter plates (Microlon, F-Shape; Greiner Bio-One GmbH, Essen, Germany) were coated with tracheal aspirate fluids overnight at 4°C. After washing with Tris-buffered saline (TBS) containing 0·1% Tween 20 (TBS-T), the plates were incubated with the DMBT1-specific monoclonal mouse antibody Hyb213–06 (Antibodyshop, Gentofte, Denmark [19,20]) diluted 1 : 5000 for 1 h at room temperature. Subsequently, the wells were incubated with a horseradish peroxidase-conjugated anti-mouse antibody (1 : 5000) for 1 h at room temperature. The bound enzyme was detected by adding TMB-substrate solution [125 μg/ml 3,3‵,5,5‵-tetramethyl-benzidine; 125 μg/ml in 0·1 M citrate buffer pH 4·5 with 0·05% (v/v) H2O2]. After incubation for 20 min in the dark, the reaction was stopped by addition of 2 M HCl. The intensity of the dye reaction was measured at 450 nm in an ELISA reader (EL800; Bio-Tec Instruments Inc., Winooski, VT, USA). The DMBT1 concentration of each sample was determined at least three times, and the DMBT1 level was calculated as percentage of total protein concentration in order to adjust for differential dilution factors. Thus, DMBT1 was expressed as (μg DMBT1/μg total protein) × 100.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analyses
Proteins of the tracheal aspirate samples were separated under non-reducing conditions on 4–15% polyacrylamide gels (Phast Gels™, Amersham Biosciences, Freiburg, Germany). Afterwards, proteins were transferred onto polyvinylidine difluoride (PVDF) membranes (Millipore, Schwalbach, Germany), which were blocked subsequently at 4°C overnight with 10% (w/v) milk and 3% (v/v) bovine serum albumin in TBS containing 0·1% Tween 20 (TBS-T). All blots were made in duplicate to detect DMBT1 with the monoclonal antibody Hyb213–06 (Antibodyshop [19,20]), and to detect the secretory component of IgA (sIgA) with an anti-serum from rabbit against the secretory component (Dako, Hamburg, Germany) diluted 1 : 5000 and 1 : 1000 in blocking solution, respectively. As secondary antibodies, goat anti-mouse-IgG and goat anti-rabbit-IgG conjugated to alkaline-phosphatase (Santa-Cruz Biotechnology, Heidelberg, Germany) were used at a dilution of 1 : 10 000 in blocking solution. Incubation steps were performed for 2 h at room temperature, followed by several washing steps with TBS-T. The membranes were developed with 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (BCIP/NBT) according to standard protocol.
Immunohistochemistry
Paraffin-embedded lung sections (thickness: 3–4 mm) of five stillborn infants and 12 neonates who died 1–42 days after birth was used for immunohistochemistry. The sections were examined at the Institute of Pathology (University of Heidelberg). The study was approved by the responsible Ethics Committee. Gestational ages at birth ranged from 20 to 41 weeks. None of these infants was included in the prospective analysis of DMBT1 in tracheal aspirates. For the detection of DMBT1 we used the monoclonal anti-DMBT1h12, as described earlier [21], and a polyclonal anti-serum (anti-DMBT1p84) generated against the human rDMBT1 in rabbit. Immunohistochemical studies with anti-DMBT1h12 were carried out manually, whereas those with anti-DMBT1p84 were carried out on an automated Ventana Discovery stainer (Ventana Medical Systems, Tucson, AZ, USA) according to a protocol described previously [21]. Peroxidase activity was detected with 3-amino-9-ethylcarbazole (AEC) as substrate (Sigma Chemicals, St. Louis, MO, USA). As a standard negative control, the antibodies were substituted by equal amounts of normal mouse IgG (Santa Cruz Biotechnology). All controls were negative. For image acquisition, digital analySIS 3·0 software, a 3CCD colour video 1705 camera (Sony Corp., Tokyo, Japan) and an Olympus BX-50 microscope (Olympus Optical, Hamburg, Germany) were used. We developed a score for determining the intensity and extent of DMBT1 signals. Sections displaying no signals were scored 0. DMBT1-positive tissues were scored semiquantitatively from 1 (weak staining) to 3 (highly intense staining). Each sample was scored independently by two investigators.
Statistics
The clinical data collected for each infant were correlated with pulmonary DMBT1 levels. Included in the analysis were gestational age, postnatal age, birth weight, SGA, gender, maternal infection, premature rupture of the membranes (>24 h), fetal lung maturation, asphyxia, respiratory distress syndrome (grade 1–4, according to Giedion et al. [22]), bronchopulmonary dysplasia (additional oxygen required at 36 weeks of corrected gestational age with abnormal radiological pulmonary findings), infection, survival, treatment with indomethacin and corticosteroids and operation.
Statistical analyses were performed with the sas software package, release 8·02. To provide a mathematical equation in order to estimate the DMBT1 levels in tracheal aspirates, we developed a general multiple model using the sas procedure proc reg. For this model, we included all potentially explanatory factors which were constant for each infant. We applied a backward selection method with a significance level of α = 0·05 to determine all significant variables in this multiple analysis.
Because the DMBT1 levels showed high interindividual variation between different infants, more analyses were conducted to examine associations between DMBT1 levels in tracheal aspirates and parameters that may vary within an individual infant. To analyse the effect of these variable parameters on individual DMBT1 levels, statistical models with only one explanatory factor were used, adjusted for each individual infant. Variable parameters were postnatal age, occurrence of infection, treatment with corticosteroids or indomethacin and operation. P-values smaller than 0·05 were regarded as statistically significant.
Results
Pulmonary DMBT1 levels
Relative DMBT1 levels expressed as percentage of total protein concentration in tracheal aspirates studied at days 1–3 of postnatal life increased significantly (P = 0·042) from [mean ± standard error of the mean (SEM)] 0·68 ± 0·14 at 23–25 weeks to 2·94 ± 0·77 at 34–38 weeks of gestation (Fig. 1). Twenty-two infants were SGA and 60 were appropriate-for-gestational-age (AGA). SGA infants (gestational age 23–33 weeks) without infection studied at 1–10 days of life showed higher DMBT1 levels than AGA infants with comparable gestational and postnatal age (1·28 ± 0·25 versus 1·03 ± 0·12). Figure 2 shows that DMBT1 levels increased with increasing postmenstrual age (defined as gestational age plus chronological age).
Fig. 1.
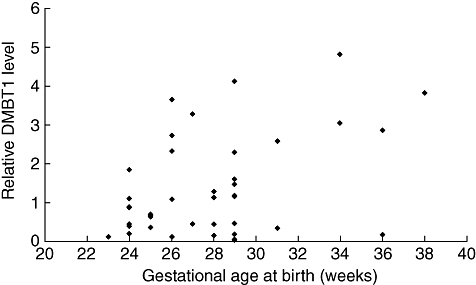
Relative Deleted in Malignant Brain Tumours 1 (DMBT1) levels, expressed as (μg DMBT1/μg total protein) × 100, at days 1–3 of postnatal life in preterm infants related to gestational age. The included infants showed no infections at this age.
Fig. 2.
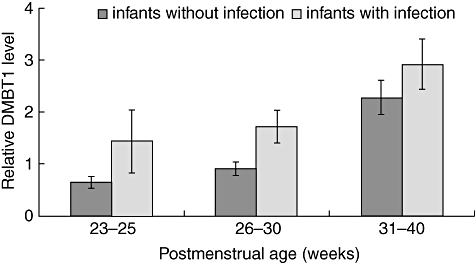
Relative Deleted in Malignant Brain Tumours 1 (DMBT1) levels of preterm infants related to postmenstrual age. Infants with infections showed higher DMBT1 levels than infants without infection. Results are means ± standard error of the mean and are expressed as (μg DMBT1/μg total protein) × 100.
The multiple models used for statistical analyses of factors associated significantly (P < 0·05) with the relative DMBT1 levels resulted in the following mathematical equation:
where y is the DMBT1 level expressed as percentage of the total protein concentration in tracheal aspirates. The variables gestational age in days (x1; P < 0·0001), postnatal age in days (x2; P < 0·0001) and SGA (x3 = 1, if the infant was SGA and 0 if not; P = 0·0179) significantly influenced the DMBT1 level.
Relative DMBT1 protein levels increased upon infection
A total of 65 tracheal aspirates were collected from 36 preterm infants with infection during the observation time. These infections were accompanied by at least C-reactive protein (CRP) elevation and clinical signs of infections over a period of several days, and in most cases with positive pathological laboratory tests (e.g. neutropenia, leucocytosis, thrombopenia). However, in 42 of 65 tracheal aspirate samples (65%) infection was confirmed by evidence of positive bacterial cultures (blood, tracheal aspirate fluid, stomach aspirate fluid, catheter tip and smear of skin, ear and pharynx). The following bacteria were detected: Streptococcus species, Staphylococcus species, Staphylococcus haemolyticus, Staph. epidermidis, Klebsiella pneumoniae, Enterococcus faecalis, Escherichia coli, Enterobacter cloacae, Enterobacter aerogenes and Pseudomonas aeruginosa.
Infection increased the relative DMBT1 level, particularly in very immature infants (Fig. 2). After adjustment for each infant a significant association with postnatal age and infection was revealed, while no significant association with the remaining parameters, including indomethacin and corticosteroid treatment, was found (Table 1). Figure 3 shows time–courses for relative respiratory DMBT1 levels in four preterm infants with bacterial infections who had been studied before and during the infection. Three infants showed a marked DMBT1 response and one did not.
Table 1.
Correlations of Deleted in Malignant Brain Tumours 1 (DMBT1) levels with clinical parameters.
| Parameter | n | Test | P-value | DMBT1 level |
|---|---|---|---|---|
| Postnatal age (days) | 205 | Regr.Anal. | < 0·0001 | +0·018 per day |
| Infection | 65/205 | anova | < 0·0001 | +1·017 upon infection |
| Corticosteroids treatment | 33/205 | anova | 0·6926 | n.s. |
| Indomethacin treatment | 42/205 | anova | 0·2339 | n.s. |
| Operation* | 11/205 | anova | 0·9438 | n.s. |
n = 205 samples of 82 different patients; DMBT1 level in tracheal aspirate fluid was expressed as (μg DMBT1/μg total protein) × 100; Regr. Anal., regression analysis; anova, analysis of variance; treat., treatment; n.s., no significant change of DMBT1 levels;
operation was necessary because of omphalocele (n = 2), gastroschisis (n = 2), necrotizing enterocolitis (n = 1), volvulus (n = 1), ileus (n = 3) or hydrocephalus (n = 2).
Fig. 3.
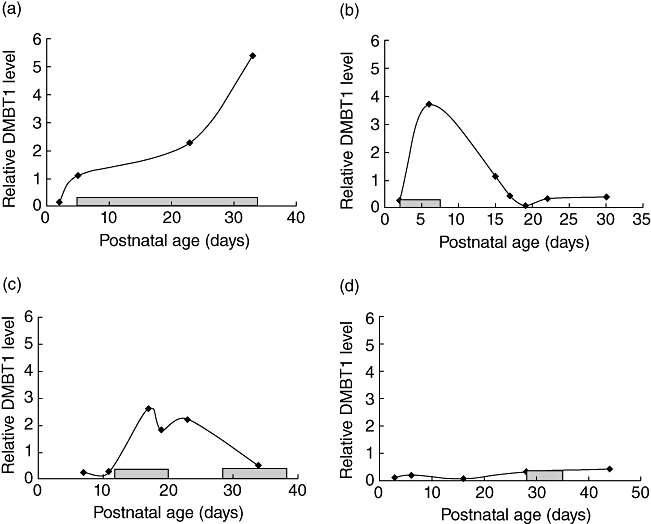
Time–courses of relative Deleted in Malignant Brain Tumours 1 (DMBT1) levels, expressed as (μg DMBT1/μg total protein) × 100, in preterm infants with infection. The infants had a gestational age of 23 (d), 24 (b, c) and 28 (a) weeks. Grey boxes along the x-axis indicate an elevated C-reactive protein (CRP) level in the respective infant. (a–c) DMBT1-responsive cases; (d) DMBT1-non-responsive case.
Western blotting of tracheal aspirate samples
To validate the results of ELISA measurements a subset of the tracheal aspirate samples was analysed additionally by Western blotting using the monoclonal antibody Hyb213-06. In this case the secretory component of IgA was used as reference protein to allow a comparison of the different samples. The results of Western blotting confirmed the specificity of the antibody used as well as reflecting the higher DMBT1 level with increased maturity and upon infection (Fig. 4).
Fig. 4.
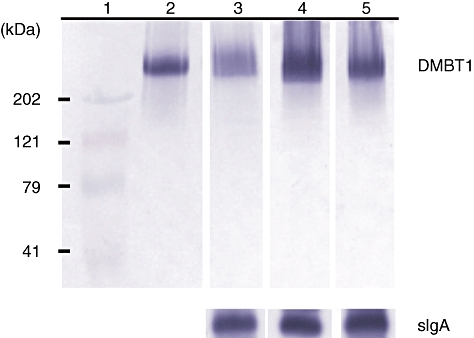
Western blotting of tracheal aspirate samples using the monoclonal antibody Hyb213-06. The secretory component of immunoglobulin A (sIgA) was used as reference protein to permit comparison of different tracheal aspirate samples. (1) Prestained protein standard. (2) Purified Deleted in Malignant Brain Tumours 1 (DMBT1)SAG. (3) Preterm infant without infection born with a gestational age of 24 weeks who has a postnatal age of 7 days. (4) Preterm infant with infection born with a gestational age of 32 weeks and who has a postnatal age of 5 days. (5) Infant without infection born with a gestational age of 36 weeks who has a postnatal age of 2 days.
Immunohistochemical analyses of DMBT1 levels in lung sections
Lung sections of nine infants without infection/chorioamnionitis and eight infants with infection/chorioamnionitis were used for immunohistochemical analyses. We used two antibodies for the assessment of DMBT1 levels in lung sections. Both antibodies showed an overlapping staining pattern with minor differences: the monoclonal antibody anti-DMBT1h12 stains preferentially DMBT1-variants produced by epithelial cells and the polyclonal antibody anti-DMBT1p84 stains preferentially variants produced by respiratory glands, and is therefore found in the mucus. Two of the neonates with infection had pneumonia, the other six infants had severe infections or septicaemia without clinical pneumonia. The levels of both variants increased with gestational age at death and were highly elevated in lung tissues of patients with infection/chorioamnionitis (Figs 5 and 6).
Fig. 5.
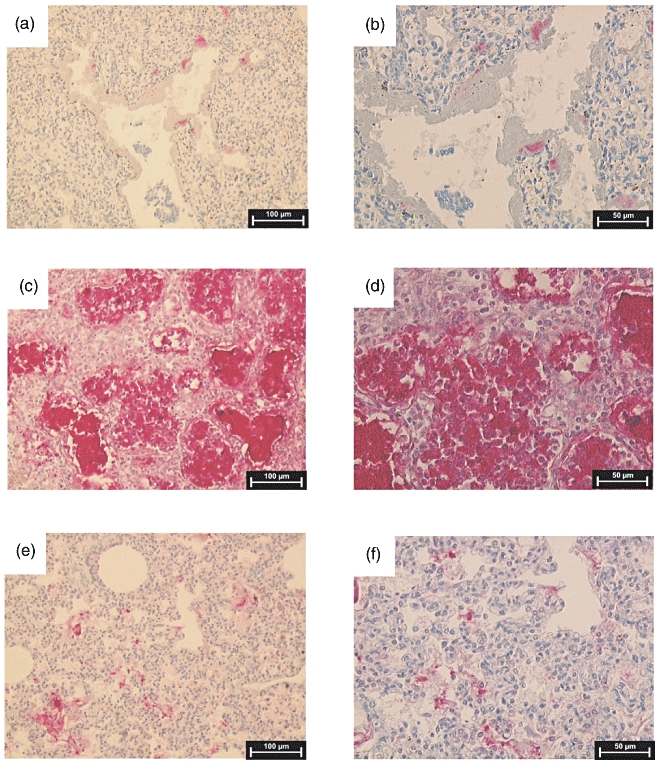
Immunohistochemical analysis of Deleted in Malignant Brain Tumours 1 (DMBT1) expression in post-mortem lung sections of preterm infants. Binding sites for anti-DMBT1p84 are displayed as red staining. (a) Lung section of a preterm infant without infection who was born at 28 weeks of gestational age and who died at 2 days of postnatal age. (b) Higher magnification of (a). (c) Lung tissue of a preterm infant born at 27 weeks of gestation who had pneumonia and died at a postnatal age of 8 days. (d) Higher magnification of (c). (e) Lung section of an infant without infection born at 36 weeks of gestation who died at a postnatal age of 2 days. (f) Higher magnification of (e).
Fig. 6.
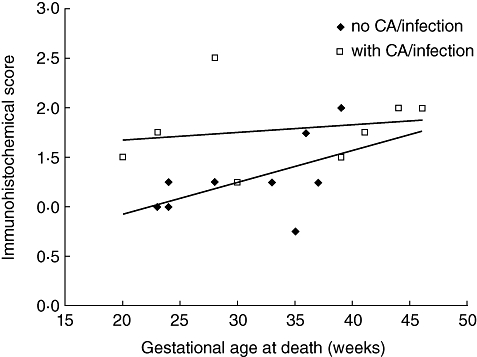
Plot of mean Deleted in Malignant Brain Tumours 1 (DMBT1) signal intensity as determined by immunohistochemistry of post-mortem lung sections (n = 17 cases). Sections displaying no signals were scored 0. DMBT1-positive tissues were scored semiquantitatively from 1 (weak staining) to 3 (highly intense staining). The signal intensity increased with increasing age and was elevated in infants with infections/chorioamnionitis. CA: chorioamnionitis.
Discussion
The present results show that DMBT1 levels in tracheal aspirates increase steadily during maturation (Fig. 1) and with postmenstrual age (Fig. 2).
DMBT1 has been shown to be involved in several mechanisms of innate immunity. DMBT1SAG is secreted by salivary glands and mediates aggregation of various streptococci, among them S. gordonii, S. mutans, S. pyogenes, S. agalalactiae, S. suis and other pathogenic bacteria such as Helicobacter pylori, Staph. aureus, E. coli and Lactobacillus casei [11,12,23]. S. agalactiae represents a group B streptococcus and is one of the major septic pathogens in neonates. Bikker et al. detected a peptide motif (SRCRP2) in the scavenger receptor cysteine-rich domains of DMBT1, which mediates bacterial binding [12]. S. gordonii DL1 (Challis) synthesizes a cell surface-anchored polypeptide and sialic acid-specific adhesion named Hsa and two Ag I/II family cell surface-anchored polypeptides named SspA and SspB, which interact with DMBT1 [23,24]. An analogous peptide of SspB was shown to bind to the SRCRP2 motif of DMBT1 [23]. Moreover, DMBT1gp340/SAG has been shown to inhibit the haemagglutination activity and infectivity of influenza A viruses and to agglutinate the virions [17]. DMBT1gp340 also interacts with HIV type 1, thereby inhibiting HIV infection [16,25]. The N-terminal SRCR-SID domain of DMBT1gp340 exhibited anti-HIV properties similar to the full-length glycoprotein [25]. Both the binding to several pathogens and to several endogenous defence factors support the idea that DMBT1 plays an important role in innate immunity [6,13–15]. In an epithelial cell line derived from lung (A549), phorbol myristate acetate induced proinflammatory expression of DMBT1, interleukin (IL)-6 and IL-8 [26].
Our data showed a significant increase of DMBT1 levels in bacterial infections, which is in accordance with its proposed function in innate immunity.
We conclude that DMBT1 is an important respiratory innate immunity component that is deficient in the perinatal period. Decreased DMBT1 level in the respiratory tract in neonates may contribute to the high risk of neonates to infections with group B streptococci (GBS), E. coli and Staph. aureus, as these bacteria are aggregated by DMBT1 [10,12]. GBS are the major pathogens producing neonatal pneumonia, and early-onset GBS sepsis is frequently transmitted via the respiratory tract [27]. The higher respiratory DMBT1 levels in SGA infants may provide protection against pulmonary infection.
Acknowledgments
This work was supported by the Wilhelm Sander-Stiftung, grant no. 99·018·3, and the Health Sciences Future Award 2005 of the HGF.
References
- 1.Hartel C, Adam N, Strunk T, Temming P, Muller-Steinhardt M, Schultz C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005;142:446–53. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zilow EP, Hauck W, Linderkamp O, Zilow G. Alternative pathway activation of the complement system in preterm infants with early onset infection. Pediatr Res. 1997;41:334–9. doi: 10.1203/00006450-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Frakking FN, Brouwer N, Zweers D, et al. High prevalence of mannose-binding lectin (MBL) deficiency in premature neonates. Clin Exp Immunol. 2006;145:5–12. doi: 10.1111/j.1365-2249.2006.03093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollenhauer J, Herbertz S, Holmskov U, et al. DMBT1 encodes a protein involved in the immune defense and in epithelial differentiation and is highly unstable in cancer. Cancer Res. 2000;60:1704–10. [PubMed] [Google Scholar]
- 5.Mollenhauer J, Wiemann S, Scheurlen W, et al. DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3-26.1 is deleted in malignant brain tumours. Nat Genet. 1997;17:32–9. doi: 10.1038/ng0997-32. [DOI] [PubMed] [Google Scholar]
- 6.Holmskov U, Mollenhauer J, Madsen J, et al. Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D. Proc Natl Acad Sci USA. 1999;96:10794–9. doi: 10.1073/pnas.96.19.10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollenhauer J, Helmke B, Muller H, et al. Sequential changes of the DMBT1 expression and location in normal lung tissue and lung carcinomas. Genes Chromosomes Cancer. 2002;35:164–9. doi: 10.1002/gcc.10096. [DOI] [PubMed] [Google Scholar]
- 8.Mollenhauer J, Deichmann M, Helmke B, et al. Frequent downregulation of DMBT1 and galectin-3 in epithelial skin cancer. Int J Cancer. 2003;105:149–57. doi: 10.1002/ijc.11072. [DOI] [PubMed] [Google Scholar]
- 9.Madsen J, Tornoe I, Nielsen O, et al. CRP-ductin, the mouse homologue of gp-340/deleted in malignant brain tumors 1 (DMBT1), binds gram-positive and gram-negative bacteria and interacts with lung surfactant protein D. Eur J Immunol. 2003;33:2327–36. doi: 10.1002/eji.200323972. [DOI] [PubMed] [Google Scholar]
- 10.Loimaranta V, Jakubovics NS, Hytonen J, Finne J, Jenkinson HF, Stromberg N. Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties. Infect Immun. 2005;73:2245–52. doi: 10.1128/IAI.73.4.2245-2252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakobphol A, Xu F, Hoang VM, et al. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J Biol Chem. 2000;275:39860–6. doi: 10.1074/jbc.M006928200. [DOI] [PubMed] [Google Scholar]
- 12.Bikker FJ, Ligtenberg AJ, End C, et al. Bacteria binding by DMBT1/SAG/gp-340 is confined to the VEVLXXXXW motif in its scavenger receptor cysteine-rich domains. J Biol Chem. 2004;279:47699–703. doi: 10.1074/jbc.M406095200. [DOI] [PubMed] [Google Scholar]
- 13.Tino MJ, Wright JR. Glycoprotein-340 binds surfactant protein-A (SP-A) and stimulates alveolar macrophage migration in an SP-A-independent manner. Am J Respir Cell Mol Biol. 1999;20:759–68. doi: 10.1165/ajrcmb.20.4.3439. [DOI] [PubMed] [Google Scholar]
- 14.Ligtenberg AJ, Bikker FJ, De Blieck-Hogervorst JM, Veerman EC, Nieuw Amerongen AV. Binding of salivary agglutinin to IgA. Biochem J. 2004;383:159–64. doi: 10.1042/BJ20040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boackle RJ, Connor MH, Vesely J. High molecular weight non-immunoglobulin salivary agglutinins (NIA) bind C1Q globular heads and have the potential to activate the first complement component. Mol Immunol. 1993;30:309–19. doi: 10.1016/0161-5890(93)90059-k. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z, Golub E, Abrams WR, Malamud D. gp340 (SAG) binds to the V3 sequence of gp120 important for chemokine receptor interaction. AIDS Res Hum Retroviruses. 2004;20:600–7. doi: 10.1089/0889222041217400. [DOI] [PubMed] [Google Scholar]
- 17.Hartshorn KL, White MR, Mogues T, Ligtenberg T, Crouch E, Holmskov U. Lung and salivary scavenger receptor glycoprotein-340 contribute to the host defense against influenza A viruses. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1066–76. doi: 10.1152/ajplung.00057.2003. [DOI] [PubMed] [Google Scholar]
- 18.End C, Lyer S, Renner M, et al. Generation of a vector system facilitating cloning of DMBT1 variants and recombinant expression of functional full-length DMBT1. Protein Expr Purif. 2005;41:275–86. doi: 10.1016/j.pep.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Holmskov U, Lawson P, Teisner B, et al. Isolation and characterization of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-D binding molecule. J Biol Chem. 1997;272:13743–9. doi: 10.1074/jbc.272.21.13743. [DOI] [PubMed] [Google Scholar]
- 20.Bikker FJ, Ligtenberg AJ, van der Wal JE, et al. Immunohistochemical detection of salivary agglutinin/gp-340 in human parotid, submandibular, and labial salivary glands. J Dent Res. 2002;81:134–9. [PubMed] [Google Scholar]
- 21.Mollenhauer J, Herbertz S, Helmke B, et al. Deleted in Malignant Brain Tumors 1 is a versatile mucin-like molecule likely to play a differential role in digestive tract cancer. Cancer Res. 2001;61:8880–6. [PubMed] [Google Scholar]
- 22.Giedion A, Haefliger H, Dangel P. Acute pulmonary X-ray changes in hyaline membrane disease treated with artificial ventilation and positive end-expiratory pressure (PEP) Pediatr Radiol. 1973;1:145–52. doi: 10.1007/BF00974058. [DOI] [PubMed] [Google Scholar]
- 23.Jakubovics NS, Kerrigan SW, Nobbs AH, et al. Functions of cell surface-anchored antigen I/II family and Hsa polypeptides in interactions of Streptococcus gordonii with host receptors. Infect Immun. 2005;73:6629–38. doi: 10.1128/IAI.73.10.6629-6638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamada T, Kawashima M, Watanabe H, Tagami J, Senpuku H. Molecular interactions of surface protein peptides of Streptococcus gordonii with human salivary components. Infect Immun. 2004;72:4819–26. doi: 10.1128/IAI.72.8.4819-4826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, Lee S, Abrams W, Weissman D, Malamud D. The N-terminal SRCR-SID domain of gp-340 interacts with HIV type 1 gp120 sequences and inhibits viral infection. AIDS Res Hum Retroviruses. 2006;22:508–15. doi: 10.1089/aid.2006.22.508. [DOI] [PubMed] [Google Scholar]
- 26.Kang W, Nielsen O, Fenger C, et al. The scavenger receptor, cysteine-rich domain-containing molecule gp-340 is differentially regulated in epithelial cell lines by phorbol ester. Clin Exp Immunol. 2002;130:449–58. doi: 10.1046/j.1365-2249.2002.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doran KS, Chang JC, Benoit VM, Eckmann L, Nizet V. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J Infect Dis. 2002;185:196–203. doi: 10.1086/338475. [DOI] [PubMed] [Google Scholar]


