Abstract
The pathological mechanism by which Aβ causes neuronal dysfunction and death remains largely unknown. Deficiencies in fast axonal transport (FAT) were suggested to play a crucial role in neuronal dysfunction and loss for a diverse set of dying back neuropathologies including Alzheimer's disease (AD), but the molecular basis for pathological changes in FAT were undetermined. Recent findings indicate that soluble intracellular oligomeric Aβ (oAβ) species may play a critical role in AD pathology. Real-time analysis of vesicle mobility in isolated axoplasms perfused with oAβ showed bidirectional axonal transport inhibition as a consequence of endogenous casein kinase 2 (CK2) activation. Conversely, neither unaggregated amyloid beta nor fibrillar amyloid beta affected FAT. Inhibition of FAT by oAβ was prevented by two specific pharmacological inhibitors of CK2, as well as by competition with a CK2 substrate peptide. Furthermore, perfusion of axoplasms with active CK2 mimics the inhibitory effects of oAβ on FAT. Both oAβ and CK2 treatment of axoplasm led to increased phosphorylation of kinesin-1 light chains and subsequent release of kinesin from its cargoes. Therefore pharmacological modulation of CK2 activity may represent a promising _target for therapeutic intervention in AD.
Keywords: Alzheimer's disease, Axonal transport, Beta amyloid oligomer, CK2, Kinesin
The complex functional changes and histopathology of Alzheimer disease (AD) make it one of the most challenging disorders faced by modern medicine. Histopathological hallmarks of AD include distinctive extracellular and intracellular aggregates of amyloid beta (Aβ) and tau (1, 2). Synaptic dysfunction and axonopathy appear to be the earliest lesions in AD as corroborated by reduced immunoreactivity of synaptophysin and other synaptic markers in terminal fields of brain-affected areas (3). AD-affected neurons appear to die eventually as a consequence of synaptic dysfunction and loss, following a typical dying-back pattern of neuronal degeneration.
The amyloid hypothesis (4), a dominant concept in AD research for many years, has been revised in recent years to include the notion that smaller soluble Aβ aggregates may play an early and significant role in AD. Soluble oligomers of Aβ (oAβ) have been shown to be neurotoxic both in vivo and in vitro (5–7) as well as altering synaptic structure and function (8). Moreover, oAβ levels correlate with impairments in cognitive function, learning, and memory (9, 10), but the molecular basis of these effects are uncertain.
Intracellular Aβ was first described by Wertkin et al. (11). Immunogold electron microscopy showed that intraneuronal Aβ is pre- and postsynaptically enriched in both AD human brain and AD transgenic animal models in association with dystrophic neurites and abnormal synaptic morphology (12–14). Spatial and temporal analyses of intraneuronal oAβ accumulation show that it precedes plaque formation in both AD animal models and Down's syndrome patients, suggesting that oAβ is an early intracellular toxic agent in AD (14, 15). Aβ-induced neurodegeneration was seen in areas affected in AD, such as the cerebral cortex, hippocampus and amygdala, but was absent in hindbrain and cerebellum of transgenic animals expressing intraneuronal Aβ (16). Similarly, transgenic flies expressing human wild-type or Arctic mutant E22G Aβ42 show neurodegeneration proportional to the degree of intraneuronal oAβ accumulation (17). In addition, microinjection of heterogeneous Aβ42 into cultured human primary neurons at 1 pM concentration induced neuronal cell death (18).
Although Aβ is generated and accumulated in tissues other than brain (19) neurons are selectively affected by intracellular Aβ (18). This suggests that intracellular Aβ must disrupt a process essential for proper function and survival of neurons. Of all of the cell types in an organism, neurons exhibit the greatest dependence on intracellular transport of proteins and membrane-bounded organelles (MBO), i.e., the machinery of fast axonal transport (FAT). Axons, unlike dendrites and cell bodies, lack the machinery for protein synthesis, and consequently essential molecules and organelles must be transported from the cell body into axons throughout life for proper neuronal function and survival. This distinctive axonal attribute renders neurons critically dependent on FAT.
Genetic, biochemical, pharmacological, and cell biological research has shown that a reduction in FAT is sufficient to trigger an adult-onset distal axonpathy and neurodegeneration. For example, point mutations affecting functional domains in kinesin or dynein motors can produce late-onset dying-back neuropathies in sensory or motor neurons (20, 21). Furthermore, dysregulation of FAT has been proposed as a pathological mechanism in several neurological disorders including AD (22, 23), Kennedy's disease (24, 25), Huntington's disease (25), and Parkinson's disease (26). These findings highlight the importance of FAT for neuronal survival.
In this work, we analyzed the intraneuronal effects of different Aβ42 structural/conformation peptide assemblies on FAT in isolated squid axoplasms. Intracellular oAβ, but not intracellular unaggregated amyloid beta (uAβ) or fibrillar amyloid beta (fAβ), inhibited both anterograde and retrograde FAT at nanomolar concentrations. FAT inhibition resulted from activation of endogenous casein kinase 2 (CK2) by oAβ. The effect of oAβ on FAT was prevented by two unrelated CK2 pharmacological inhibitor 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT) and tetrabromocinnamic acid (TBCA) as well as by an excess of a specific CK2 substrate peptide. Consistent with these data, perfusion of axoplasms with active CK2 induces a comparable inhibition of FAT. Both oAβ and CK2 increase kinesin-1 light chains (KLCs) phosphorylation by CK2, leading to kinesin-1 release from vesicular cargoes and inhibition of FAT. We propose that modulation of CK2 activity represents a promising _target for pharmacological intervention in AD.
Results
oAβ Is a Potent Inhibitor of FAT.
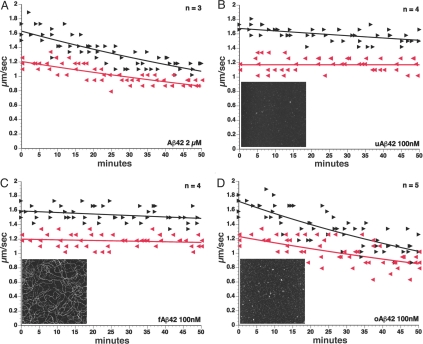
Our previous studies found reduced anterograde FAT of specific synaptic cargoes in different AD murine models known to accumulate intracellular Aβ in the axonal compartment progressively (23). To evaluate the intraxonal effects of Aβ on FAT directly, we perfused heterogeneous preparations of synthetic Aβ42 into isolated extruded axoplasms dissected from the squid Loligo pealeii. Perfusion of synthetic Aβ42 at 2 μM severely reduced both kinesin-1-based anterograde and cytoplasmic dynein (cDyn)-based retrograde FAT (Fig. 1A). Reconstitution of synthetic Aβ in aqueous solutions produces a mixture of different Aβ assemblies caused by preexisting molecular structures in lyophilized stocks (27). To determine the contribution of specific Aβ structural assemblies to inhibition of FAT, we prepared Aβ42 under defined conditions that yield uniform solutions of oligomers or fibrils (27). These unaggregated, oligomeric, and fibrillar Aβ42 preparations (100 nM) were perfused into isolated axoplasms to evaluate the effects on FAT. Neither uAβ (Fig. 1B) nor fAβ (Fig. 1C) altered FAT in either direction. In contrast, perfusion of 100 nM oAβ inhibited both directions of FAT significantly (Fig. 1D), and inhibition of FAT was seen as low as 10 nM oAβ (data not shown). Collectively, these results indicate that oAβ has a toxic activity that inhibits FAT when present intracellularly. This toxic effect is independent of transcription and translation, as extruded axoplasms are separated from cell bodies. The oAβ inhibition of FAT is also independent of energy metabolism changes, because 5 mM ATP is added to assays of vesicle motility with oAβ, thereby ruling out the possibility that oAβ affects FAT by altering mitochondrial ATP production.
Fig. 1.
Soluble oAβ inhibits FAT in both anterograde and retrograde directions. (A) FAT rates were evaluated on isolated extruded axoplasms perfused with X/2 buffer containing synthetic heterogeneous 1–42 Aβ peptide (Aβ42). Perfusion with Aβ42 produced a significant reduction of FAT rates in both directions. Lines represent the best-fit exponential of rates for vesicles moving in the anterograde (black) and retrograde (gray) direction of FAT. Each arrowhead represents a measurement of the rate for vesicles in the specified direction at a given time in an axoplasm. The letter n represents the number of independent axons tested. To determine the effects of specific conformers of Aβ42, we used defined structural assemblies as described in the text. Neither unaggregated uAβ42 (B) nor fibrillar fAβ (C) had an effect on FAT at 100 nM. In contrast, oligomeric oAβ42 (D) dramatically inhibited both directions of FAT. These results suggest that intracellular oAβ is a potent FAT inhibitor. (Insets to B-D) Representative 2 × 2 μm x-y, 10-nm total z-range AFM images of unaggregated, fibrillar, and oligomeric Aβ.
oAβ-Induced FAT Inhibition Results from Endogenous CK2 Activation.
To determine the specific molecular mechanism by which oAβ inhibits FAT, the characteristic profile of FAT inhibition was evaluated. The ability of nanomolar levels of oAβ to inhibit FAT suggested that the molecular mechanism of inhibition is likely to be enzymatic (24) rather than a physical blockade. Abnormal phosphorylation of different neuronal cytoskeletal proteins is a characteristic hallmark in AD and our previous studies in isolated axoplasm have identified multiple kinase and phosphatase activities that can inhibit FAT (22, 24, 26, 28). A number of different phosphotransferase activities are altered in brains of AD patients, AD animal models, and cell lines exposed to Aβ. The list of abnormal kinase activities in AD is extensive, among them several unrelated serine/threonine protein kinases including GSK3 (23, 29, 30), p38 (31), JNK (31), cdk5 (32, 33), and CK2 (34). Interestingly, many of these enzymes are involved in regulation of FAT (28, 29).
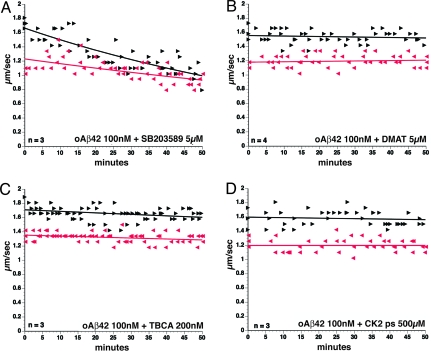
Similarities in the profile of FAT inhibition produced by activation of several members of the stress-activated protein kinases (SAPK) and oAβ (24) led us to test the role of axonal SAPK in oAβ-induced FAT inhibition. Co-perfusion of homogeneous oAβ42 (Fig. 2A) with 5 μM SB203580, a well-characterized inhibitor for multiple SAPKs (24), did not prevent inhibition FAT by oAβ, making a role for endogenous SAPKs unlikely.
Fig. 2.
Activation of endogenous CK2 mediates oAβ42-induced FAT inhibition. (A) Co-perfusion of SAPK inhibitor SB203580 with oAβ did not prevent FAT inhibition. However, co-perfusion of oAβ with a specific CK2 inhibitor such as DMAT (B) or TBCA (C) prevented FAT inhibition. Furthermore, co-perfusion of oAβ with a specific CK2 substrate peptide (D) also prevented oAβ42-induced FAT inhibition. All of these results suggest that oAβ42-induced FAT inhibition is mediated by activation of endogenous CK2 activity.
CK2 is another kinase that can inhibit both anterograde and retrograde FAT (28). Based on results showing that heterogeneous Aβ could directly stimulate CK2 kinase activity in vitro (35), the role of endogenous CK2 activation in oAβ-induced FAT inhibition was evaluated. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT) is a potent and highly specific ATP-competitive inhibitor of CK2 (36) derived from 4,5,6,7-tetrabromo-2-azabenzimidazole (TBB). Co-perfusion of oAβ42with DMAT (Fig. 2B) blocked the oAβ-inhibitory effects on both directions of FAT entirely. A newly developed CK2 inhibitor also derived from TBB, tetrabromocinnamic acid (TBCA), has proved to be even more specific for CK2 (37), and TBCA also blocked inhibition of FAT by oAβ (Fig. 2C). Finally, a peptide substrate specific for CK2 was used as a competitive inhibitor of CK2 activity, and it also prevented inhibition of FAT by oAβ (Fig. 2D). Thus, selective inhibition of CK2 can prevent oAβ-induced inhibition of FAT.
Perfusion of oAβ Increased Phosphorylation of Squid Kinesin-1 Light Chains by Endogenous CK2 Activity.
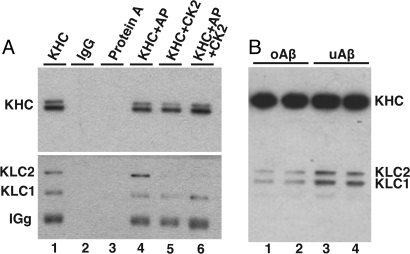
To assess the ability of CK2 to phosphorylate a molecular motor for FAT, we evaluated the phosphorylation status of squid kinesin-1 after oAβ perfusion using the 63–90 monoclonal antibody against KLCs (38). The 63–90 antibody recognizes dephosphorylated KLCs (22, 23). KLCs in immunoprecipitates (IP) from murine brain were recognized by 63–90 (Fig. 3A, lane 1) and immunoreactivity increased after treatment with Antarctic phosphatase (Fig. 3A, lane 4). When kinesin-1 IP were treated with CK2, KLCs immunoreactivity is dramatically reduced (Fig. 3A, lanes 5 and 6), consistent with a CK2 phosphorylation site within the 63–90 epitope. When the extent of endogenous squid KLCs phosphorylation was evaluated by immnunoblot with 63–90 (Fig. 3B), KLC immunoreactivity was significantly reduced in axoplasms perfused with oAβ relative to uAβ-perfused axoplasms. These data suggest that oAβ activates endogenous CK2 activity, leading to a concomitant increase in squid KLC phosphorylation.
Fig. 3.
oAβ induced squid KLCs phosphorylation by endogenous CK2. Antibody 63–90 preferentially recognized KLCs when they were dephosphorylated. (A) Immunoblot analysis of IP kinesin-1 from murine brain. Kinesin-1 was immunoprecipitated using H2 antibody and analyzed by H2 (KHCs) and 63–90 (KLCs) antibodies. Of note, 63–90 immunoreactivity increased when IP kinesin-1 was dephosphorylated by Antarctic phosphatase (AP) (lane 4), as can be observed when comparing lane 1 and lane 4. Conversely, 63–90 immunoreactivity decreased when IP kinesin-1 was phosphorylated with CK2 (lane 5) or when dephosphorylated by AP before CK2 phosphorylation (lane 6). Control immunoprecipitates with normal murine IgG (lane 2) and protein A agarose beads (lane 3) are shown as controls. These results indicate that 63–90 is a KLCs antibody sensitive to CK2 phosphorylation. (B) Phosphorylation of squid KLCs by CK2 upon oAβ perfusion. Of note, 63–90 immunoreactivity decreased when axoplasms were perfused with oAβ compared with uAβ. These data are consistent with the suggestion that perfusion of oAβ leads to an increase in squid KLCs phosphorylation by endogenous CK2.
CK2 Mimics the Inhibitory Effect of oAβ on FAT by Directly Phosphorylating Kinesin-1.
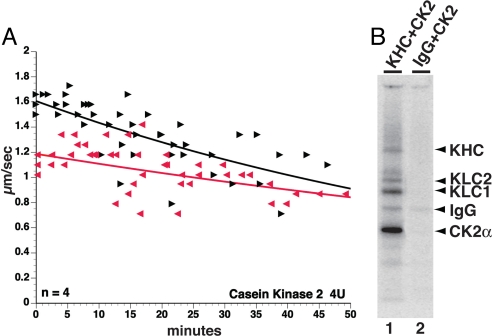
If oAβ effects on FAT are caused by activation of CK2, then recombinant CK2 should have the same effect on FAT when perfused into axoplasm. Indeed, both directions of FAT were inhibited by CK2, and the inhibition seen with CK2 was qualitatively indistinguishable from the oAβ effects on FAT (Fig. 4A). In vitro kinase assays showed that both kinesin-1 heavy chain (KHC) and KLCs from murine brain are phosphorylated by recombinant CK2 (Fig. 4B), consistent with previous results (39). Together these experiments suggest that inhibition of FAT by oAβ is mediated by activation of endogenous CK2 and phosphorylation of kinesin-1.
Fig. 4.
CK2 inhibited FAT and phosphorylated both KHC and KLC. (A) CK2 mimicked the extent and inhibitory profile of oAβ42 on bidirectional FAT. (B) Both KHC and KLCs immunoprecipitated (IP) from murine brains with H2 antibody were phosphorylated by active CK2 in vitro. Autoradiogram showing incorporation of 32P into immunoprecipitates (IP) of KHC and KLCs (lane 1) incubated with CK2. IP with normal murine IgG was also incubated with CK2 as a control for nonspecific binding to beads and IgG. These experiments indicate that CK2 is capable of directly phosphorylating both KHC and KLCs.
oAβ-Induced FAT Inhibition Results from Kinesin-1 Release of Its Vesicular Cargoes.
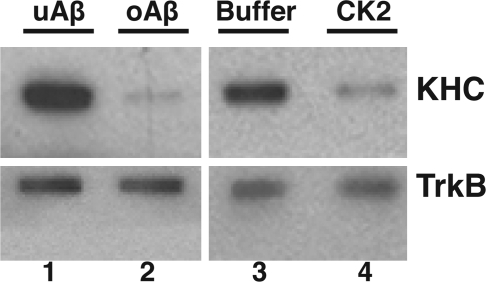
Phosphorylation of kinesin-1 by various kinases can regulate its activities and affect anterograde FAT (22, 24, 28). Previous studies on phosphorylation of KLC at the 63–90 epitope suggest that this site regulates binding of kinesin-1 to vesicle cargoes (23, 40). To determine whether oAβ leads to release of kinesin-1 from cargoes, we isolated vesicle fractions by flotation assays from axoplasms treated for 50 minutes with either uAβ or oAβ, and evaluated the association of kinesin-1 with membrane fractions by immunoblot with the kinesin-1 KHC-specific antibody H2. A dramatic reduction of kinesin-1 immunoreactivity was seen on axonal vesicles from oAβ-perfused relative to uAβ-perfused axoplasms (Fig. 5, lanes 1 and 2). There was a comparable reduction of kinesin-1 immunoreactivity on axonal vesicles perfused with active CK2 relative to those perfused with CK2 buffer control (Fig. 5, lanes 3 and 4). An antibody to the integral membrane protein Trk (41) was used to verify the presence of vesicles and to serve as a loading control. Collectively, these results suggest that one mechanism by which oAβ mediates FAT inhibition is by increasing CK2 activity and phosphorylating KLCs, leading to release of kinesin-1 from vesicles.
Fig. 5.
Induced inhibition of FAT by oAβ and CK2 was caused by kinesin-1 release from cargo vesicles. Based on evidence that oAβ and CK2 both increase phosphorylation of KLCs, the effects of treatment on kinesin-1 association with cargo vesicles were evaluated. Vesicles purified from axoplasms perfused with uAβ, oAβ, CK2 buffer, or active CK2 were assayed by immunoblot for KHC and TrkB. TrkB immunoreactivity shows that the vesicle load was equivalent; but kinesin-1 was significantly lower in oAβ (lane 2) and CK2 (lane 4) vesicle fractions than in vesicle fractions from a sister axoplasm from the same squid treated with uAβ (lane 1) or CK2 buffer (lane 3). Both oAβ and CK2 lead to increased phosphorylation of KLCs and reduced binding of kinesin-1 to vesicles. Total KLC and KHC levels in axoplasm were not altered by treatments (data not shown). These results indicate that the inhibition of FAT oAβ activates endogenous CK2 activation and leads to the subsequent release of kinesin-1 from its cargos.
Discussion
Questions about the toxicity of Aβ peptides emerged when Aβ was identified as the main component of senile plaques in AD. Efforts to identify the pathogenic form of Aβ indicated that oAβ was a major toxic component of Aβ fractions in AD (5, 42). Intracellular oAβ42 accumulation as a consequence of altered amyloid precursor protein metabolism (43) or by reuptake of previously secreted Aβ42 (44) is well documented. Regardless of its origin, intracellular Aβ accumulation may represent a key event in AD pathogenesis. However, the nature of oAβ toxicity remained uncertain, and a pathological mechanism to explain the characteristic pathological features of AD (i.e., neuronal specificity, selective vulnerability of particular neuronal populations, and late onset of symptoms) was elusive.
Synaptic dysfunction has been suggested as the initial pathological change leading to neuronal death in multiple progressive neuropathologic conditions of the central and peripheral nervous system, including AD (45, 46). Collectively, these can be described as dying-back neuropathies or distal axonopathies that evolve from synaptic dysfunction followed by _target detachment progressing to neuronal death (47). Often changes in FAT are associated with these pathologic conditions, although kinesin and dynein motors are expressed in essentially all eukaryotic cells. Point mutations in either kinesin-1 or cDyn that affect function of the motor are associated with reduction of FAT and produce dying-back neuropathies in specific neuronal populations (20, 21). Dysregulation of FAT has been tightly linked to several late-onset progressive neurological diseases associated with the early synaptic failure and loss typical of dying-back neuropathies including polyQ-expansion diseases (24, 25), Parkinson's disease (26), and AD (22, 23).
The critical roles that molecular motors play in dying-back neuropathies are general enough to define this category of neurological disease as a “dysferopathy” in which the pathologic condition is associated with alterations in FAT (26). AD is perhaps an archetypical dysferopathy, given the early loss of synaptic function followed much later by neuronal death (45) and the accumulating evidence for disruption of FAT in AD (23, 29). In particular, striking changes in phosphotransferase activities implicated in regulation of FAT are a key feature of AD. Altered regulation of FAT markedly reduces transport of synaptic proteins and mitochondria in AD brain and AD murine models that accumulate intracellular oAβ (23). Dysregulation of FAT results in reduced delivery of critical synaptic elements required for integrity, maintenance, and function of synapses, leading to synaptic failure and a dying-back pattern of neurodegeneration.
A number of observations indicate that intracellular oAβ plays a role in AD pathogenesis. For example, oAβ has been observed in association with membranous organelles as well as in the cytoplasm of neuronal processes either in association with microtubules or in empty areas within axonal processes (13). Furthermore, oAβ is reported to be toxic when introduced into the cytosol of cultured primary neurons (18). Therefore, we decided to investigate whether intraneuronal oAβ would directly interfere with FAT. In this work, we provide direct evidence that intraneuronal Aβ is a strong inhibitor of both anterograde and retrograde FAT (Figs. 1A and 1C). Initial experiments showed that a mixture of heterogeneous Aβ moieties significantly inhibited both directions of FAT when perfused in isolated axoplasm (Fig. 1A). When different conformational forms of Aβ were compared, only oAβ inhibited FAT at concentrations that were physiologically plausible (10–100 nM) (Fig. 1D). Neither uAβ (Fig. 1B) nor fAβ (Fig. 1C) forms of Aβ affected FAT at 100 nM. Much higher levels of fAβ (2 μM) did inhibit FAT (data not shown); but whether this effect was caused by low levels of oAβ contamination (1–2%) or by reduced biological activity for fAβ could not be determined.
The ability of soluble oAβ to inhibit FAT at stoichiometries an order of magnitude or more lower than affected molecular motors effectively eliminated the possibility that oAβ represented a steric interference as previously suggested. Pathogenic forms of Huntingtin and androgen receptor (24, 25) as well as tau filaments (40) and mutant presenilin 1 (23), all inhibit FAT by activation of a kinase or phosphatase. A variety of kinases have been found to affect FAT in axoplasm (28), and multiple kinase activities are elevated in AD (GSK, JNK, CK2). These provide candidate kinase activities that might be elevated by oAβ.
Perfusion of isolated axoplasms with active GSK3 (22), JNK (24) or CK2 (28) results in inhibition of FAT. Two different mechanisms have been identified for inhibition of kinesin-based FAT. When KLCs are phosphorylated by GSK3 (22, 23, 40), binding of kinesin-1 to cargoes is reduced. Alternatively, phosphorylation of KHC by JNK inhibits the ability of kinesin-1 to bind microtubules (24). Neuropathogenic forms of Huntingtin or androgen receptor (AR) increased activity of JNK and inhibition of bidirectional FAT in extruded axoplasms (24, 25). This profile of FAT inhibition was similar to oAβ effects, but co-perfusion of oAβ with SB203580, an inhibitor of JNK and p38 kinases, did not block effects of oAβ on FAT (Fig. 2A). GSK3 was not a candidate to mediate oAβ effects of FAT, because GSK3 inhibits only anterograde FAT (22), whereas oAβ affects both anterograde and retrograde FAT.
Previous studies in axoplasm indicated that CK2 could inhibit both directions (Fig. 4A) (28). Co-perfusion of axoplasms with oAβ and selective CK2 inhibitors (DMAT or TBCA) completely blocked effects of oAβ on FAT (Figs. 2B and 2C). To eliminate the possibility that a secondary kinase _target was responsible for oAβ effects, we co-perfused axoplasms with oAβ and a CK2-specific peptide substrate, which served as a competitive inhibitor of axoplasmic CK2 and prevented inhibition of FAT (Fig. 2D). In addition, Aβ was reported to increase the kinase activity of CK2 in vitro (35). Although the molecular mechanism by which oAβ induces CK2 activation remains to be determined, preliminary studies suggest that oAβ can activate recombinant CK2 in vitro and increase phosphorylation of the CK2 specific peptide in the absence of cell lysates (G.P., Y.A., and S.B., unpublished). These results suggest that oAβ-induced FAT inhibition results from activation of endogenous CK2.
CK2 phosphorylates both KHC and KLCs in vitro (Fig. 4B) (39), and phosphorylation of endogenous KLCs in axoplasm was significantly increased by perfusion of either CK2 or oAβ as determined by reduced immunoreactivity with the phospho-sensitive 63–90 monoclonal antibody (Fig. 3B). Previous studies with GSK3 showed that the 63–90 epitope was also sensitive to GSK3 activity (22, 23, 40). Significantly, CK2 was a priming kinase for GSK3 modification of KLC and GSK3 did not phosphorylate KLC without a priming phosphorylation (22).
The functional consequence of GSK3-mediated phosphorylation of KLC was release of kinesin-1 from its vesicle cargoes (22, 23, 40). Consistent with this observation, the amount of kinesin-1 on axoplasmic vesicles was substantially reduced relative to controls with both oAβ and CK2 perfused axoplasms (Fig. 5). Thus, phosphorylation of KLCs by CK2 activity leads to release of kinesin-1 from cargoes, either directly or in combination with endogenous GSK3 activity. These experiments strongly suggest that intraneuronal oAβ leads to abnormal activation of axonal CK2, which phosphorylates kinesin-1, leading to removal the anterograde motor from vesicles and inhibition of FAT. However, unlike GSK3, which affects only anterograde FAT, CK2, and oAβ inhibit both directions of FAT, suggesting that CK2 affects cDyn function as well.
Although hyperphosphorylation of neuronal proteins in AD has long been recognized, the question of how specific kinases are activated in AD has seldom been addressed. The activation of CK2 by oAβ provides a partial answer to this critical question in AD pathogenesis. These data identify kinesin-1 and cDyn as key _targets of CK2 in AD. By affecting the function of motors critical for FAT and maintenance of neuronal connectivity, a direct link from oAβ to neuronal degeneration can be defined. This is not to suggest that FAT and molecular motors are the only CK2 _targets that contribute to AD pathology. CK2 has hundreds of known substrates (48), including ones critical for cell survival. As shown in the accompanying paper on the squid giant synapse (49), CK2 activated by oAβ can also affect synaptic function either through an effect on FAT or through actions on specific synaptic protein _targets (50).
Effective therapeutic intervention in progressive neurological disorders depends on a clear understanding of the molecular mechanisms associated with the disease in question. In this manuscript we have shown that dysregulation of CK2 by oAβ is capable of inhibiting the vital neuronal process of FAT. Therefore, we propose that pharmacological regulation of CK2 activity represents a promising _target for therapeutic intervention in AD, particularly when combined with treatments that help manage GSK3 activity as well.
Materials and Methods
Antibodies and Reagents.
The following antibodies were used: H2, a monoclonal antibody (mAb) against kinesin-1 heavy chains (51); 63–90, a monoclonal antibody (mAb) that preferentially recognizes dephosphorylated kinesin-1 light chains (22); and TrkB, a rabbit polyclonal antibody from Santa Cruz Biotechnology. Protein kinase inhibitors were obtained from Calbiochem, including SB 203580 and CK2 inhibitors (DMAT and TBCA). Inhibitors were diluted in DMSO or ethanol as appropriate and kept at −80 °C until use. CK2 substrate peptide was obtained from Sigma. Active CK2 and Antarctic Phosphatase were from New England Biolabs.
Preparation of Aβ42 Solutions.
For heterogeneous Aβ solutions, synthetic Aβ42 peptide (Bachem) was prepared as previously described (52). These heterogeneous Aβ solutions were perfused into extruded axoplasms isolated from the squid Loligo pealeii at 2-μM concentration. Vesicle motility was evaluated for 50 minutes after perfusion. For defined solutions of unaggregated, oligomeric, and fibrillar Aβ42 conformations, synthetic Aβ42 from California Peptide, Inc. were meticulously prepared as previously described (27). Solutions of defined Aβ42 conformations were perfused as above at 100 nM.
Atomic Force Microscopy.
Peptide solutions were characterized using a NanoScope IIIa scanning probe work station equipped with a MultiMode head using a vertical engage E-series piezoceramic scanner (Veeco, Santa Barbara, CA). AFM probes were single-crystal silicon microcantilevers with 300-kHz resonant frequency and 42 Newton/meter spring constant model OMCL-AC160TS-W2 (Olympus). A 20-μl quanity of sample solution (diluted from 100 μM to 10 μM (or 30 μM for fibrils) in deionized MilliQ water) was spotted on freshly cleaved mica, incubated at room temperature for 3 minutes, rinsed with 0.02 μm of filtered (Whatman Anotop 10) MilliQ water (Millipore), and blown dry with tetrafluoroethane (CleanTex MicroDuster III). For Aβ solutions in F12 media, the cleaved mica was pretreated with 3 μl of 1M HCl and rinsed before addition of sample. Some samples were imaged under dry helium. Image data were acquired at scan rates between 1 and 2 Hz with drive amplitude and contact force kept to a minimum. Data were processed to remove vertical offset between scan lines by applying zero order flattening polynomials using Nanoscope software (Version 5.31r1, Veeco).
Motility Studies in Isolated Axoplasm.
Axoplasms were extruded from giant axons of the squid Loligo pealeii provided by the Marine Biological Laboratory as previously described in detail (53). Vesicle motility was analyzed using a Zeiss Axiomat microscope equipped with DIC optics. Vesicle velocities were assessed as previously described (54).
Immunochemical Methods.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophorisis (SDS-PAGE) on 4–12% Bis-Tris gels (NuPage minigels, Invitrogen), using Mops Running Buffer (Invitrogen) and transferred to polyvinylidene fluoride (PVDF) membranes as previously described (52). Immunoblots were blocked with 5% nonfat dried milk, in phosphate-buffered saline, pH 7.4, and probed with different polyclonal and monoclonal antibodies. Primary antibody binding was detected with horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibody (Jackson Immunoresearch) and visualized by chemiluminescence (ECL, Amersham). Kinesin immunoprecipitation was performed from murine brain by our published procedure (55). Isolation of membrane vesicle fractions from axoplasms was as previously described (40). Two axoplasms from the same animal were prepared and incubated with appropriate effectors (CK2 buffer, active CK2, uAβ, or oAβ) and vesicle fractions evaluated by immunoblot using H2 and Trk antibodies. Trk served as protein loading control and vesicle fraction marker.
In Vitro Kinesin-1 Phosphorylation.
Immunoprecipitated kinesin-1 was either phosphorylated by CK2 (250 U) or was previously dephosphorylated by Antartic Phosphatase (15 U) following NEB protocols and then phosphorylated by CK2. Kinase reactions were started by addition of 100 μM of radiolabeled ATP. After 1 hour at 30 °C, reactions were stopped by addition of 60 μl sample buffer. Proteins were separated by SDS-PAGE and the gels dried and exposed to a phosphoroimager screen for analysis (55).
Statistical Analysis.
All experiments were repeated at least three times. Unless otherwise stated, the data were analyzed by two-sample t test for pairwise comparisons or analysis of variance followed by a post hoc Student-Newman-Keul's test to make all possible comparisons. Data were expressed as mean ± SEM; significance was assessed at P < 0.05 or 0.01 as noted.
Acknowledgments.
The authors thank Bin Wang and Agnieszka Kaminska for their excellent technical assistance. Research supported in part by grants from National Institutes of Health (NS23320, NS41170, and NS43408) (S.B.), Muscular Dystrophy Association (S.B.), Amyotrophic Lateral Sclerosis Association (G.M., S.B.), fellowships from the Marine Biological Laboratory (to G.M.), and American Parkinson's Disease Association (G.M.)
Footnotes
The authors declare no conflict of interest.
References
- 1.Maeda S, et al. Increased levels of granular tau oligomers: An early sign of brain aging and Alzheimer's disease. Neurosci Res. 2006;54:197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Terry RD, Davies P. Dementia of the Alzheimer type. Annu Rev Neurosci. 1980;3:77–95. doi: 10.1146/annurev.ne.03.030180.000453. [DOI] [PubMed] [Google Scholar]
- 3.Masliah E, Terry RD, DeTeresa RM, Hansen LA. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989;103:234–239. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- 4.Hardy JA, Higgins GA. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 5.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlgren KN, et al. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesne S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 10.Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 11.Wertkin AM, et al. Human neurons derived from a teratocarcinoma cell line express solely the 695-amino acid amyloid precursor protein and produce intracellular beta-amyloid or A4 peptides. Proc Natl Acad Sci USA. 1993;90:9513–9517. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouras GK, et al. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi RH, et al. Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi RH, et al. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med. 2001;125:489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- 16.LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC, Jay G. The Alzheimer's A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet. 1995;9:21–30. doi: 10.1038/ng0195-21. [DOI] [PubMed] [Google Scholar]
- 17.Crowther DC, et al. Intraneuronal Abeta, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer's disease. Neuroscience. 2005;132:123–135. doi: 10.1016/j.neuroscience.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid beta peptide1–42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002;156:519–529. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joachim CL, Mori H, Selkoe DJ. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989;341:226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- 20.Hafezparast M, et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 21.Reid E, et al. A Kinesin Heavy Chain (KIF5A) Mutation in Hereditary Spastic Paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;23:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pigino G, Morfini G, Mattson MP, Brady ST, Busciglio J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morfini G, et al. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci. 2006;9:907–916. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- 25.Szebenyi G, et al. Neuropathogenic forms of Huntingtin and androgen receptor inhibit fast axonal transport. Neuron. 2003;40:41–52. doi: 10.1016/s0896-6273(03)00569-5. [DOI] [PubMed] [Google Scholar]
- 26.Morfini G, et al. 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc Natl Acad Sci USA. 2007;104:2442–2447. doi: 10.1073/pnas.0611231104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- 28.Morfini G, Szebenyi G, Richards B, Brady ST. Regulation of kinesin: Implications for neuronal development. Dev Neurosci. 2001;23:364–376. doi: 10.1159/000048720. [DOI] [PubMed] [Google Scholar]
- 29.Morfini G, Pigino G, Beffert U, Busciglio J, Brady ST. Fast axonal transport misregulation and Alzheimer's disease. Neuromol Med. 2002;2:89–99. doi: 10.1385/NMM:2:2:089. [DOI] [PubMed] [Google Scholar]
- 30.Pei JJ, et al. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol. 1997;56:70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Pei JJ, et al. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer's disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis. 2001;3:41–48. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- 32.Lee MS, Tsai LH. Cdk5: One of the links between senile plaques and neurofibrillary tangles? J Alzheimers Dis. 2003;5:127–137. doi: 10.3233/jad-2003-5207. [DOI] [PubMed] [Google Scholar]
- 33.Morfini G, et al. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masliah E, et al. Casein kinase II alteration precedes tau accumulation in tangle formation. Am J Pathol. 1992;140:263–268. [PMC free article] [PubMed] [Google Scholar]
- 35.Chauhan A, Chauhan VP, Murakami N, Brockerhoff H, Wisniewski HM. Amyloid beta-protein stimulates casein kinase I and casein kinase II activities. Brain Res. 1993;629:47–52. doi: 10.1016/0006-8993(93)90479-7. [DOI] [PubMed] [Google Scholar]
- 36.Pagano MA, et al. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: A novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun. 2004;321:1040–1044. doi: 10.1016/j.bbrc.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 37.Pagano MA, et al. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem. 2007;8:129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- 38.Stenoien DS, Brady ST. Immunochemical analysis of kinesin light chain function. Molec Biol Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donelan MJ, et al. Ca2+-dependent dephosphorylation of kinesin heavy chain on beta-granules in pancreatic beta-cells. Implications for regulated beta-granule transport and insulin exocytosis. J Biol Chem. 2002;277:24232–24242. doi: 10.1074/jbc.M203345200. [DOI] [PubMed] [Google Scholar]
- 40.Lapointe NE, et al. The amino terminus of tau inhibits kinesin-dependent axonal transport: Implications for filament toxicity. J Neurosci Res. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno H, et al. Nerve growth factor acutely reduces chemical transmission by means of postsynaptic TrkA-like receptors in squid giant synapse. Proc Natl Acad Sci USA. 1998;95:14997–15002. doi: 10.1073/pnas.95.25.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein WL. Abeta toxicity in Alzheimer's disease: Globular oligomers (ADDLs) as new vaccine and drug _targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 43.Busciglio J, et al. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron. 2002;33:677–688. doi: 10.1016/s0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 44.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 45.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 46.Wishart TM, Parson SH, Gillingwater TH. Synaptic vulnerability in neurodegenerative disease. J Neuropathol Exp Neurol. 2006;65:733–739. doi: 10.1097/01.jnen.0000228202.35163.c4. [DOI] [PubMed] [Google Scholar]
- 47.Coleman M. Axon degeneration mechanisms: Commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 48.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 49.Moreno HH, et al. Synaptic transmission block by pre-synaptic injection of oligomeric amyloid beta. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0900944106. 10.1073.0900944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shata A, Saisu H, Odani S, Abe T. Phosphorylated synaphin/complexin found in the brain exhibits enhanced SNARE complex binding. Biochem Biophys Res Commun. 2007;354:808–813. doi: 10.1016/j.bbrc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 51.Deboer SR, et al. Conventional kinesin holoenzymes are composed of heavy and light chain homodimers. Biochemistry. 2008;47:4535–4543. doi: 10.1021/bi702445j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pigino G, Pelsman A, Mori H, Busciglio J. Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and tau phosphorylation. J Neurosci. 2001;21:834–842. doi: 10.1523/JNEUROSCI.21-03-00834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brady ST, Lasek RJ, Allen RD. Video microscopy of fast axonal transport in isolated axoplasm: A new model for study of molecular mechanisms. Cell Motility. 1985;5:81–101. doi: 10.1002/cm.970050203. [DOI] [PubMed] [Google Scholar]
- 54.Brady ST, Richards BW, Leopold PL. Assay of vesicle motility in squid axoplasm. Meth Cell Biol. 1993;39:191–202. doi: 10.1016/s0091-679x(08)60171-5. [DOI] [PubMed] [Google Scholar]
- 55.Morfini G, Pigino G, Brady ST. Approaches to kinesin-1 phosphorylation. In: Sperry A, editor. Molecular Motors: Methods and Protocols, Methods in Molecular Biology. Vol 392. Clifton, NJ: Humana Press; 2006. pp. 51–70. [DOI] [PubMed] [Google Scholar]