Abstract
Background
There is uncertainty over when to pursue intensive glucose control among older diabetes patients.
Objective
To assess the impact of comorbid illnesses and functional status, mediated through background mortality, on the expected benefits of intensive glucose control.
Design
Decision analysis.
Data Sources
Major clinical studies in diabetes and geriatrics.
_target population
Patients aged 60–80 years with type 2 diabetes and varied life expectancies estimated from a mortality index validated at the population level.
Time Horizon
Patient lifetime.
Perspective
Health care system.
Interventions
Intensive glucose control (HbA1C <7.0%) versus moderate control (HbA1C <7.9%).
Outcome Measures
Lifetime differences in incidence of complications and average quality-adjusted days.
Results of Base Case Analysis
Healthy older patients of different age groups had expected benefits of intensive glucose control ranging from 51 to 116 quality-adjusted days. Within each age group, the expected benefits of intensive control steadily declined as the level of comorbid illness and functional impairment increased (mortality index score 1–26 points). For patients 60–64 years of age with new-onset diabetes, the benefits declined from 106 days at baseline good health (life expectancy 14.6 years), to 44 days with 3 additional index points (life expectancy 9.7 years), and to 8 days with 7 additional index points (life expectancy 4.8 years). A similar decline in benefits occurred among patients with prolonged duration of diabetes.
Results of Sensitivity Analysis
Expected benefits of intensive control declined with rising index scores with alternative model assumptions (e.g., Framingham models).
Limitations
Lack of diabetes clinical trial data for frail, older patients, use of a mortality index not validated for use in predicting individual level life expectancies, and lack of accounting for adverse effects of intensive control.
Conclusions
Among older diabetes patients, the presence of multiple comorbid illnesses or functional impairments is a more important predictor of limited life expectancy and diminishing expected benefits of intensive glucose control, than age alone.
Keywords: Diabetes mellitus, geriatrics, life expectancy, comorbid illnesses, functional status
Background
Intensive glucose control (glycosylated hemoglobin (HbA1C) levels <7% (1)) has been found to significantly lower the risk of multiple complications in patients with diabetes compared to moderate glucose control (HbA1C ~ 7.9%) (2–4). The importance of intensive glucose control has led to significant public health efforts to improve the delivery of diabetes care (5).
Despite its promise, the benefits of intensive glucose control remain uncertain for the heterogeneous population of older patients living with diabetes. This uncertainty is reflected in wide variation in practice by clinical specialty (6, 7) and in vastly different therapeutic recommendations from diabetes opinion leaders (8–10). This uncertainty arises from a lack of clinical trial data evaluating the benefits of long-term intensive glucose control in older patients, especially those with significant comorbid illnesses or functional impairments.
In 2003, the first guideline to acknowledge the unique care considerations of older diabetes patients was published (11). The guideline recommended an individualized approach to diabetes care that has been subsequently endorsed by multiple medical organizations (12, 13, 14). A central concept introduced in the guideline is that providers should consider _targeting glucose control levels based on life expectancy. Patients who have a life expectancy less than five years are felt to be unlikely to benefit from intensive glucose control levels, while patients with extended life expectancy are thought to be good candidates for intensive glucose control.
While these recommendations represent a conceptual advance in diabetes care, there has been very little evaluation of the recommendations. Comorbid illness and functional status are well known determinants of life expectancy (15, 16), but the extent to which these characteristics might influence the expected benefits of intensive glucose control is unknown. In addition, there are concerns about using limited life expectancy as a sole means of determining glucose control levels. Many older patients with limited life expectancy may also have prolonged duration of diabetes, a clinical characteristic that may enlarge the expected benefits of intensive control. How these competing characteristics might interact and influence decisions is unknown.
One approach to gaining insight into these questions is to utilize existing clinical evidence in decision analyses (17–19). An advantage of decision analysis is that it allows one to examine clinical questions for patients that might be typically excluded from clinical trials. Recent advances in prediction models have occurred in the fields of diabetes (20) and geriatrics (21). These advances enable us to evaluate how comorbid illnesses and functional status may alter the expected benefits of intensive glucose control in older type 2 diabetes patients.
Methods
Overview
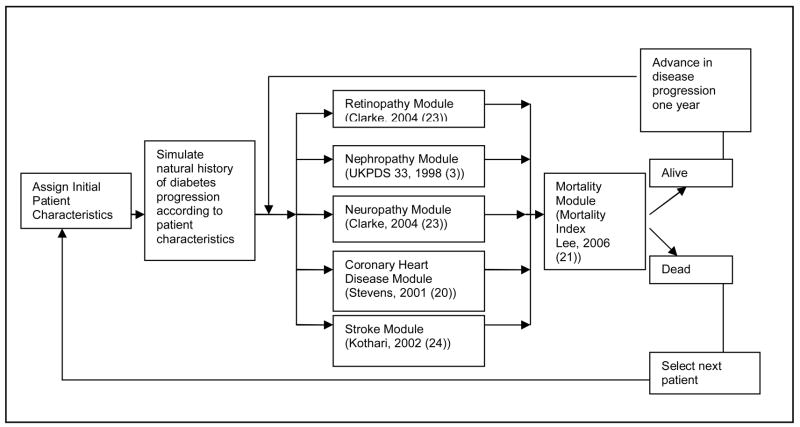
This decision analysis is an integration of multiple prediction models from the fields of diabetes and geriatrics. All prediction models were housed within the structure of an existing model of diabetes complications (National Institutes of Health (NIH) Model) (17, 22). This Monte Carlo simulation model is framed by simultaneous progression of disease through individual diabetes-related complications and mortality (Figure 1). Within a 1-year cycle length, patients move from one disease state to another or stay in the current disease state until death or age 95 (Microsoft Excel 2000, Microsoft, Seattle, WA and @Risk 4.0, Palisades, Inc., Newfield, NY). For each specific model setting (e.g., population characteristics, glucose level), the model is run 10,000 iterations (each iteration representing a patient life).
Figure 1. Model of diabetes complications in older patients.
The structure of the decision analytic model is presented in this figure. Hypothetical patients move through the model from left to right for each cycle length (one year). Based on initial patient clinical characteristics, patients are subject to the risk of various complications related to diabetes as well as mortality. Patients who survive a given year repeat the cycle until death.
In the following sections, we describe the individual prediction models, the population of interest, the comparison treatments, the outcomes of interest, and sensitivity analyses. Please see the Technical Appendix for details.
Diabetes Complications
The diabetes complication models in this analysis are all derived from United Kingdom Prospective Diabetes Study (UKPDS) results (3, 20, 23–24). Prediction models for all major diabetes-related complications have been developed by the UKPDS study group. These models have been internally validated and externally validated with cardiovascular trial data (25). We could not use the UKPDS prediction model for end-stage renal disease because this model does not include glucose control as a predictor. Instead, we modeled the development of microalbuminuria and proteinuria which are linked to the intensity of glucose control (19, 26). For probabilities under moderate control, we used prediction models developed using optimization procedures to fit observations from the UKPDS control arm to a functional form used in the original NIH model (27) (Technical Appendix). To determine the transition probabilities for intensive glucose control, we used a multiplier derived from the comparison of the overall results of UKPDS for individual complications. For the transition between proteinuria to end-stage renal disease we used probabilities from an observational study (28).
Incorporating functional status and comorbidity into background mortality
We used mortality rates from a 4-year mortality index developed from the Health and Retirement Study (21), rather than mortality rates from life tables (17, 22, 29). This index was developed and validated with a split-sample approach. The index has a total score of 26 and each comorbid illness or functional impairment contributes one to two points to the index score. To calculate background mortality rates for the general population, we subtracted cardiovascular mortality rates for the general population from the mortality rates associated with each index score (30). These mortality rates were multiplied by 2.75 as previously done to reflect higher background mortality rates for patients with diabetes (22).
The baseline index score for each hypothetical subgroup included points for age group and gender. We then systematically increased the mortality index score by as much as 14 additional points. Apart from these changes, we retained the NIH model assumptions about mortality due to other specific causes of death (31–33).
Population of interest
We performed simulations for hypothetical patients with type 2 diabetes at ages 60 to 80 years of age with no prior history of diabetes-related complications. The patients were assumed to have the demographic and clinical characteristics of diabetes patients over 60 years of age, found in the National Health and Nutrition Examination Surveys (NHANES)(1999–2002) (34).
Hypothetical cohorts were divided into 5-year age groups with a uniform age distribution. The age groups correspond with major groupings of older diabetes patients. We also varied the duration of diabetes for such patients (new onset, 0–5 years, 5–10 years, and 10–15 years). We assumed that the population had the gender and ethnic/racial distributions observed in NHANES. We did not assume any additional impact of race on complication rates, because the major ethnic minorities included in the UKPDS data do not completely correspond with the major ethnic minorities in the United States.
Comparison treatments
We compared the projected health effects of moderate glucose control (HbA1C 7.9%) and intensive glucose control (HbA1C 7.0%)(11, 35). We assumed that patients maintained these glucose control levels throughout their lives. In sensitivity analyses, we also compared HbA1C of 9.0% versus 7.9% and HbA1C of 7.0% versus 6.5% (36).
Other treatment assumptions
All other elements of care were held constant in the two scenarios (37–39). Hypothetical patients had cardiovascular risk factor levels selected from the age, race, and gender specific subgroup distributions for systolic blood pressure, diastolic blood pressure, total cholesterol, high density lipoprotein cholesterol, and smoking for diabetes patients found in NHANES (1999–2002).
Outcomes of interest
Outcomes of interest included the lifetime incidence of individual complications and life expectancy. The benefits of intensive versus moderate glucose control are reported as an average absolute risk reduction in complications and added days of life. The primary outcome of interest was the average difference in quality-adjusted days. To calculate quality-adjusted benefits, we used utility weights for major complications used in prior analyses (19, 27, 40–44). We assumed no disutility of life with different treatments (45). When multiple health states occurred, we used the minimum health state method (46).
Sensitivity Analysis
The UKPDS prediction models assume that the glucose level is a modifiable risk factor for coronary heart disease in type 2 diabetes, when in fact this remains a highly debated and studied topic (47). To assess the impact of this assumption, we replaced the UKPDS models for coronary heart disease and stroke with Framingham models (48, 49). We also conducted sensitivity analyses on other important model assumptions (e.g., background mortality rate inflator). Subgroup analyses were performed for men, women, Whites, African-Americans, and Latinos.
Role of the Funding Source
The funding sources had no role in the design, conduct, or analysis of the study or in the decision to submit the manuscript for publication.
Results
Base Case Results
The model predicted that cardiovascular complications would be the most frequently experienced complications in all patient subgroups (44–54% lifetime incidence coronary heart disease) (Table 1). Microvascular complications had lower lifetime incidences that varied with starting age of the simulation and duration of diabetes. The incidence of end-stage renal disease and amputation declined with increasing starting age but rose with increasing duration of diabetes. Conversely, incidence of blindness increased with increasing starting age but declined with increasing disease duration; these patterns reflect the inclusion of age at onset of diabetes as a predictor in the UKPDS blindness model.
Table 1.
Baseline predictions for moderate glucose control and expected benefits of intensive glucose control in older patients with no comorbid illnesses or functional impairments.*
| Age, years | Blindness | End-stage renal disease | Amputation | Coronary heart disease | Life expectancy | Quality-adjusted life expectancy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inc., %, (95% CI) |
ARR, %, (95% CI) |
Inc., %, (95% CI) |
ARR, %, (95% CI) |
Inc., %, (95% CI) |
ARR, %, (95% CI) |
Inc., %, (95% CI) |
ARR, %, (95% CI) |
Years, mean, (95% CI) |
Days gained, mean, (95% CI) |
Years, mean, (95% CI) |
QAD gained, mean, (95% CI) |
||
| New-onset diabetes |
60–64 | 7.0 (6.5–7.5) |
1.2 (0.9–1.4) |
3.1 (2.7–3.4) |
0.8 (0.6–1.0) |
5.2 (4.8–5.6) |
1.3 (1.1–1.6) |
52.2 (51.3–53.2) |
2.8 (2.5–3.2) |
14.6 (14.4–14.7) |
114 (101–128) |
13.1 (13.0–13.3) |
106 (95–117) |
| 65–69 | 7.8 (7.3–8.3) |
1.3 (1.0–1.5) |
1.8 (1.6–2.1) |
0.6 (0.4–0.8) |
5.8 (5.4–6.3) |
1.6 (1.4–1.9) |
49.5 (48.5–50.4) |
2.6 (2.3–2.9) |
12.1 (11.9–12.2) |
90 (79–102) |
10.7 (10.6–10.8) |
82 (73–91) |
|
| 70–74 | 8.4 (7.8–8.9) |
1.2 (0.9–1.4) |
0.9 (0.7–1.1) |
0.3 (0.2–0.4) |
4.7 (4.2–5.1) |
1.4 (1.1–1.6) |
47.4 (46.4–48.3) |
2.4 (2.1–2.7) |
9.8 (9.6–9.9) |
73 (64–83) |
8.5 (8.4–8.6) |
63 (56–70) |
|
| 75–79 | 8.8 (8.2–9.3) |
1.3 (1.2–1.6) |
0.4 (0.3–0.5) |
0.1 (<0.1–0.2) |
3.5 (3.2–3.9) |
0.9 (0.7–1.1) |
44.8 (43.8–45.7) |
2.7 (2.3–3.0) |
7.8 (7.7–7.9) |
64 (55–72) |
6.7 (6.6–6.8) |
52 (46–58) |
|
| Duration of diabetes 10–15 years |
60–64 | 3.7 (3.3–4.0) |
0.4 (0.2,–0.5) |
8.5 (7.9–9.0) |
1.1 (0.8–1.4) |
11.7 (11.1–12.3) |
2.1 (1.8–2.5) |
53.8 (52.8–54.8) |
2.1 (1.7–2.5) |
13.5 (13.3–13.6) |
132 (117–147) |
11.7 (11.6–11.9) |
116 (103–129) |
| 65–69 | 4.3 (3.8–4.7) |
0.5 (0.3–0.8) |
5.6 (5.1–6.0) |
0.9 (0.6–1.2) |
13.1 (12.4–13.8) |
2.4 (2.0–2.8) |
52.2 (51.2–53.2) |
2.2 (1.9–2.6) |
11.2 (11.0–11.3) |
110 (97–123) |
9.6 (9.4–9.7) |
99 (88–110) |
|
| 70–74 | 4.7 (4.3–5.1) |
0.5 (0.3–0.7) |
3.4 (3.1–3.8) |
0.6 (0.4–0.8) |
11.2 (11.1–12.4) |
2.0 (1.6–2.3) |
50.3 (49.4–51.3) |
2.3 (2.0–2.7) |
9.1 (9.0–9.2) |
81 (70–91) |
7.7 (7.6–7.8) |
72 (64–81) |
|
| 75–79 | 5.4 (4.9–5.8) |
0.3 (0.1–0.6) |
2.0 (1.7–2.3) |
0.4 (0.2–0.5) |
10.9 (10.3–11.5) |
3.1 (2.7–3.4) |
48.9 (47.9–49.9) |
3.1 (2.7–3.4) |
7.3 (7.2–7.4) |
60 (51–68) |
6.0 (5.9–6.1) |
51 (45–58) |
|
Moderate glucose control= glycosylated hemoglobin 7.9%. Intensive glucose control=glycosylated hemoglobin 7.0%. Inc.= remaining lifetime incidence. ARR= absolute risk reduction. QAD= quality-adjusted days. CI= confidence interval. Virtual patients with duration of diabetes in ranges 0–5 years and 5–10 years had results that were intermediate between patients with new onset diabetes and 10–15 years of diabetes at the start of simulations.
Life expectancy declined with increasing starting age and increasing duration of diabetes. The model’s life expectancy predictions for healthy older patients with new onset diabetes matched expectations from epidemiological studies of mortality and diabetes (50, 51). Quality-adjusted life expectancy was 1–1.5 years less than unadjusted life expectancy.
The overall magnitude of expected benefits of achieving intensive glucose control compared to moderate glucose control declined as the age of hypothetical patients rose (Table 1). With rising starting simulation age, the level of absolute risk reduction declined for end-stage renal disease and amputation but remained stable for blindness and coronary heart disease. Life expectancy and quality of life benefits declined with rising age. The expected benefit of intensive glucose control was 106 (95% Confidence Intervals (CI), 95–117) quality-adjusted days at age 60–64 years of age and declined to 52 (CI, 46–58) days at 75–79 years of age among individuals with no comorbid illness or functional impairment. Increasing duration of diabetes had the opposite effect of increasing the overall expected quality of life benefits of intensive glucose control. For 60–64 year old patients, the overall quality of life benefit increased from 106 days for new-onset diabetes to 114 days for duration of diabetes beyond five years.
Larger differences in the expected benefits of intensive glucose control were observed within each age group with changes in the mortality index score (Figure 2, Appendix Figure 1). As the index score increased, life expectancy declined. In the case of patients 60–64 years of age with new diabetes, life expectancy declined from 14.6 (CI, 14.4–14.7) years at baseline, to 9.7 (CI, 9.6–9.9) years with 3 additional index points, and to 4.8 (CI, 4.7–4.9) years with 7 additional index points. As life expectancy declined, so did expected benefits. Over the same interval of index points, life expectancy benefits declined from 114 (CI, 101–128) days to 41 (CI, 34–48) days to 5 (CI, 3–8) days, and quality-adjusted benefits declined from 106 (CI, 95–117) days to 44 (CI, 38–50) days to 8 (CI, 5–10) days.
Figure 2. Expected quality of life benefits of intensive glucose control for 60–64 year old and 75–79 year old patients.
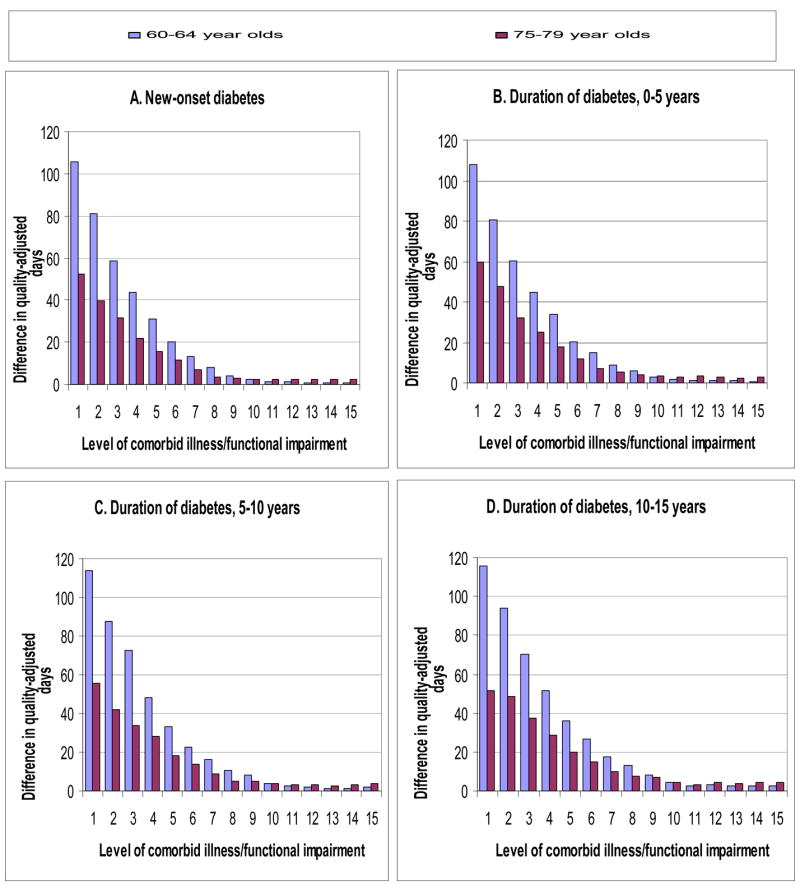
**Level of comorbid illness and functional impairment is indicated by additional points on the mortality index score (1–2 points per illness or impairment). Expected benefits for 65–69 year old and 70–74 year old patients are intermediate to those of 60–64 year old and 75–79 year old subgroups (see Technical Appendix).
The negative associations between life expectancy, benefits, and the mortality index score were also observed for patients with greater duration of diabetes. Again, life expectancy declined with an increasing index score. For patients 60–64 years of age and 10–15 years of diabetes, life expectancy declined from 13.5 (CI, 13.3–13.6) years at baseline to 8.0 (CI, 7.9–8.1) years with 4 additional index points and to 3.9 (CI, 3.8–4.0) with 8 additional index points. Over the same intervals, the expected benefits declined from 116 (CI, 103–129) quality-adjusted days, to 36 (CI, 29–43) days, to 8 (CI, 6–11) days (Figure 2d).
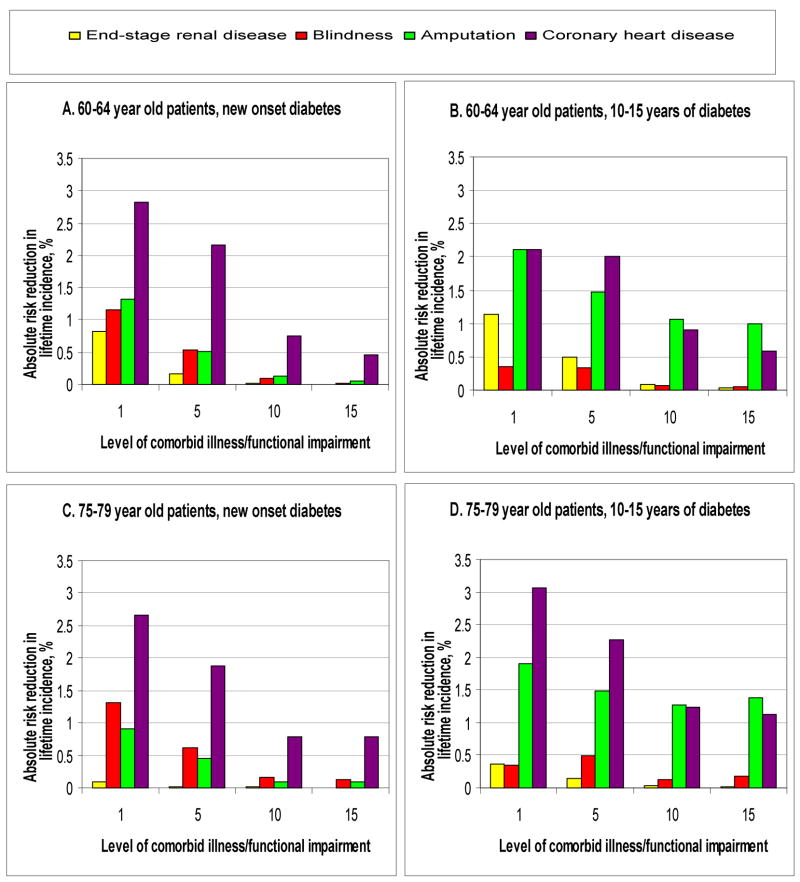
When examining these interrelationships by specific complications, we found distinct patterns for different complications (Figures 3, Appendix Figure 2). For end-stage renal disease, there is a decline in absolute risk reduction as the mortality index score rises, even among patients with extended duration of diabetes (Figures 3b and 3d). On the other hand, the benefits of preventing amputation declined but remained close to an absolute risk reduction over 0.5% percent at high index scores, when duration of diabetes exceeded five years (Figure 3b and 3d). These distinct patterns reflect differences in the size of baseline incidence rates and differences in assumptions regarding how glucose control affected individual complication rates.
Figure 3. Expected differences in lifetime incidence of specific complications for 60–64 year old and 75–79 year old patients.
**Level of comorbid illness and functional impairment is indicated by additional points on the mortality index score (1–2 points per illness or impairment). The relationships between absolute risk reductions for specific events and the mortality index score are not consistently monotonic because we assessed fairly wide ranges of duration of diabetes (5 years) and the individual complication models vary in their responsiveness to this variable. Expected differences for 65–69 year old and 70–74 year old patients are intermediate to those of 60–64 year old and 75–79 year old subgroups (see Technical Appendix).
Sensitivity analyses
When we used Framingham models, the predicted rates of cardiovascular disease were lower and life expectancies were higher than in the base case analysis, but expected benefits of intensive glucose control were lower. For patients 60–64 years of age with new diabetes and moderate glucose control, the incidence of coronary heart disease declined from 52% to 37 (CI, 36–38)% and life expectancy increased from 14.6 to 16.1 (CI, 15.9–16.3) years. Despite longer life expectancy, the expected benefits of intensive control were found to be less than half of those observed in the base case (e.g,44 (CI, 38–50) instead of 106 quality-adjusted days) due to the absence of glucose control as a predictor in the Framingham models. With all of these changes, the basic relationship between the mortality index score and expected benefits observed earlier was maintained. Expected benefits for the same 60–64 year old patients declined from 44 (CI, 38–50) days to 16 (CI, 13–19) days with 3 additional index points and to 3 (CI, 2–4) days with 7 additional index points.
In other sensitivity analysis, we generally found that results did not deviate significantly from the base case analysis. When we assumed no inflation of the non-diabetic background mortality rate, life expectancies increased by 2–3 years and the magnitude of expected benefits increased (e.g., 106 to 126 (CI, 115–137) quality-adjusted days for healthy 60–64 year old patents with new diabetes). Expected benefits continued to decline with a rising mortality index score but at slightly higher index scores compared to the base case. For example, in the same 60–64 year old patients mentioned earlier, expected benefits were reduced to less than 20 quality-adjusted days with 10 additional index points instead of 7 index points. In subgroup analyses, we found small differences in these patterns between men and women; for 60–64 year old patients, benefits dropped below 20 quality-adjusted days for men at 5 additional index points while women had this decline at 7 additional index points.
Different comparisons of glucose control levels altered the baseline magnitude of benefits of achieving lower glucose control _targets, but did not significantly alter the importance of the index score on the expected benefits. For healthy 60–64 year old patients with new diabetes the benefit was 131 quality-adjusted days for a comparison HbA1C of 9.0% and 7.9% and 52 days for a comparison HBA1C of 7.0% and 6.5%. In the case of the first comparison, the benefit declined to 10 days with 7 additional index points. In the case of the second comparison, the benefit declined to 3 days with 7 additional index points.
In our analyses of utility assumptions, we found that model results were highly sensitive to differences in treatment state utilities. If the every day quality of life experience of intensive glucose control was lower than moderate glucose control by more than 0.02 (i.e., a 2% reduction in daily quality of life from perfect health, 1.00 vs. 0.98), intensive control became a harmful therapy. Model results did not change significantly when using lower utilities for complication states.
Discussion
Our results illustrate that limited life expectancy is an important determinant of the magnitude of the expected benefit of intensive glucose control compared to moderate glucose control, even in settings of advanced duration of diabetes. The results suggest that five years of life expectancy is an acceptable threshold for identifying older patients who are unlikely to benefit from intensive control. We found that patients with a life expectancy of five years or less were likely to only gain 20 quality-adjusted days with intensive glucose control.
If life expectancy is important, there is a need for practical approaches to identifying older diabetes patients with limited life expectancy. We found that a combination of multiple comorbid illnesses and functional impairments is a more important predictor of limited life expectancy and diminishing benefits of intensive glucose control, than age alone. For patients 60–64 years of age, the presence of a combination of four longstanding comorbid illnesses or functional impairments is associated with a total mortality index score of 8–10, life expectancy less than five years, and significantly reduced benefits of 8–13 quality-adjusted days. On average, life expectancy is less than five years for 60–64 year old patients with 7 additional index points, 65–69 years old patients with 6 additional index points, 70–74 year old patients with 5 additional index points, and 75–79 year old patients with 4 additional points. In our analyses, we assumed that comorbid illnesses or functional impairments were permanent, when in reality these characteristics are dynamic (52). The dynamic nature of these characteristics suggests that decisions regarding the intensity of glucose control need to be routinely revisited.
These analyses provide insights into how benefits of intensive glucose control may vary by clinical characteristics, but they should not be the sole consideration when determining the goals of diabetes care for an individual patient. The mortality index used in our model was developed and validated at a population level and our model generates estimates that represent the average effects for subgroups of patients. The experiences of individual patients will still vary from these average effects. Apart from these considerations regarding benefits, concerns regarding the adverse consequences of pursuing intensive glucose control may be particularly relevant when identifying glucose control _targets for some older patients (11, 53, 54). There is current uncertainty regarding the potential harms of pursuing near-normal glucose levels (_target HbA1C<6.0%) in older patients. The glucose control portion of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial was recently terminated because of excessive deaths in the very intensive glucose control arm (55); on the other hand, there have been no excessive deaths attributable to very intensive glucose control in the ADVANCE study (56). In the case of pursuing an HbA1C in the range of 7.0%, clinical trials have not documented harms like those observed in the ACCORD trial; however, there are likely to be subgroups of older patients at particularly high risk of hypoglycemia and geriatric syndromes even with this traditional glucose control _target. The current uncertainty related to pursuing near-normal glucose levels underscores the importance of carefully tailoring diabetes care goals and plans to individual older patients based on expected benefits and harms of therapy. A critical piece of this process is the acknowledgement of patients’ treatment preferences which should be important determinants of treatment decisions (45, 53).
The validity of our findings depends on the quality of our decision analytic model. We attempted to enhance the validity of our analyses by using the most up-to-date prediction models from the fields of both diabetes and geriatrics. In addition, the results of our model have face validity in comparisons with expectations from epidemiological studies of diabetes and related complications (51). Our results regarding life expectancy and the estimated benefits of intensive glucose control are also highly comparable to those of other well-known diabetes complication prediction models such as the Centers for Disease Control Cost-Effectiveness Model of Diabetes Complications and the UKPDS Outcomes Model (19, 23).
There are several limitations of our analysis. There is a lack of directly available clinical trial data for older patients and patients with comorbid illness or functional impairment. Without such data, our analysis relied on prediction models for diabetic complications developed from UKPDS trial results. Our findings are therefore attributable to the particularities of these prediction models and their study populations. In addition, we made a number of assumptions in our analysis, such as not accounting for the adverse consequences of intensive control, that biased the analysis favorably towards intensive control. Despite this bias, our analysis illustrates a steady decline of expected benefits with a rising mortality index score.
The results of this study provide support for the recommendations of geriatric diabetes care guidelines. The challenge for older patients and their providers is in deciding how to best apply results from clinical trials to the care of an individual. In the absence of directly applicable trial data, methods such as decision analysis allow us to integrate data from existing clinical trials with advancing knowledge about the health experiences of older diabetes patients and provide insight into the care of individuals. These personalized estimates provide a starting point for discussions between older diabetes patients and their providers regarding the value of pursuing a complex therapy such as intensive glucose control.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the assistance of Priya John, MPH in preparing this manuscript.
Grant support: This study was supported by a NIA Career Development Award (Dr. Huang, K23 AG021963), a NIDDK Diabetes Research and Training Center (Drs. Huang, Zhang, Chin, and Meltzer P60 DK20595), the Chicago Center of Excellence in Health Promotion Economics (Drs. Huang, Chin, and Meltzer), a NICHD Small Grant (Dr. Zhang, R03 HD056073), and a NIDDK Midcareer Investigator Award in Patient-Oriented Research (Dr. Chin, K24 DK071933).
Footnotes
Address for reprint requests: Elbert S. Huang, MD, MPH
The University of Chicago
5841 S. Maryland Avenue, MC 2007
Chicago, IL 60637
ehuang@medicine.bsd.uchicago.edu
Current author addresses: Elbert S. Huang, MD, MPH
The University of Chicago
5841 S. Maryland Avenue, MC 2007
Chicago, IL 60637
Qi Zhang, PhD
3138 Health Sciences Building
School of Community and Environmental Health
Old Dominion University
Norfolk, VA 23529
Niren Gandra, BA
10 Buick Street, Box 8298
Boston, MA 02215
Marshall H. Chin, MD, MPH
The University of Chicago
5841 S. Maryland Avenue, MC 2007
Chicago, IL 60637
David O. Meltzer, MD, PhD
The University of Chicago
5841 S. Maryland Avenue, MC 2007
Chicago, IL 60637
Reproducible Research Statement
Protocol: not available
Statistical code: Readers with questions regarding the simulation model used in this analysis may send inquiries to Dr. Huang (ehuang@medicine.bsd.uchicago.edu). The model is not available without establishing written agreements with the authors.
Data: not available
References
- 1.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(Supplement 1):S4–S36. [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The Effect Of Intensive Treatment Of Diabetes On The Development And Progression Of Long-Term Complications In Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.U. K. Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complication Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming BB, Greenfield S, Engelgau MM, et al. The diabetes quality improvement project. Diabetes Care. 2001;24(10):1815–1820. doi: 10.2337/diacare.24.10.1815. [DOI] [PubMed] [Google Scholar]
- 6.Chin MH, Zhang JX, Merrell K. Specialty differences in the care of older patients with diabetes. Med Care. 2000 Feb;38(2):131–140. doi: 10.1097/00005650-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Chin MH, Su AW, Jin L, Nerney MP. Variations in the care of the elderly persons with diabetes among endocrinologists, general internists, and geriatricians. J Gerontol. 2000;55A(10):M601–M606. doi: 10.1093/gerona/55.10.m601. [DOI] [PubMed] [Google Scholar]
- 8.Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006 Apr 26;295(16):1935–1940. doi: 10.1001/jama.295.16.1935. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamson MJ. A 74-year-old woman with diabetes. JAMA. 2007;297(2):196–204. doi: 10.1001/jama.297.2.196. [DOI] [PubMed] [Google Scholar]
- 10.Hayward RA, Hofer TP, Vijan S. Intensive glucose control in elderly adults. JAMA. 2007 May 23;297(20):2195. doi: 10.1001/jama.297.20.2195-a. author reply 2196. [DOI] [PubMed] [Google Scholar]
- 11.Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidlines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl Guidelines):S265–280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2004;27(Supplement 1):S15–S35. doi: 10.2337/diacare.27.2007.s15. [DOI] [PubMed] [Google Scholar]
- 13.Standards of medical care in diabetes--2007. Diabetes Care. 2007 Jan;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 14.Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c _targets. A guidance statement from the American College of Physicians. Ann Intern Med. 2007 Sep 18;147(6):417–422. doi: 10.7326/0003-4819-147-6-200709180-00012. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Saliba D, Elliott M, Rubenstein LV, et al. The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 17.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM: II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care. 1997;20(5):735–744. doi: 10.2337/diacare.20.5.735. [DOI] [PubMed] [Google Scholar]
- 18.Vijan S, Hofer TP, Hayward RA. Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Ann Intern Med. 1997;127(9):788–795. doi: 10.7326/0003-4819-127-9-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 19.The CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction, for type 2 diabetes. JAMA. 2002;287(19):2542–2551. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- 20.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci (Lond) 2001 Dec;101(6):671–679. [PubMed] [Google Scholar]
- 21.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006 Feb 15;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 22.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM: I. Model construction and assumptions. Diabetes Care. 1997;20(5):725–734. doi: 10.2337/diacare.20.5.725. [DOI] [PubMed] [Google Scholar]
- 23.Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS 68) Diabetologia. 2004;47:1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 24.Kothari V, Stevens RJ, Adler AI, et al. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002 Jul;33(7):1776–1781. doi: 10.1161/01.str.0000020091.07144.c7. [DOI] [PubMed] [Google Scholar]
- 25.The Mount Hood 4 Modeling Group. Computer modeling of diabetes and its complications. Diabetes Care. 2007;30(6):1638–1646. doi: 10.2337/dc07-9919. [DOI] [PubMed] [Google Scholar]
- 26.Huang ES, Zhang Q, Brown SES, Drum ML, Meltzer DO, Chin MH. The cost-effectiveness of improving diabetes care in U.S. federally-qualified community health centers. Health Serv Res. 2007;42(6 Part 1):2174–2193. doi: 10.1111/j.1475-6773.2007.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Diabetes Control and Complications Trial Research Group. Lifetime benefits and costs of intensive therapy as practiced in the Diabetes Control and Complications Trial. JAMA. 1996;276:1409–1415. [PubMed] [Google Scholar]
- 28.Humphrey LL, Ballard DJ, Frohnert PP, Chu CP, O’Fallon WM, Palumbo PJ. Chronic renal failure in non-insulin-dependent diabetes mellitus. A population-based study in Rochester, Minnesota. Ann Intern Med. 1989;111(10):788–796. doi: 10.7326/0003-4819-111-10-788. [DOI] [PubMed] [Google Scholar]
- 29.Andersen R, DeTurk P. National Vital Statistics Report. 6. Vol. 50. Hyattesville, Maryland: National Center for Health Statistics; 2002. [PubMed] [Google Scholar]
- 30.Anderson KM, Odell PM, Wilson PWF, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1990;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 31.Hunink MGM, Goldman L, Tosteson ANA, et al. The recent decline in mortality from coronary heart disease, 1980–1990. JAMA. 1997;277(7):535–542. [PubMed] [Google Scholar]
- 32.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975–1989. Neurology. 1998;50:208–216. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Renal Data System. USRDS 1994 Annual Data Report: Appendix D17. Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1994. [Google Scholar]
- 34.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men; evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health. 2001;91(1):76–83. doi: 10.2105/ajph.91.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saaddine JB, Cadwell B, Gregg EW, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med. 2006 Apr 4;144(7):465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 36.The American Association of Clinical Econdocrinologists Medical Guidelines for the Management of Diabetes Mellitus: the AACE System of Intensive Diabetes Self-Management—2002 Update. Endocrine Practice. 2002;9(Suppl 1):41–82. [Google Scholar]
- 37.Saydah SH, Fradkin JE, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 38.Grant RW, Pirraglia PA, Meigs JB, Singer DE. Trends in complexity of diabetes care in the United States from 1991 to 2000. Arch Intern Med. 2004;164(10):1134–1139. doi: 10.1001/archinte.164.10.1134. [DOI] [PubMed] [Google Scholar]
- 39.Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Medicine. 2005;2(12):e353. doi: 10.1371/journal.pmed.0020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in healthcare. Annu Rev Public Health. 2000;21:587–611. doi: 10.1146/annurev.publhealth.21.1.587. [DOI] [PubMed] [Google Scholar]
- 41.Dasbach EJ, Fryback DG, Thornbury JR. Health utility preference differences. Med Decis Making. 1992;12(4):351. [Google Scholar]
- 42.Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Medicine. 2004;30(6):549–556. doi: 10.1016/s1262-3636(07)70154-4. [DOI] [PubMed] [Google Scholar]
- 43.Tennvall GR, Apelqvist J. Prevention of diabetes-related foot ulcers and amputations: a cost-utility analysis based on Markov model simulations. Diabetologia. 2001;44:2077–2087. doi: 10.1007/s001250100013. [DOI] [PubMed] [Google Scholar]
- 44.Rosen A, Hamel MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S. Cost-effectiveness of full medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes. Annals of Internal Medicine. 2005;143(2):89–99. doi: 10.7326/0003-4819-143-2-200507190-00007. [DOI] [PubMed] [Google Scholar]
- 45.Huang ES, Jin L, Shook M, Chin MH, Meltzer DO. The impact of patient preferences on the cost-effectiveness of intensive glucose control in older patients with new onset diabetes. Diabetes Care. 2006;29(2):259–264. doi: 10.2337/diacare.29.02.06.dc05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naglie G, Krahn MD, Naimark D, Redelmeier DA, Detsky AS. Primer on medical decision analysis: part 3- estimating probabilities and utilities. Med Decis Making. 1997;17:136–141. doi: 10.1177/0272989X9701700203. [DOI] [PubMed] [Google Scholar]
- 47.Huang ES, Meigs JB, Singer DE. The effect of interventions to prevent cardiovascular disease in patients with type 2 diabetes mellitus. Am J Med. 2001;111:633–642. doi: 10.1016/s0002-9343(01)00978-0. [DOI] [PubMed] [Google Scholar]
- 48.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 49.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 50.Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes. In: Harris MI, editor. Diabetes in America. Bethesda, MD: National Institute of Health: National Institute of Diabetes and Digestive and Kidney Diseases; 1995. pp. 233–258. [Google Scholar]
- 51.Arias E. United States Life Tables, 2003. Natl Vital Stat Rep. 2006;54(14) [PubMed] [Google Scholar]
- 52.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004 Apr 7;291(13):1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 53.Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30(10):2478–2483. doi: 10.2337/dc07-0499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161:1653–1659. doi: 10.1001/archinte.161.13.1653. [DOI] [PubMed] [Google Scholar]
- 55.National Heart, Lung, and Blood Institute. For safety, NHLBI changes intensive blood sugar treatment strategy in clinical trial of diabetes and cardiovascular disease. 2008 February 6; http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2551 Press Release. Retrieved on 2008-02-22.
- 56.The George Institute for International Health. Major diabetes study does not confirm increased risk of death reported by U.S. trial. 2008 February 18; http://www.advance-trial.com/static/html/virtual/contents.asp?P=39 Press Release. Retrieved on 2008-03-19.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.