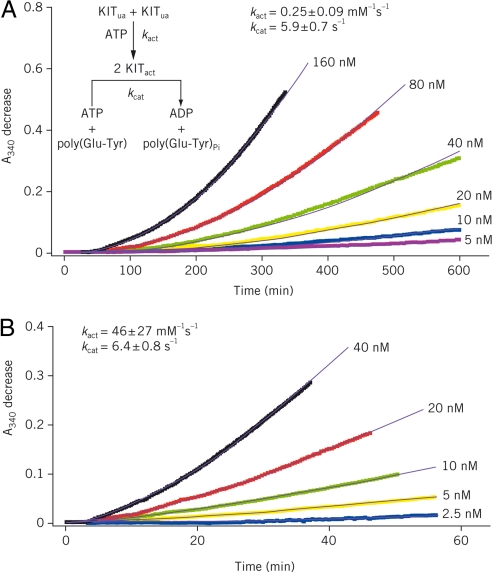
Fig. 5.
KIT A-loop mutant D816H autoactivates at a much faster rate than WT enzyme. WT KIT (A) and D816H mutant (B) autoactivation was conducted in a reaction mixture containing 4 mM ATP and various starting concentrations of unactivated (ua) KIT as indicated. Autoactivation was monitored in a real-time manner by coupling the activation product [activated (act) KIT] to the kinase activity assay reaction described in Methods by using a saturating poly(Glu-Tyr) concentration (1.5 mg/ml). ADP production resulting from the reaction catalyzed by active KIT was followed by measuring absorbances at 340 nm (A340; colored traces) in the ATP-regeneration system described in Methods. Fitting the data to Eq. 1 in Methods gave the simulated results shown as solid black curves and second-order rate constants (kact) of 0.25 ± 0.09 and 46 ± 27 mM−1s−1 for WT KIT and D816H mutant, respectively.