Abstract
Exercise promotes weight loss and improves insulin sensitivity. However, the molecular mechanisms mediating its beneficial effects are not fully understood. Obesity correlates with increased production of inflammatory cytokines, which in turn, contributes to systemic insulin resistance. To test the hypothesis that exercise mitigates this inflammatory response, thereby improving insulin sensitivity, we developed a model of voluntary exercise in mice made obese by feeding of a high fat/high sucrose diet (HFD). Over four wk, mice fed chow gained 2.3 ± 0.3 g, while HFD mice gained 6.8 ± 0.5 g. After 4 wk, mice were subdivided into four groups: chow-no exercise, chow-exercise, HFD-no exercise, HFD-exercise and monitored for an additional 6 wk. Chow-no exercise and HFD-no exercise mice gained an additional 1.2 ± 0.3 g and 3.3 ± 0.5 g respectively. Exercising mice had higher food consumption, but did not gain additional weight. As expected, GTT and ITT showed impaired glucose tolerance and insulin resistance in HFD-no exercise mice. However, glucose tolerance improved significantly and insulin sensitivity was completely normalized in HFD-exercise animals. Furthermore, expression of TNF-α, MCP-1, PAI-1 and IKKβ was increased in adipose tissue from HFD mice compared with chow mice, whereas exercise reversed the increased expression of these inflammatory cytokines. In contrast, expression of these cytokines in liver was unchanged among the four groups. These results suggest that exercise partially reduces adiposity, reverses insulin resistance and decreases adipose tissue inflammation in diet-induced obese mice, despite continued consumption of HFD.
Keywords: insulin resistance, cytokine, adiposity, high-fat diet
the prevalence of obesity in Western countries has reached epidemic proportions (32, 33). Obesity is associated with a chronic low-grade proinflammatory metabolic state that contributes to insulin resistance, the metabolic syndrome, type 2 diabetes, cardiovascular disease, and several cancers (9, 32, 46). The pathogenesis of this inflammation remains poorly understood.
A complex interaction of peripheral and central pathways regulates food intake, and obesity occurs when there is a significant imbalance between food intake and energy expenditure (8). Although genetics play an important role in the regulation of body weight homeostasis, physical activity and diet are also important environmental contributors to body weight regulation (37). Furthermore, high-fat diet-induced obesity is associated with adipose tissue inflammation, and it was recently shown that the IκB kinase-β (IKKβ)/NF-κB pathway, a key component of the inflammatory cascade, is activated in diet-induced obesity, as well as in a genetic model of obesity and insulin resistance (ob/ob) (2, 47). In addition, inhibition of NF-κB and its upstream activator IKKβ by salicylates, or _targeted disruption of IKKβ, reversed obesity-induced insulin resistance in vitro and in vivo (2, 3, 47). Thus, the IKKβ/NF-κB pathway appears to be a key mediator of obesity-induced insulin resistance (1, 2, 47).
Exercise has been shown to improve insulin sensitivity in obese individuals even in the absence of weight loss (6). However, the mechanisms underlying the beneficial effects of exercise have yet to be fully elucidated. In animal models, forced exercise on treadmills leads to reduced body weight and improved lipid profiles as well as to reductions in systemic inflammation and insulin resistance (12, 13, 18). However, potential effects on insulin sensitivity and inflammation in key _target organs such as liver and adipose tissue are not well defined. Furthermore, forced-exercise models may be problematic, as they are stressful. This suggests that voluntary exercise may be a better model. A few studies have indicated that voluntary exercise can slow the onset of weight gain in genetically obese rodent models such as the MC4R knockout mouse and the agouti (Ay) mouse (4, 23). Overall, though, data on the effects of voluntary exercise on obesity and associated effects on expression of liver and adipose tissue inflammatory markers in animal models remain limited.
We therefore developed such a model using mice made obese through feeding of a high-fat and high-sucrose diet (HFD). After the onset of obesity, mice were housed individually. Mice in chow-fed and HFD-fed exercise groups were housed with a functional running wheel, whereas chow-fed and HFD-fed mice in the no-exercise groups received an identical but nonfunctional fixed wheel.
Using our murine model of diet-induced obesity, we examined the effects of voluntary exercise on adiposity, insulin resistance, and liver and adipose tissue inflammation. Specific parameters evaluated included body weight, glucose tolerance, and insulin sensitivity. Liver and adipose tissue inflammatory markers assessed included TNF-α, monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), and IKKβ, as well as leptin and adiponectin for adipose tissue.
RESEARCH DESIGN AND METHODS
Animals.
These studies were approved by the Beth Israel Deaconess Medical Center (BIDMC) Animal Care and Use Committee. Twelve-week-old male mice (29 male C57BL/6, Jackson Laboratory, Bar Harbor, ME) were purchased and divided into two groups: chow diet (n = 13) and HFD (n = 16). Chow diet purchased from Purina was Formulab Diet 5008 (3.71 kcal/g), which contains 6.5% fat by weight and provides 16.7% total calories from fat. HFD purchased from Research Diets, D12451 (4.7 kcal/g) contains 24% fat by weight and provides 45% calories from fat. Mice were housed in the BIDMC Animal Facility and maintained at 22°C under a 14:10-h alternating light-dark cycle, which is standard for this facility. After 4 wk, chow-fed and HFD-fed mice were further subdivided into no-exercise and exercise groups designated as chow-no exercise (n = 6), chow-exercise (n = 7), HFD-no exercise (n = 8), and HFD-exercise (n = 8), respectively. These four groups were monitored for an additional 6 wk. Mice were housed individually and received either a functional running wheel (Bio-Serv, Frenchtown, NJ) or an identical locked, nonfunctional running wheel. Body weights of animals were measured three times per week, and food intake was measured weekly.
Locomotor activity.
Exercise monitoring was performed between the fourth and fifth weeks after mice had been exposed to the running wheels. Total activity was assessed using two techniques. To assess wheel running activity, we used an infrared camera to record four mice running over a 6-h period from the beginning of the dark cycle and for a 6-h period from the beginning of the light cycle. The recordings were then reviewed, and total revolutions were recorded by an observer. A fluorescent marker was fixed to the wheel so that rotations could be counted as they were observed, using a hand-held manual counter. We also assessed relative locomotor activity over 24 h in mice in the absence of running wheels, using a monitoring system from Columbus Instruments (Columbus, OH) that reports activity as sequential beam breaks on an infrared grid.
Glucose tolerance tests.
Intraperitoneal glucose tolerance tests (GTT) were performed after mice had undergone 6 wk of exercise. Running wheels were removed from cages in the evening before metabolic testing. Mice were fasted overnight (1700–0800) and were subsequently injected with glucose (2 g/kg body wt ip). Tail blood was collected at −1, 15, 30, 60, and 120 min. Blood glucose concentrations were measured using a glucometer (Elite, Bayer, Mishawaka, IN).
Insulin tolerance tests.
Insulin tolerance tests (ITT) were also performed after 6 wk of exercise. Between 1400 and 1500, fed mice were injected with regular insulin (0.75 U/kg, Eli Lilly, Indianapolis, IN), and tail-blood samples were obtained at −1, 15, 30, 60, and 120 min after the insulin injection.
Quantitative real-time PCR.
Total RNA was extracted from liver and adipose tissues using the Ultraspec RNA Isolation System (Biotecx, Houston, TX) according to the manufacturer's instructions. cDNA was synthesized using oligo(dT) primers with the Advantage RT-for-PCR kit (BD Biosciences, San Jose, CA). Primers spanned intronic regions to generate 300- to 400-bp PCR products. PCR amplifications were quantified using the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Primer sequences were as follows: TNF-α (Fwd: 5′-GACCCTCACACTCAGATCATCTTCT-3′, Rev: 5′-CCACTTGGTGGTTTGCTACGA-3′), MCP-1 (Fwd: 5′-GCTGACCCCAAGAAGGAATG-3′, Rev: 5′-GTGCTTGAGGTGGTTGTGGA-3′), and PAI-1 (Fwd: 5′-ACAGCCAACAAGAGCCAATC-3′, Rev: 5′-ATAGCCAGCACCGAGGACAC-3′). Mouse gene PCR primer sets (RT2) for IKKβ (Cat no. PPM03198A, Reference Sequence Accession no. NM_010546) were purchased from SuperArray Biosciences (Frederick, MD). Results were normalized against cyclophilin (Fwd: 5′-GGTGGAGAGCACCAAGACAGA-3′, Rev: 5′-GCCGGAGTCGACAATGATG-3′).
Plasma leptin, adiponectin, and insulin.
Plasma leptin and adiponectin were measured using commercially available ELISA kits (leptin and insulin: Crystal Chem, Downers Grove, IL; adiponectin: LINCO Research, St. Charles, MO) according to manufacturers' instructions. Plasma insulin was measured using a mouse insulin assay (Crystal Chem) as described by the manufacturer.
Retinol-binding protein-4.
Serum retinol-binding protein-4 (RBP4) was measured by quantitative Western blotting standardized to full-length recombinant RBP4, as described previously (15). Signals were quantified with GeneSnap software (Synoptics/Syngene, Frederick, MD).
Body composition.
Fat and lean body mass were assessed using dual-energy X-ray absorptiometry (DEXA; Lunar PIXImus2 mouse densitometer, GE Medical Systems, Madison, WI) as described by the manufacturer. Mice were anesthetized by intraperitoneal injection of a (1:1) mixture of tribromoethanol and tert-amyl alcohol, 0.015 ml/g body wt. The animals were then scanned, and total body fat and lean body mass were determined using an analysis program provided by the manufacturer.
Statistical analysis.
Values are reported as group means ± SE. Interactions between diet and exercise on physiological parameters were analyzed by two-way ANOVA. One-way ANOVA and independent t-test were also used. A probability value of <0.05 was considered statistically significant. Statistical comparisons were made using StatView (Abacus Concepts, Berkley, CA).
RESULTS
Voluntary exercise mitigates diet-induced obesity.
Mice that had access to running wheels ran a total of 3,402 ± 1,822 m during the first 6 h of the dark cycle and 135 ± 75 m during the first 6 h of the light cycle. This is consistent with other reports, which showed that male C57BL/6 mice will run on an average of 10 km over 24 h (5). Relative activity of mice that had exercised was increased over control mice, even in the absence of running wheels. When monitored in cages using infrared beam breaks, mice with previous access to running wheels had 1,319 ± 239 beam breaks/h during the dark cycle, whereas mice housed with fixed running wheels had only 638 ± 124 beam breaks/h during the dark cycle. During the light cycle, exercising mice were also more active and had 279 ± 31 beam breaks/h compared with nonexercised mice, which had an average of 129 ± 14 beam breaks/h.
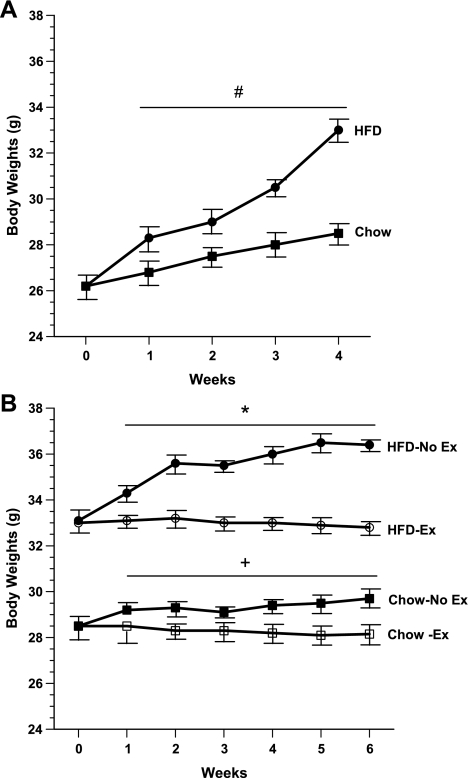
To validate the effects of HFD on body weight and body composition, 12-wk-old male C57BL/6 mice were divided into two groups, a chow-fed group (n = 13) and a HFD-fed group (n = 16). As expected, the HFD group gained substantially more body weight. After 4 wk of ad libitum feeding, HFD mice gained 6.8 ± 0.5 g compared with 2.3 ± 0.3 g for the chow group. HFD mice weighed 33 ± 1.3 g vs. 28.5 ± 0.8 g for chow-fed animals (Fig. 1A).
Fig. 1.
Body weights of Chow vs. high-fat diet (HFD) mice. A: 12-wk-old male C57BL/6 were fed either chow (n = 13) or HFD (n = 16) for 4 wk and body weights were recorded. B: Chow and HFD mice were subsequently subdivided into 4 groups: chow-no exercise (n = 6), chow-exercise (n = 7), HFD-no exercise (n = 8), and HFD-exercise (n = 8), and body weights were monitored for an additional 6 wk. Mice were housed individually, and running wheels were provided for exercise groups. #P < 0.05 vs. Chow; *P < 0.05 vs. HFD-Exercise; +P < 0.05 vs. Chow-Exercise.
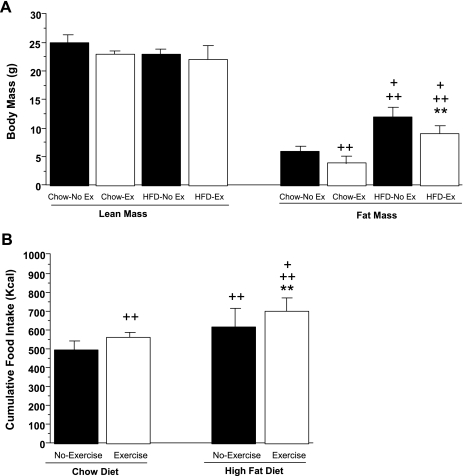
After 4 wk, chow-fed and HFD-fed mice were further subdivided into chow-no exercise (n = 6), chow-exercise (n = 7), HFD-no exercise (n = 8), and HFD-exercise (n = 8) groups, respectively. These four groups were monitored for an additional 6 wk. As shown in Fig. 1B, both chow-no exercise and HFD-no exercise mice continued to gain weight during this period, whereas no further increase in body weight occurred in chow-exercise and HFD-exercise mice. Over the course of 6 wk, chow-no exercise and HFD-no exercise mice gained an average of 1.2 ± 0.3 and 3.3 ± 0.5 g, respectively. Body composition analysis by DEXA (Fig. 2A) showed that the chow-no exercise and HFD-no exercise groups had significantly greater fat mass (6 ± 0.7 and 12 ± 0.8 g, respectively) compared with chow-exercise and HFD-exercise groups (4 ± 0.2 and 9 ± 0.7 g, respectively). By comparison, lean body mass was unchanged among the four groups (Fig. 2A). Furthermore, the reduced fat body mass in chow-exercise and HFD-exercise mice persisted despite an ∼12% increase in cumulative food intake in both these groups (Fig. 2B).
Fig. 2.
Lean and fat body mass composition (A) and cumulative food intake (B) of chow-no exercise, chow-exercise, HFD-no exercise, and HFD-exercise mice, respectively, after 6 wk of chow vs. HFD with and without exercise paradigm. DEXA scanning was used to measure body composition. +P < 0.05 vs. Chow-exercise; ++P < 0.05 vs. Chow-no exercise; **P < 0.05 vs. HFD-no exercise.
Voluntary exercise reverses HFD-induced insulin resistance.
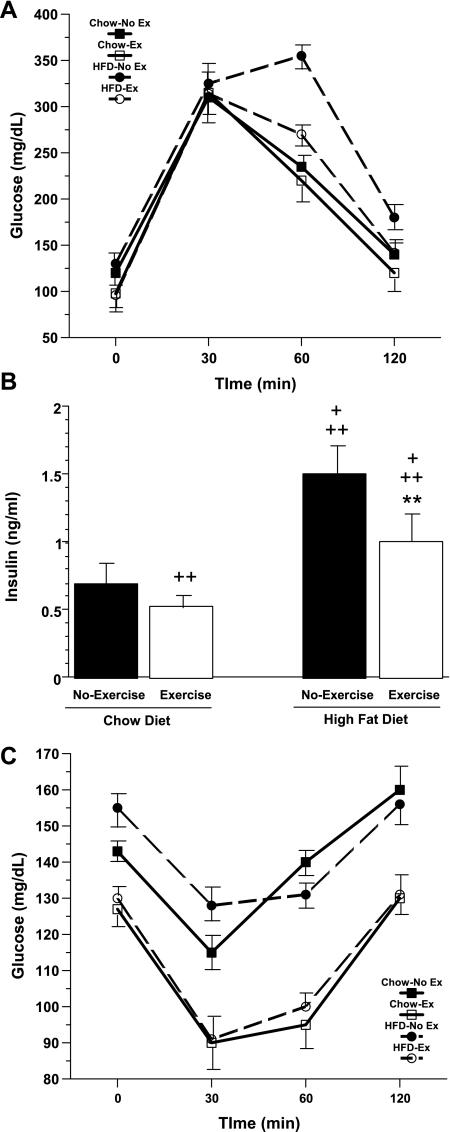
Numerous studies have shown a strong correlation between consumption of HFD and the onset of insulin resistance in rodents and humans (30). To evaluate the effects of exercise on HFD-induced insulin resistance, GTT and ITT were performed in chow-no exercise, chow-exercise, HFD-no exercise, and HFD-exercise mice. Glucose tolerance was assessed by calculating the incremental area under the curve (AUC). GTT showed that chow-exercise mice had slightly better glucose tolerance (6% decrease in glucose AUC) than chow-no exercise mice (Fig. 3A). Compared with chow-no exercise mice, HFD-no exercise mice had significantly worse glucose tolerance, as evidenced by a 25% increase in their glucose AUC (P < 0.05). Indeed, among the four groups, HFD-no exercise animals had the worst glucose tolerance. However, voluntary exercise (HFD-exercise group) markedly improved this impaired glucose tolerance. Overall, a 20% reduction in glucose AUC was seen in HFD-exercise mice compared with HFD-no exercise mice (P < 0.05).
Fig. 3.
A: glucose tolerance test (GTT) of chow-no exercise, chow-exercise, HFD-no exercise, and HFD-exercise mice, respectively, after 6-wk exercise period. Dashed lines represent HFD mice. Animals were fasted overnight (1700-0800). Chow-exercise mice had slightly better glucose tolerance (6% decrease in glucose AUC) than chow-no exercise mice. By comparison, HFD-no exercise mice had significantly worse glucose tolerance than chow-no exercise mice (25% increase in glucose AUC), whereas HFD-exercise mice showed a significant improvement in glucose tolerance (20% reduction in glucose AUC) compared with HFD-no exercise mice. B: fasting plasma insulin levels. Blood was collected just prior to starting GTT. +P < 0.05 vs. chow-exercise; ++P < 0.05 vs. chow-no exercise; **P < 0.05 vs. HFD-no exercise. C: insulin tolerance test (ITT) was performed after 6-wk exercise period. Fed mice were injected between 1400 and 1500 with regular insulin (0.75 U/kg body wt). Chow-exercise and HFD-exercise mice displayed similar insulin sensitivity, and both these groups were significantly more insulin sensitive (P < 0.05) than either chow-no exercise or HFD-no exercise mice.
These findings were consistent with fasting plasma insulin levels (Fig. 3B), which were lower in chow-fed than in HFD-mice and were reduced in both groups by voluntary exercise (chow-no exercise: 0.7 ± 0.05 ng/ml; chow-exercise: 0.53 ± 0.02 ng/ml; HFD-no exercise 1.5 ± 0.06 ng/ml; HFD-exercise: 1.0 ± 0.04 ng/ml). Among the four groups, chow-exercise had the lowest insulin levels and HFD-no exercise had the highest insulin levels. In addition, as illustrated in Fig. 3C, ITT showed that among the four groups chow-exercise mice and HFD-exercise mice were significantly more insulin sensitive than their sedentary counterparts (P < 0.05). Furthermore, despite continued consumption of HFD, HFD-exercise mice demonstrated similar insulin sensitivity to chow-exercise mice.
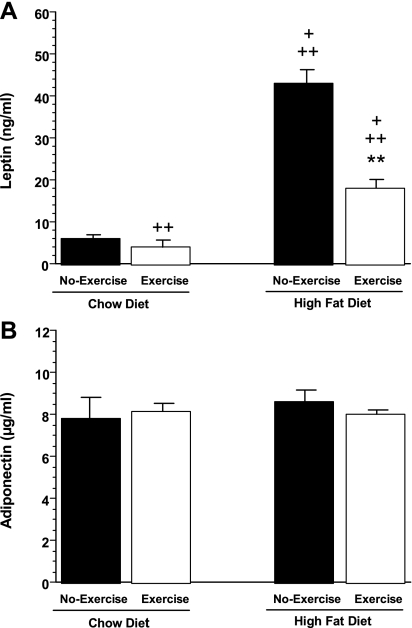
Effects of exercise on two key mediators of energy balance and insulin sensitivity, the adipocyte-derived hormones leptin and adiponectin, were also evaluated. As shown in Fig. 4A, and consistent with their increased adiposity, plasma leptin levels were increased sevenfold in HFD-no exercise mice compared with chow-no exercise animals. Furthermore, exercise attenuated this increase in leptin in HFD mice. Plasma leptin levels were reduced by 58% in HFD-exercise mice compared with HFD-no exercise mice. In addition, plasma leptin levels were also reduced by 33% in chow-exercise mice compared with chow-no exercise mice. In contrast, no significant differences were seen in adiponectin levels among the four groups (Fig. 4B). We also measured circulating levels of another potential mediator of insulin sensitivity, serum RBP4 but saw no significant differences in mean RBP4 levels among the four groups: (chow-no exercise: 20.1 ± 5.6 μg/ml; chow-exercise: 23.4 ± 6.0 μg/ml; HFD-no exercise 21.1 ± 7.2 μg/ml and HFD-exercise: 20.9 ± 7.9 μg/ml).
Fig. 4.
Plasma leptin (A) and adiponectin (B) levels in chow-no exercise, chow-exercise, HFD-no exercise, and HFD-exercise mice, respectively, after 6-wk exercise period. +P < 0.05 vs. chow-exercise; ++P < 0.05 vs. chow-no exercise; **P < 0.05 vs. HFD-no exercise.
Effects of voluntary exercise on expression of adipose tissue and liver inflammatory markers.
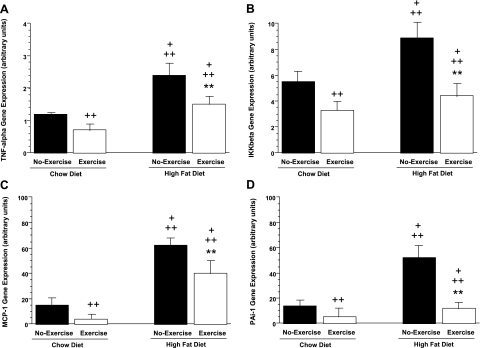
To begin characterizing the effects of voluntary exercise on HFD-induced inflammation, we examined expression of several key inflammatory markers in adipose tissue and liver by using real-time PCR. Inflammatory markers evaluated included TNF-α, PAI-1, MCP-1, and IKKβ. Consumption of HFD markedly increased expression of these inflammatory markers in adipose tissue. As shown in Fig. 5, A–D, relative to chow-no exercise animals, HFD-no exercise mice showed increased expression of each of these inflammatory markers in perigonadal fat. TNF-α and IKKβ expression increased twofold and 38% respectively, and there was a fourfold increase in expression of both MCP-1 and PAI-1. The increased expression of TNF-α and IKKβ was reversed by voluntary exercise. HFD-exercise mice showed a 40% reduction in TNF-α mRNA levels and a 50% decrease in IKKβ expression compared with HFD-no exercise mice. Similarly, MCP-1 and PAI-1 gene expression were decreased in HFD-exercise mice by 35 and 77%, respectively, compared with HFD-no exercise mice. Furthermore, voluntary exercise reduced expression of these inflammatory markers in chow-exercise compared with chow-no exercise mice. TNF-α and IKKβ mRNAs were reduced by 42 and 49%, respectively, whereas MCP-1 and PAI-1 mRNAs were reduced by 73 and 64%, respectively.
Fig. 5.
Effect of 6-wk exercise period on tumor necrosis factor-α (TNF-α; A), IκB kinase-β (IKKβ; B), monocyte chemoattractant protein-1 (MCP-1; C), and plasminogen activator inhibitor-1 (PAI-1; D) gene expression (A-D) in perigonadal fat from Chow vs. HFD mice. +P < 0.05 vs. chow-exercise; ++P < 0.05 vs. chow-no exercise; **P < 0.05 vs. HFD-no exercise.
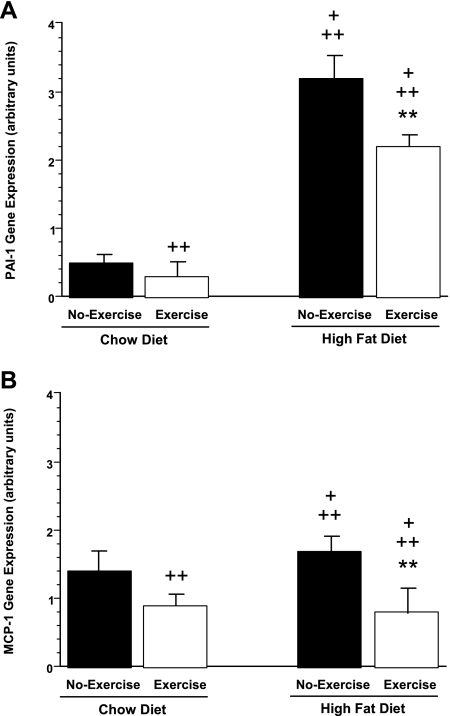
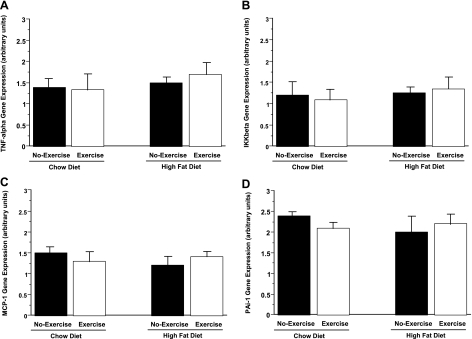
On the other hand, mesenteric fat showed a differential expression of these inflammatory markers compared with perigonadal fat. In mesenteric fat, HFD-no exercise mice showed a sixfold and a 21% increase in PAI-1 and MCP-1 mRNA, respectively, compared with chow-no exercise mice (Fig. 6, A and B). Exercise reduced expression of both markers in HFD mice by 31 and 53%, respectively. Similarly, expression of PAI-1 and MCP-1 were reduced in chow-exercise compared with chow-no exercise mice (40 and 36%, respectively). However, in contrast to the changes seen in perigonadal fat, expression of TNF-α and IKKβ in mesenteric fat was unchanged among the four animal groups (not shown). Further indication of the tissue-specific expression pattern of inflammatory markers in response to the HFD/exercise paradigm was seen in liver, where expression of TNF-α, IKKβ, PAI-1, and MCP-1 was not significantly different among the four groups (Fig. 7, A–D).
Fig. 6.
PAI-1 and MCP-1 gene expression are increased by HFD and reduced by exercise in mesenteric fat from chow-no exercise, chow-exercise, HFD-no exercise, and HFD-exercise mice. +P < 0.05 vs. chow-exercise; ++P < 0.05 vs. chow-no exercise; **P < 0.05 vs. HFD-no exercise.
Fig. 7.
TNF-α, IKKβ, MCP-1, and PAI-1 gene expression in liver are not significantly different among chow-no exercise, chow-exercise, HFD-no exercise, and HFD-exercise mice.
DISCUSSION
Insulin resistance, a common metabolic perturbation in obese individuals, is a known risk factor for the development of type 2 diabetes, cardiovascular disease, and other chronic diseases (18, 38, 39). Thus, interventions that improve insulin sensitivity constitute an important therapeutic strategy. In humans, exercise is known to increase insulin sensitivity (14, 18, 24) and is a helpful method of body weight control. However, the mechanisms that mediate exercise-induced improvements in insulin sensitivity when weight is not normalized in obese individuals are minimally understood; furthermore, few animal models have been developed to address this issue. In the present study, we found that voluntary exercise in diet-induced obese mice reduced adiposity despite continued consumption of HFD. In addition, exercise normalized insulin sensitivity independently of changes in adiponectin levels and mitigated adipose tissue inflammation in these animals.
Mice spontaneously exercised when functional running wheels were made available. Voluntary exercise was associated with a partial reduction in body fat mass, a concomitant improvement in glucose tolerance, and complete normalization of insulin sensitivity in mice with diet-induced obesity. The reduction in adiposity among animals with access to running wheels while being maintained on a HFD was not due to reduced feeding, as total food intake was increased in HFD-exercise mice, as well as in chow-exercise mice, compared with their respective no-exercise counterparts. Instead, the reduced adiposity was secondary to increased exercise-associated energy expenditure.
Lean body mass remained similar among chow-no exercise, chow-exercise, HFD-no exercise, and HFD-exercise groups. However, fat body mass was significantly increased in HFD mice compared with chow animals. Furthermore, exercise reduced fat body mass in HFD as well as in chow mice. Although fat mass was reduced in HFD-exercise mice compared with HFD-no exercise animals, it still remained higher than in chow-no exercise and chow-exercise mice. Thus, although voluntary exercise almost completely normalized the metabolic phenotype of obese HFD mice, it did not fully reverse their increased adiposity.
Nevertheless, the partial reduction in fat mass was associated with a complete reversal of obesity-induced insulin resistance. ITT showed that HFD-exercise mice had similar insulin sensitivity to chow-exercise mice and that both these groups were significantly more sensitive than either chow-no exercise mice or HFD-no exercise mice. Similarly, fasting plasma glucose and insulin levels were higher in HFD mice compared with chow mice but were reduced by exercise in both these groups. In aggregate, these results indicate that the reduction in fat mass secondary to voluntary exercise substantially improved insulin sensitivity in both chow and HFD mice. In the case of the latter, normalization of insulin sensitivity persisted, despite continued consumption of HFD.
In addition to the effects of chronic exercise on insulin sensitivity, acute bouts of exercise may also have effects on insulin sensitivity, raising the possibility that the improved insulin sensitivity in our exercise model may have been due to the timing of the last exercise period relative to GTT and ITT. This possibility cannot be excluded, as the system utilized in our study measured cumulative exercise and was not designed to distinguish between the relative contributions of the most recent exercise bout and the benefits conferred by chronic exercise training.
Consistent with their increased adiposity, plasma leptin levels were substantially elevated in HFD-no exercise mice and were significantly decreased in tandem with the reduced adiposity seen in HFD-exercise mice. A similar pattern of lower plasma leptin was seen in chow-exercise compared with chow-no exercise mice. In contrast, plasma adiponectin levels were similar among the four groups. In humans, adiponectin levels are reduced with increasing adiposity (11, 21, 43, 44). However, a number of studies have reported increased adiponectin levels in mice and rats fed HFD to induce obesity (10, 28, 29) and it has been proposed that this increase may represent an initial response to counteract diet-induced obesity and insulin resistance (10). In the current study, although HFD-no exercise mice showed a trend toward increased adiponectin levels, this increase was not statistically significant. Furthermore, our results suggest that adiponectin was not a contributing factor to the exercise-induced improvement in insulin sensitivity seen in our model. Although plasma adiponectin is known to increase with weight loss and has been attributed to improved insulin action (11, 21, 43, 44), the effects of exercise on plasma adiponectin levels are less well defined. In support of our findings, a separate study has reported that exercise training improves insulin action in overweight humans independently of changes in plasma adiponectin levels (22). Thus, the mechanisms of exercise-induced improvements in insulin sensitivity may differ to some degree from those underlying weight loss-induced improvements in insulin sensitivity.
We also evaluated changes in serum RBP4 as a potential mechanism of normalized insulin sensitivity in our HFD/exercise model. RBP4 is a liver- and adipocyte-derived factor that is elevated in serum from insulin-resistant mice and humans with obesity and type 2 diabetes (16, 35, 45). In addition, transgenic overexpression of human RBP4 or injection of recombinant RBP4 into normal mice induces insulin resistance (45), and pharmacological or genetic reductions in circulating RBP4 levels increase insulin sensitivity in mice (45). It has been proposed that reductions in circulating RBP4 may contribute to increased insulin sensitivity in obese individuals after weight loss (17, 45). However, in the current study, normalization of insulin sensitivity in our HFD/exercise model was not mediated by alterations in serum RBP4.
It is now well established that obesity correlates with dysregulated secretion of several adipose tissue-derived cytokines (collectively termed adipokines), which in turn contributes to a chronic subclinical inflammation seen in obese individuals. This inflammation, in turn, has been linked to the pathology of insulin resistance as well as several other metabolic and cardiovascular disorders (27, 36, 38–40, 42). Studies in models of rodent and human obesity have indicated that inflammatory cytokines such as TNF-α are markedly upregulated in adipose tissue (19, 20, 26, 31, 42). TNF-α has been shown to induce insulin resistance (7, 20), and null mutations in either the genes encoding TNF-α or its receptors have been shown to ameliorate obesity-induced insulin resistance (41). In addition, a recent study has suggested that the release of adipose tissue cytokines may rapidly induce insulin resistance in human skeletal muscle cells via the IKKβ/NF-κB pathway (25), a key signaling pathway in tissue inflammation. Furthermore, Cai et al. (3), have shown that transgenic activation of the IKKβ/NF-κB pathway in mouse liver induces insulin resistance.
Our findings on changes in adipokine expression suggest that these alterations may play a key role in exercise-induced improvements in insulin sensitivity. HFD/exercise-associated alterations in expression of these inflammatory cytokines occurred in a tissue-specific pattern. Whereas expression of all four markers was increased in perigonadal fat from HFD mice, only PAI-1 and MCP-1 expressions were increased in mesenteric fat from HFD mice. Furthermore, the HFD-induced increases in the aforementioned adipokines were almost completely reversed by exercise. By comparison, no changes in expression were noted in liver for any of the inflammatory markers tested. It is possible, however, that changes in liver inflammatory markers in our HFD/exercise model may manifest themselves during a more prolonged study period. To our knowledge, these findings are the first demonstration of this differential expression pattern within adipose tissue depots and between adipose tissue and liver. In contrast, a separate study has reported increased TNF-α production in mesenteric fat from voluntary exercised rats fed a high-sucrose diet to induce insulin resistance compared with nonexercised rats fed the same diet. However, this study was in rats as opposed to mice and manifested itself over a more prolonged 12-wk exercise period (34).
It is noteworthy that exercise reversed the increased adipokine expression despite continued consumption of HFD and despite the fact that HFD-exercise mice still had significantly higher fat mass than chow-fed mice. On the other hand, in our model, exercise-induced normalization of insulin sensitivity occurred independently of changes in expression of liver inflammatory cytokines. Thus, the reduction in adipokine expression in perigonadal and mesenteric adipose tissue in HFD-exercise mice may contribute to the normalized insulin sensitivity phenotype seen in these animals. A significant exercise-induced decrease in adipokine expression was also observed in chow-fed mice. It is possible that the exercise-induced decrease in adipokine expression may occur in part secondarily to increased utilization of circulating fatty acids. However, the mechanisms of exercise-induced downregulation of adipokine expression remain unknown and await further study.
In summary, we have demonstrated that voluntary exercise partially reversed diet-induced obesity in mice despite continued consumption of a HFD and reversed obesity-associated insulin resistance in these animals. Furthermore, the exercise-mediated normalization of insulin sensitivity was accompanied by decreased expression of adipose tissue-derived proinflammatory factors. Taken together, these results are consistent with the hypothesis that exercise-induced improvements in insulin sensitivity may be mediated, in part, by mitigation of adipose tissue inflammation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants PPG-DK-56116 and RO1-DK-56113 (E. Maratos-Flier), by a postdoctoral fellowship award from the Natural Science and Engineering Council of Canada (J. Y. Jeon), and by NIDDK Grant KO1-DK-063080 (R. L. Bradley).
Acknowledgments
We thank Dr. Timothy E. Graham for measuring the serum RBP4.
Present address of J. Y. Jeon: Dept. of Sport and Leisure Studies, Yonsei University, Seoul, Korea.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11: 191–198, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu S, Fisler JS, Espinal GM, Havel PJ, Stern JS, Warden CH. The yellow agouti mutation alters some but not all responses to diet and exercise. Obes Res 12: 1243–1255, 2004. [DOI] [PubMed] [Google Scholar]
- 5.De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol 290: R926–R934, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Duncan GE, Perri MG, Theriaque DW, Hutson AD, Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care 26: 557–562, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem 268: 26055–26058, 1993. [PubMed] [Google Scholar]
- 8.Flier J, Maratos-Flier E. Energy homeostasis and body weight. Curr Biol 10: R215–217, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Flier JS Obesity wars: molecular progress confronts an expanding epidemic. Cell 116: 337–350, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Franckhauser S, Munoz S, Elias I, Ferre T, Bosch F. Adipose overexpression of phosphoenolpyruvate carboxykinase leads to high susceptibility to diet-induced insulin resistance and obesity. Diabetes 55: 273–280, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98: 2005–2010, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthier MS, Couturier K, Charbonneau A, Lavoie JM. Effects of introducing physical training in the course of a 16-week high-fat diet regimen on hepatic steatosis, adipose tissue fat accumulation, and plasma lipid profile. Int J Obes Relat Metab Disord 28: 1064–1071, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol 94: 2127–2134, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49: 235–261, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia 50: 814–823, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 354: 2552–2563, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Haider DG, Schindler K, Prager G, Bohdjalian A, Luger A, Wolzt M, Ludvik B. Serum retinol-binding protein-4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab 92: 1168–1171, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Henriksen EJ Invited review. Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol 93: 788–796, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Hulver MW, Zheng D, Tanner CJ, Houmard JA, Kraus WE, Slentz CA, Sinha MK, Pories WJ, MacDonald KG, Dohm GL. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab 283: E861–E865, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell-Luevano C. Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochem Biophys Res Commun 326: 638–644, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev 27: 1–35, 1999. [PubMed] [Google Scholar]
- 25.Kamon J, Yamauchi T, Muto S, Takekawa S, Ito Y, Hada Y, Ogawa W, Itai A, Kasuga M, Tobe K, Kadowaki T. A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Commun 323: 242–248, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 95: 2111–2119, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diabetes Rep 5: 70–75, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Yu X, Pan W, Unger RH. Gene expression profile of rat adipose tissue at the onset of high-fat-diet obesity. Am J Physiol Endocrinol Metab 282: E1334–E1341, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Lopez IP, Milagro FI, Marti A, Moreno-Aliaga MJ, Martinez JA, De Miguel C. Gene expression changes in rat white adipose tissue after a high-fat diet determined by differential display. Biochem Biophys Res Commun 318: 234–239, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Lovejoy JC The influence of dietary fat on insulin resistance. Curr Diabetes Rep 2: 435–440, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Mito N, Hosoda T, Kato C, Sato K. Change of cytokine balance in diet-induced obese mice. Metabolism 49: 1295–1300, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 286: 1195–1200, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Nara M, Kanda T, Tsukui S, Inukai T, Shimomura Y, Inoue S, Kobayashi I. Running exercise increases tumor necrosis factor-alpha secreting from mesenteric fat in insulin-resistant rats. Life Sci 65: 237–244, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Polonsky KS Retinol-binding protein 4, insulin resistance, and type 2 diabetes. N Engl J Med 354: 2596–2598, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Schaffler A, Muller-Ladner U, Scholmerich J, Buchler C. Role of adipose tissue as an inflammatory organ in human diseases. Endocr Rev 27: 449–467, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Schrauwen P, Westerterp KR. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr 84: 417–427, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Trayhurn P Adipose tissue in obesity—an inflammatory issue. Endocrinology 146: 1003–1005, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389: 610–614, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Wellen KE, Hotamisligil GS. Inflammation, stress, diabetes. J Clin Invest 115: 1111–1119, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436: 356–362, 2005. [DOI] [PubMed] [Google Scholar]
- 46.You T, Yang R, Lyles MF, Gong D, Nicklas BJ. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am J Physiol Endocrinol Metab 288: E741–E747, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or _targeted disruption of Ikkbeta. Science 293: 1673–1677, 2001. [DOI] [PubMed] [Google Scholar]