Abstract
Both impulsivity and novelty-seeking have been suggested to be behavioral markers of the propensity to take addictive drugs. However, their relevance for the vulnerability to compulsively seek and take drugs, which is a hallmark feature of addiction, is unknown. We report here that whereas high reactivity to novelty predicts the propensity to initiate cocaine self-administration, high impulsivity in contrast predicts the development of addiction-like behavior in rats, including persistent or compulsive drug taking in the face of aversive outcomes. This study provides experimental evidence that a shift from impulsivity to compulsivity occurs during the development of addictive behavior, thereby providing important insights into the genesis and neural mechanisms of drug addiction.
Compulsive cocaine use has been hypothesized to result from a failure in top-down executive control over maladaptive habit learning (1, 2). In neural terms this may reflect the diminishing influence of prefrontal cortical function, as behavioral control devolves from ventral to dorsal striatum (1). In behavioral terms, we predict that the development of addiction reflects a shift from impulsivity to compulsivity (3).
Human studies have implicated individual differences in different forms of impulsivity and sensation-seeking in vulnerability to drug use and abuse (4-6). However, whether the enhanced impulsivity observed in drug addicts (7, 8) pre-dates the onset of compulsive drug use or is a consequence of protracted exposure to drugs has not been fully established. In addressing this issue experimentally, we operationalized these human traits in experimental animals as an inability to wait before performing an appropriate response, one phenotype of impulsivity (9) measured as premature responses in a 5-choice serial reaction-time task (5-CSRTT) of sustained visual attention (10), as distinct from locomotor reactivity to a novel environment, a sensation-seeking phenotype (11). These animal models support the existence of a “vulnerable phenotype” that predisposes to drug addiction. Thus outbred rats exhibiting high levels of novelty-induced locomotor activity, called high responder (HR), show increased sensitivity to the reinforcing effects of addictive drugs and self-administer lower doses of psychostimulants than low responder (LR) littermates (11). Impulsivity, on the other hand, correlates with ethanol intake (12) and predicts instead the escalation of cocaine self-administration (10, 13), which may be more indicative of a necessary stage in the transition to compulsive drug-seeking. Whilst these studies have addressed the initiation of drug taking, they have not captured the essential feature of addiction, namely the persistence of drug seeking in the face of negative consequences, a characteristic incorporated into recent animal models based on the DSMIV criteria for substance dependence (14, 15). Therefore, we have employed a model of addiction based upon individual differences in compulsive cocaine use (14) to investigate the contrasting contribution of high impulsivity (HI) and high reactivity to novelty (HR) to the development of compulsive drug-taking.
In this model we have operationally defined three addiction-like criteria in rats which correspond to those of the DSM-IV description of substance dependence (16), namely: (i) increased motivation to take the drug, (ii) an inability to refrain from drug-seeking and (iii) maintained drug use despite aversive consequences (see 17 for details). Thus, rats positive for none of the three criteria (0 criteria rats) are resistant to addiction, whereas rats meeting the 3 addiction-like criteria (3 criteria rats) are considered ‘addicted’, and represent 15-20% of the population initially exposed to cocaine (14), a proportion that is similar to that observed in human populations (18).
To compare the propensity of rats with high impulsivity (HI) and high responders to novelty (HR) both to acquire cocaine self-administration and to make the transition to compulsive cocaine taking, we first identified HI and LI rats in the 5-CSRTT (10) then HR and LR rats in a novelty-induced locomotor activity procedure (11). Subsequently, we compared the propensity of these different groups to acquire cocaine self-administration and to develop the three addiction-like criteria following protracted self-administration (17).
HI and LI rats did not differ in their novelty-induced locomotor activity, conversely, HR and LR rats were not impulsive (Fig. 1A-B).
Fig. 1. Impulsivity and novelty-induced locomotor activity: two distinct phenotypes.
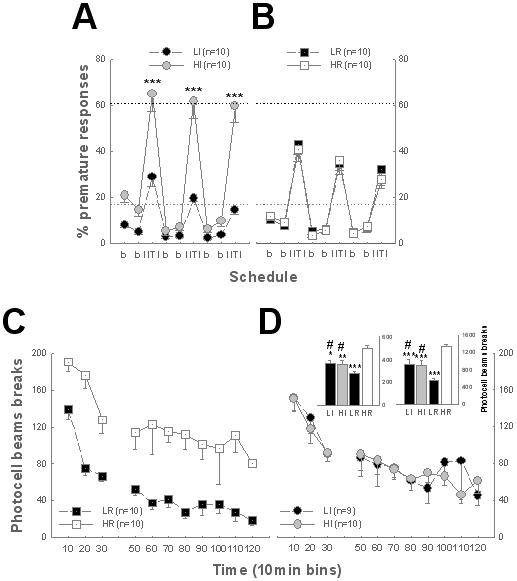
A-B. During long ITI sessions in the 5-CSRTT, HI rats showed more premature responses than LI rats [Group: F3,36 = 14.4, p < 0.01, Schedule: F8,288 = 130.22, p < 0.01, Schedule × Group: F24, 288 = 7.01, p < 0.01] (***: p < 0.001) (A) and HR (p < 0.01) or LR rats (p < 0.05) (B). HR rats did not differ from LR rats nor from LI subjects (B). C-D. HR rats were more reactive to novelty than LR rats [first 30 min (left histogram) or total duration of the session (right histogram): Group: F3,35 = 12.17, p < 0.01, F3,35 = 17.63, p < 0.01, respectively, Group × Time: F6,70 = 1.26, ns and F30,350 < 1, respectively], (p < 0.001). HI and LI subjects differed from both HR (p < 0.01) and LR rats (# p < 0.01) but never from each other. * versus HR, p < 0.05, ** p < 0.01, *** p < 0.01. Black and grey dotted lines represent the average premature responses during the last two long inter-trial intervals for HI and LI rats, respectively.
As predicted (11), HR rats were more prone to acquire cocaine self-administration than LR rats, showing an upward shift in the cocaine dose-response curve (Fig. 2). However, HI rats did not differ from LI rats in their acquisition of cocaine self-administration.
Fig. 2. Novelty-induced locomotor activity predicts the propensity to acquire cocaine SA.
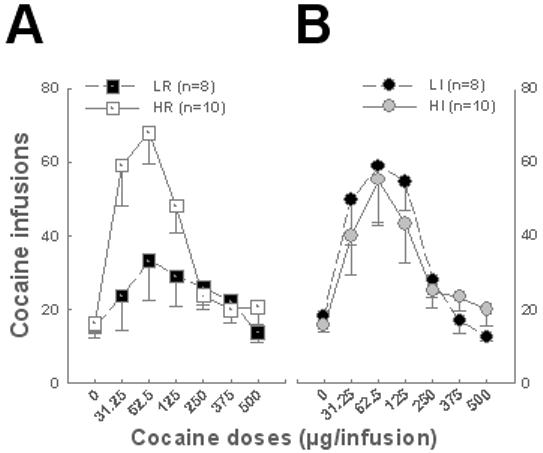
A. HR rats showed an upward shift of the cocaine dose-response curve compared to LR littermates [Group: F1, 16 = 4.9, p < 0.05, Dose: F6, 96 = 11.73, p < 0.01, and Group × Dose: F6, 96 = 4.39, p < 0.01], HR rats infusing more cocaine at the lowest three doses than vehicle (p < 0.01). B. HI and LI subjects did not differ in their number of cocaine infusions self-administered [Group: F1,16 < 1, Dose: F6, 96 = 10.79, p < 0.01, Group × Dose: F6, 96<1].
After 40 days of cocaine self-administration we measured the three addiction-like behaviors in a cohort of 23 rats (17) so that each rat was defined as showing 0, 1, 2 and 3 of these behaviours (Table S2) as well as an addiction score, calculated as the sum of the standardized scores of each of the addiction-like criteria (17). Thus, 0, 1, 2 and 3 criteria rats were linearly distributed along an addiction scale, corresponding operationally to the Addiction Severity Index (ASI) in humans (17, 19). On this scale 3 criteria rats had scores higher than all the other groups (Fig. 3A), especially when compared with 0 criteria animals, from which they differed for each of the addiction-like behaviors (Fig S1). Only the 0 criteria rats had highly negative addiction scores.
Fig. 3. Highly impulsive rats closely resemble 3 criteria rats.
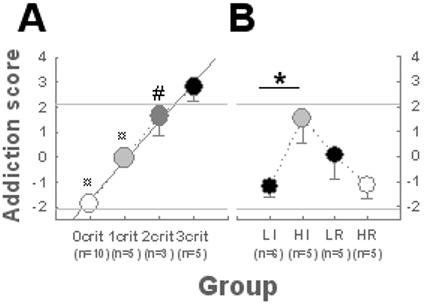
After protracted self-administration 0, 1, 2 or 3 criteria rats were identified. A. When ranked on a linear addiction scale [R2 = 0.99, Group: F3,19 = 34.43, p < 0.01], 3 criteria rats had addiction scores (2.8 ± 0.6) above the standard deviation (2.1), and higher than all the other groups (vs 0 and 1 criteria rats: ¤ p < 0.01, vs 2 criteria rats: # p ≤ 0.05). B. HI rats displayed higher addiction scores than LI rats [F1,9 = 7.55, p < 0.05: *] whereas HR did not differ from LR rats. Only HI rats did not differ from 3 criteria rats for their addiction score [F5,30 = 10.13, p < 0.01], displaying higher addiction scores than 0 criteria (p < 0.01), and HR rats (p < 0.05). HR, LR and LI rats did not differ from 0 criteria rats.
Whereas reactivity to novelty predicts the vulnerability to acquire cocaine self-administration, it is high impulsivity that predicts the transition from controlled to compulsive cocaine taking. HI rats displayed higher addiction scores than LI rats whereas, in marked contrast, HR rats did not differ from LR rats (Fig. 3B). LI, HR and LR rats were represented mainly in the 0 and 1 criteria populations, whereas HI rats were largely represented in the 2-3 criteria populations. Additionally, only HI rats were more frequently represented in the 3 criteria group than in the 0 criteria group (Table S1).
A factor analysis revealed that impulsivity and addiction-like behaviour are explained by the same factor that was itself orthogonal to reactivity to novelty, thereby identifying an impulsivity / addiction construct (Fig. S2). Thus HI rats did not differ from 3 criteria rats in any of their addiction-like behaviors (Figs. 4A and S3).
Fig. 4. Impulsivity predicts the transition to compulsivity.
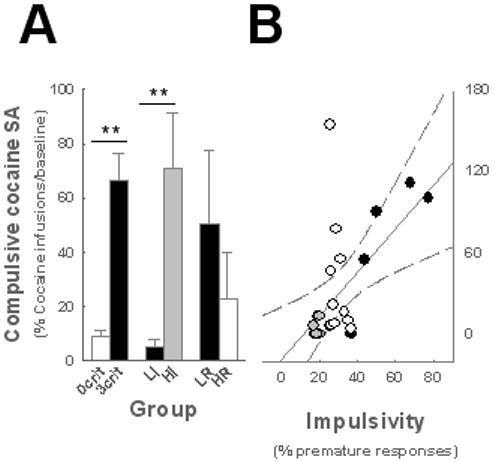
HI rats (n = 5) displayed higher resistance to punishment than LI rats (n = 6) [F1, 9 = 12.79, p < 0.01] whereas HR (n = 5) rats did not differ from LR rats (n = 5). When compared to 0 and 3 criteria rats for their resistance to punishment [Group: F5,30 = 10.13, p < 0.01], only HI rats were similar to 3 criteria rats, showing greater resistance to punishment than 0 criteria, LI and HR rats (p < 0.05). LI and HR rats differed from 3 criteria but not from 0 criteria rats (A). **p < 0.01. B. Impulsivity predicts compulsive cocaine self-administration (R=0.42, p<0.05). Grey and black shadings represent LI and HI rats, respectively.
More specifically, the high addiction score of HI rats derived from the development of compulsive cocaine self-administration. HI rats displayed greater resistance to punishment of the drug taking response than did LI, HR and LR rats (Fig. 4A) and, at the population level, correlational analysis revealed that impulsivity predicts compulsivity (Fig. 4B). However HI rats did not differ from LI, HR or LR rats in their total intake of cocaine (Fig. S3); therefore the development of compulsive cocaine taking observed specifically in the highly impulsive rats cannot be attributed to differential exposure to cocaine. Since clinical investigations generally compare addicted subjects to drug-naïve controls, we analyzed whether animals vulnerable and resistant to addiction differed in their impulsivity and locomotor reactivity to novelty prior to any exposure to cocaine. This analysis showed that 3 criteria rats were more impulsive but not more reactive to novelty than 0 criteria rats prior to cocaine self-administration (Fig. S4).
These data allow us to identify one variety of impulsivity, measured as an inability to wait and sample predictive stimuli before responding (20), as a key behavioral marker specific for the vulnerability to progress to compulsive cocaine use, the hallmark of addiction. Our results are in accord with observations that (i) highly impulsive humans are over-represented in drug addicted populations (21), (ii) impulsivity or sensation-seeking may pre-date compulsive drug use (22, 23) and (iii) there is a high comorbidity between drug addiction and disorders characterized by impulsive behavior, such as attention-deficit-hyperactivity disorder (ADHD) (21).
The results indicate that the relationship between high impulsivity and addiction-like behavior is completely independent of the initial propensity to acquire cocaine self-administration (Fig. S2) (17), an observation consistent with the demonstration that impulsivity is unrelated to the subjective effects of oral amphetamine administration (24). Instead, this early vulnerability to cocaine’s reinforcing effects was predicted by high locomotor reactivity to novelty. Our observations further suggest that the subjective and behavioral responses to cocaine during initial exposure to the drug do not determine the subsequent progression to addiction, as might perhaps have been previously suspected (11).
Our study also provides experimental evidence that high levels of impulsivity can antedate the onset of compulsive drug use and thereby emphasizes the importance of pre-existing impulsivity observed in addicts (2, 4, 7). Moreover, by demonstrating the link between impulsivity and compulsivity in the development of addiction, these data provide a major impetus for investigating the neurobiological mechanisms underlying this transition. One candidate is the apparent devolution of control over drug-seeking behavior from the ventral to the dorsal striatum (25) which has been shown to depend upon the cascading, serial ascending circuitry that links these striatal domains via its regulatory dopaminergic innervation arising in the midbrain (26, 27). This hypothesis is further supported by the observation that the early vulnerability to escalate cocaine intake shown by highly impulsive rats is predicted by low D2/D3 dopamine receptor levels in the ventral, but not the dorsal striatum (10). In contrast, chronic exposure to cocaine in monkeys (28) and drug abusers (29) is associated with low D2/3 dopamine receptor availability, predominantly in the dorsal striatum.
Supplementary Material
Acknowledgments
This work has been supported by grants from the Foundation Fyssen to D.B. and the UK Medical Research Council No. G9536855 to B.J.E. and completed within the Behavioural and Clinical Neuroscience Institute which is supported by a joint grant from the MRC and the Wellcome Trust. The authors would like to thank D. Theobald for his assistance and Dr M. Solinas and A. Rauscent for their comments on this manuscript. Behavioural experiments and statistical analyses were designed and performed by D.B. The manuscript was prepared by D.B, A.C.M, J.W.D, T.W.R and B.J.E. The authors declare that they have no competing financial interests.
References
- 1.Everitt BJ, Robbins TW. Nat Neurosci. 2005;8:1481. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 2.Jentsch JD, Taylor JR. Psychopharmacology (Berl) 1999;146:373. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Le Moal M. Neuropsychopharmacology. 2001;24:97. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 4.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Nat Neurosci. 2005;8:1450. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 5.Chakroun N, Doron J, Swendsen J. Encephale. 2004;30:564. doi: 10.1016/s0013-7006(04)95471-1. [DOI] [PubMed] [Google Scholar]
- 6.Wills TA, Vaccaro D, McNamara G. J Subst Abuse. 1994;6:1. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 7.Dom G, D’Haene P, Hulstijn W, Sabbe B. Addiction. 2006;101:50. doi: 10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- 8.Zilberman ML, Tavares H, Hodgins DC, el-Guebaly N. J Addict Dis. 2007;26:79. doi: 10.1300/J069v26n01_10. [DOI] [PubMed] [Google Scholar]
- 9.Evenden JL. Psychopharmacology (Berl) 1999;146:348. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 10.Dalley JW, et al. Science. 2007;315:1267. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piazza PV, Deminiere JM, Le Moal M, Simon H. Science. 1989;245:1511. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 12.Poulos CX, Le AD, Parker JL. Behav Pharmacol. 1995;6:810. [PubMed] [Google Scholar]
- 13.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Psychopharmacology (Berl) 2005;178:193. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 14.Deroche-Gamonet V, Belin D, Piazza PV. Science. 2004;305:1014. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 15.Vanderschuren LJ, Everitt BJ. Science. 2004;305:1017. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 16.Diagnostic and Statistical Manual of Mental Disorders. ed 4. American Psychiatric Association; 2000. revised version 2000. [Google Scholar]
- 17.Materials and methods are available as supporting material on Science Online.
- 18.Anthony JC, Warner LA, Kessler RC. Exp Clin Psychopharmacol. 1994;2:244. [Google Scholar]
- 19.Rikoon SH, Cacciola JS, Carise D, Alterman AI, McLellan AT. J Subst Abuse Treat. 2006;31:17. doi: 10.1016/j.jsat.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Evenden J. Psychopharmacology (Berl) 1999;143:111. doi: 10.1007/s002130050926. [DOI] [PubMed] [Google Scholar]
- 21.Nigg JT, et al. J Am Acad Child Adolesc Psychiatry. 2006;45:468. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 22.Dawe S, Loxton NJ. Neurosci Biobehav Rev. 2004;28:343. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Sher KJ, Bartholow BD, Wood MD. J Consult Clin Psychol. 2000;68:818. [PubMed] [Google Scholar]
- 24.White TL, Lott DC, de Wit H. Neuropsychopharmacology. 2006;31:1064. doi: 10.1038/sj.npp.1300939. [DOI] [PubMed] [Google Scholar]
- 25.Vanderschuren LJ, Di Ciano P, Everitt BJ. J Neurosci. 2005;25:8665. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belin D, Everitt BJ. Neuron. 2008;57:432. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Haber SN, Fudge JL, McFarland NR. J Neurosci. 2000;20:2369. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nader MA, et al. Nat Neurosci. 2006;9:1050. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 29.Volkow ND, et al. Synapse. 1993;14:169. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


