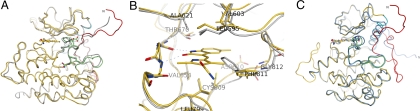
Fig. 3.
Sunitinib recognizes the autoinhibited form of KIT. WT KIT bound to sunitinib is shown in yellow (JM domain, red; A-loop, green; C α-helix, cyan). (A) WT KIT bound to sunitinib is very similar to the published autoinhibited structure of KIT (gray) (7, 8). Amino acid side chains are shown at the sites of A-loop substitutions found in sunitinib-resistant GISTs. (B) Sunitinib-binding site in the complex and apo structures. Drug binding induces a slight rearrangement of the Phe-811 side chain relative to the apo form. (C) The overall structure of the D816H mutant bound to sunitinib (darker blue) is very similar to that with WT, except for the proposed dislocation of the JM domain from its autoinhibitory position. Residue 816 is shown for both proteins.