Abstract
Background
Frailty is a common condition in elders and identifies a state of vulnerability for adverse health outcomes.
Objective
Our objective was to provide a biological face validity to the well-established definition of frailty proposed by Fried et al.
Design
Data are from the baseline evaluation of 923 participants aged ≥65 y enrolled in the Invecchiare in Chianti study. Frailty was defined by the presence of ≥3 of the following criteria: weight loss, exhaustion, low walking speed, low hand grip strength, and physical inactivity. Muscle density and the ratios of muscle area and fat area to total calf area were measured by using a peripheral quantitative computerized tomography (pQCT) scan. Analyses of covariance and logistic regressions were performed to evaluate the relations between frailty and pQCT measures.
Results
The mean age (±SD) of the study sample was 74.8 ± 6.8 y, and 81 participants (8.8%) had ≥3 frailty criteria. Participants with no frailty criteria had significantly higher muscle density (71.1 mg/cm3, SE = 0.2) and muscle area (71.2%, SE = 0.4) than did frail participants (69.8 mg/cm3, SE = 0.4; and 68.7%, SE = 1.1, respectively). Fat area was significantly higher in frail participants (22.0%, SE = 0.9) than in participants with no frailty criteria (20.3%, SE = 0.4). Physical inactivity and low walking speed were the frailty criteria that showed the strongest associations with pQCT measures.
Conclusion
Frail subjects, identified by an easy and inexpensive frailty score, have lower muscle density and muscle mass and higher fat mass than do nonfrail persons.
Keywords: Frailty, skeletal muscle, body composition, fat mass, inflammation, muscle mass, muscle density, aging, epidemiology
INTRODUCTION
Frailty is generally defined as a state of high vulnerability for adverse health outcomes, including falls, hospitalization, physical disability, and mortality (1). The challenges of finding a standard definition of frailty that could be uniformly applied and valid in different settings (2, 3) make any estimation of prevalence approximate and tentative (4). However, according to current literature, the prevalence of frailty increases with age, and the American Medical Association estimated that ≈40% of persons aged ≥80 y are frail (5).
The pathophysiologic modifications underlying frailty have not been carefully explored, but they are probably independent of aging. Evidence on the nature of biological processes that cause frailty is quite limited, probably because of their complex and multifactual nature. For example, evidence suggests that body composition, musculoskeletal and nervous systems, and inflammation act synergistically as risk factors for frailty. The lack of a standardized working definition of frailty in elders may have further slowed the research on this topic.
In the present study, the frailty syndrome was defined by using the criteria proposed by Fried et al (1) and developed in the context of the Cardiovascular Health Study. This screening instrument was developed to identify frail persons at high risk of adverse health-related outcomes in the clinical setting (1). The operational definition of frailty was based on a conceptual paradigm and was later validated by showing its ability to predict physical disability, hospitalization, and mortality in a sample of community-dwelling older persons (1).
The strong association between Fried’s frailty definition and the risk of developing health-related events might be at least partially explained by changes in body composition that occur with aging in most persons. In particular, an accelerated decline in muscle mass and quality and a parallel increase in fat mass may represent pathophysiologic changes that critically precipitate the development of the frailty syndrome.
The aim of the present study was to investigate the relation of the frailty syndrome with muscle and fat measures, as assessed by a peripheral quantitative computerized tomography (pQCT) scan, in a large sample of community-dwelling older persons. Given the known relations of inflammation with frailty (6, 7) and body composition (8, 9), we also explored whether inflammation [measured as concentrations of interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and C-reactive protein (CRP)] mediated, at least in part, the relation between changes in body composition and frailty.
SUBJECTS AND METHODS
The present study is based on data from the baseline visit of the Invecchiare in Chianti (Aging in the Chianti area, InCHIANTI) study, a prospective population-based study of older people designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council of Aging (Florence, Italy). The InCHIANTI study is aimed at the identification of risk factors for the onset of disability in the elderly, the study of physiologic subsystems critical for walking, and the definition of critical ranges for tests that evaluate the integrity of the physiologic subsystems important for walking.
The study population for these analyses included 1155 participants aged between 65 and 102 y and who were randomly selected from residents in 2 towns of the Chianti geographic area (Greve In Chianti and Bagno a Ripoli, Tuscany, Italy). The data collection started in September 1998 and was completed in March 2000. A detailed description of the sampling procedure and data collection method was previously published (10). The Italian National Research Council on Aging Ethical Committee ratified the entire study protocol.
The present analyses were performed on 923 participants; we excluded subjects in whom pQCT measures and frailty syndrome were not assessed (n = 232). The only sociodemographic characteristics in which excluded participants differed from those considered for the present analyses were the older age (80.0 y compared with 74.3 y, P < 0.001) and the lower Mini Mental State Examination (MMSE) score (20.5 compared with 25.0, P < 0.001).
Frailty syndrome
Frailty syndrome was defined on the basis of the well-established, standardized phenotype described by Fried et al (1) in the Cardiovascular Health Study. In the present study, the 5 different components of the frailty syndrome were evaluated and defined as follows:
Weight loss. Weight loss was defined as the unintentional loss of >4.5 kg in the past year.
Exhaustion. If the participant answered “Often” or “Most of the time” for the question “How often in the last week did you feel that everything you did was an effort?” included in the Center for Epidemiologic Studies-Depression scale (11), the exhaustion criterion was considered present.
Physical inactivity. Participants who performed no physical activity, spent most of the time sitting, or rarely had a short walk (or other nondemanding physical activity) in the past year were considered physically inactive.
Low walking speed. Participants were divided into 4 groups, below and above the median body height by sex to get percentile values on a 4-m walk test. If walking speed corresponded to the worst quintile for the sex and height groups, low walking speed was considered present.
Low hand grip strength. Hand grip strength was measured by a hand-held dynamometer (hydraulic hand BASELINE; Smith & Nephew, Agrate Brianza, Milan, Italy). The participants were asked to perform the task twice with each hand. The average of the best results obtained for each side was used for the present analyses. The participants were divided into 8 groups according to quartiles of body mass index (BMI; in kg/m2) by sex. If hand grip strength corresponded to the ≤20th percentile for the sex and BMI groups, low hand grip strength was considered present.
The participants who reported ≥3 of these characteristics were defined as frail, and intermediate frailty was defined as the presence of 1 or 2 components. This commonly used definition has shown predictive validity for the adverse outcomes that geriatricians tend to associate with being frail: falls, hospitalizations, disability, and death (1).
Peripheral quantitative computerized tomography measures
A pQCT scan of the right leg was performed in all participants with a recent generation device (XCT 2000; Stratec, Pforzheim, Germany) to evaluate the total, muscular, and fat cross-sectional areas of the calf. Data presented here were derived from standard 2.5-mm thick transverse scans obtained at 66% of the tibial length, starting from the tibiotarsal joint. Previous studies showed that this is the region with the largest outer calf diameter and with a small variability across persons (12). The muscle density (in mg/cm3), muscle area (in cm2), fat area (in cm2), and total area (in cm2) were calculated by using the BONALYSE software version 3.1 (BonAlyse Ltd, Jyväskylä, Finland). Different tissues in the analyses were separated according to different density thresholds, with the use of the soft tissue algorithm: a density value of 15 mg/mm3 was used to separate fat from muscle tissue and 180 mg/mm3 to separate muscle from bone tissue. In recent years, pQCT has gained popularity for the analysis of bone geometry and density and to quantify limb muscle markers. The radiation exposure of a measurement performed with a modern system is far below that of a standard hand X-ray (13). For the present study, we considered the muscle density and the ratios (expressed as percentages and defined, respectively, as muscle area ratio and fat area ratio) of muscle area and fat area to total calf area as predictors of frailty.
Covariates
Covariates included sociodemographic variables (age, sex, study site, smoking habit, and MMSE score), BMI, comorbidity (adjudicated diagnoses of angina, myocardial infarction, hypertension, stroke, congestive heart failure, peripheral artery disease, diabetes, cancer, osteoarthritis, and pulmonary disease), and biological markers (albumin, total cholesterol, and hemoglobin). Adjudicated disease diagnoses were based on self-reported history, clinical documentation, and medication use, on the basis of prestandardized criteria derived from the Women’s Health and Aging Study protocol (14). Serum lipids were measured from fresh blood samples drawn after an overnight fast. Commercial enzymatic tests (Roche Diagnostics, Mannheim, Germany) were used to measure the concentration of serum total cholesterol (interassay CV: <3.8%). Percentage serum albumin was detected by electrophoresis (Hydragel 7 Protein, Sebia, France; mean interassay coefficient: 0.8%), and its concentration was calculated from serum total proteins (Roche Diagnostics; interassay coefficient: <1%). Hemoglobin concentrations were measured by using the hematology automated autoanalyzer DASIT SE 9000 (Sysmex Corporation, Kobe, Japan).
IL-6, TNF-α, and CRP were considered as additional potential explanatory covariates in the adjusted models to evaluate whether inflammation explained the association between frailty syndrome and pQCT measures. This is consistent with the hypothesis that chronic inflammation represents a common feature of the geriatric syndrome of frailty (7) and with data showing that a proinflammatory state is a strong predictor of body composition changes (8, 9). The quantitative measurement of serum concentrations of IL-6 and TNF-α was performed with enzyme-linked immunosorbent assays from commercial kits (Biosource International, Camarillo, CA). The lower detectable concentrations for IL-6 and TNF-α were 0.10 pg/mL and 0.09 pg/mL, respectively. The mean interassay CV for IL-6 and TNF-α was 7.0%. Concentrations of CRP were measured by using a high-sensitivity enzyme-linked immunosorbent assay, a competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies. The minimum detectable concentration was 0.03 mg/L. The interassay CV was 5.0%. Because of the nonnormal distribution of the serum concentration of inflammatory markers, the present analyses were performed by using their log values.
Statistical analyses
Differences in proportions and means of covariates according to the presence of frailty syndrome were assessed by using chi-square and analysis of variance statistics, respectively. Median values with 25th–75th percentile ranges and P values based on Mann-Whitney U statistics were reported for nonnormally distributed variables. To maintain a conservative approach, all variables found to be different at the univariate analyses with a P value < 0.10 were considered as covariates to adjust the subsequent multivariate analyses. Spearman’s correlation analyses were performed between frailty syndrome score (and single items composing it) and pQCT measures of the calf. Analyses of covariance were performed to assess differences in adjusted means of calf pQCT measures according to the presence of frailty syndrome (dependent variable). Separate logistic regression models were used to identify odds ratios (ORs) and 95% CIs between the frailty syndrome components (dependent variables) and the pQCT measures (per SD increase). Statistical analyses were conducted with SPSS software (version 13.0; SPSS Inc, Chicago, IL).
RESULTS
The mean age (±SD) of the participants in the present study was 74.8 ± 6.8 y. Participants were predominantly women (55.9%) and moderately overweight (BMI: 27.4 ± 4.1). Only 4 participants (0.4%) had a BMI < 18.5, and 227 participants (24.6%) had a BMI ≥ 30.
In our sample, 81 participants (8.8%) had ≥3 frailty criteria (Table 1). The following prevalences were reported for the frailty criteria in the study sample: low walking speed, 22.4%; low hand grip strength, 20.0%; exhaustion, 17.9%; physical inactivity, 17.4%; and weight loss, 4.9%. Frail participants were older and had a higher prevalence of congestive heart failure, hypertension, osteoarthritis, peripheral artery disease, stroke, and myocardial infarction (P < 0.09), lower concentrations of albumin and hemoglobin, and higher concentrations of inflammatory markers than did nonfrail participants. The participants with frailty syndrome had lower muscle density and muscle area ratio than did nonfrail control subjects, but they had similar fat area.
TABLE 1.
Participants’ characteristics according to the presence of frailty syndrome1
| No frailty (n = 842) | Frailty (n = 81) | P2 | |
|---|---|---|---|
| Age (y) | 73.7 ± 6.63 | 80.3 ± 6.6 | < 0.001 |
| Sex (women; %) | 54.8 | 63.0 | 0.16 |
| Site (Bagno a Ripoli; %) | 51.8 | 55.6 | 0.52 |
| Smoking status | 0.31 | ||
| Never (%) | 58.4 | 64.2 | |
| Former (%) | 27.7 | 19.8 | |
| Current (%) | 13.9 | 16.0 | |
| Mini Mental State Examination Score | 25.3 ± 3.7 | 22.4 ± 4.7 | < 0.001 |
| BMI (kg/m2) | 27.4 ± 4.0 | 28.1 ± 5.1 | 0.13 |
| Clinical condition | |||
| Cancer (%) | 6.2 | 8.6 | 0.39 |
| Angina (%) | 4.4 | 6.2 | 0.46 |
| Myocardial infarction (%) | 4.4 | 8.6 | 0.09 |
| Congestive heart failure (%) | 3.6 | 12.3 | < 0.001 |
| Diabetes (%) | 10.0 | 13.6 | 0.31 |
| Hypertension (%) | 34.0 | 53.1 | 0.001 |
| Osteoarthritis (%) | 9.7 | 25.9 | < 0.001 |
| Peripheral artery disease (%) | 5.6 | 14.8 | 0.001 |
| Pulmonary disease (%) | 7.4 | 12.3 | 0.11 |
| Stroke (%) | 3.8 | 12.3 | < 0.001 |
| Biological markers | |||
| Total cholesterol (mg/dL) | 219.6 ± 39.1 | 213.1 ± 39.0 | 0.15 |
| Albumin (g/dL) | 4.2 ± 0.3 | 4.1 ± 0.3 | 0.002 |
| Hemoglobin (g/dL) | 13.8 ± 1.3 | 13.3 ± 1.5 | 0.001 |
| Inflammatory markers | |||
| Interleukin 6 (pg/mL) | 1.38 (0.82–2.06)4 | 2.04 (1.20–3.86) | < 0.001 |
| C-reactive protein (μg/mL) | 2.55 (1.26–5.40) | 4.29 (1.59–9.19) | 0.002 |
| Tumor necrosis factor α (pg/mL) | 1.96 (1.49–3.21) | 2.41 (1.69–3.71) | 0.01 |
| Body composition measures | |||
| Muscle density (mg/cm3) | 71.01 ± 3.52 | 68.73 ± 3.85 | < 0.001 |
| Muscle area/total area (%) | 70.38 ± 11.17 | 67.14 ± 12.70 | 0.01 |
| Fat area/total area (%) | 21.03 ± 10.64 | 22.65 ± 12.12 | 0.20 |
Frailty was defined as ≥ 3 of the following criteria: exhaustion for ≥ 3 d in the past week, grip strength in the lowest quintile, low physical activity, weight loss (>4.5 kg in the past year), and low walking speed (lowest quintile). n = 923.
Differences in proportions and means of covariates according to the presence of frailty syndrome assessed by using chi-square and ANOVA statistics, respectively. For nonnormally distributed variables, statistical significance was based on the Mann-Whitney U test.
x̄ ± SD (all such values).
Median; interquartile range in parentheses (all such values).
Spearman’s correlations between pQCT measures and frailty syndrome criteria are shown in Table 2. Muscle density was inversely and significantly associated with the overall frailty syndrome score and with components of low hand grip strength, physical inactivity, and low walking speed. Significant correlations were also found between percentage muscle area and fat area and the number of frailty criteria, exhaustion, and physical inactivity.
TABLE 2.
Spearman correlations between frailty syndrome variables and body composition measures1
| Muscle density | P | Percentage muscle area | P | Percentage fat area | P | |
|---|---|---|---|---|---|---|
| Frailty criteria | −0.215 | < 0.001 | −0.186 | < 0.001 | 0.129 | < 0.001 |
| Exhaustion | −0.031 | 0.35 | −0.147 | < 0.001 | 0.140 | < 0.001 |
| Low hand grip strength | −0.135 | < 0.001 | −0.024 | 0.46 | −0.024 | 0.47 |
| Low physical activity | −0.216 | < 0.001 | −0.226 | < 0.001 | 0.188 | < 0.001 |
| Weight loss | −0.014 | 0.68 | 0.010 | 0.78 | −0.015 | 0.66 |
| Low walking speed | −0.238 | < 0.001 | −0.058 | 0.08 | −0.012 | 0.72 |
n = 923.
Analyses of covariance (adjusted for age, sex, MMSE score, myocardial infarction, congestive heart failure, hypertension, osteoarthritis, peripheral artery disease, stroke, albumin concentration, and hemoglobin concentration) were performed to assess the differences in adjusted means of pQCT measures across different frailty states. Participants without frailty criteria had higher muscle density (71.1 mg/cm3, SE = 0.2) and muscle area ratio (71.2%, SE = 0.4) than did the participants with intermediate frailty (70.6 mg/cm3, SE = 0.2; and 69.0%, SE = 0.5, respectively) and those with frailty (69.8 mg/cm3, SE = 0.4; and 68.7%, SE = 1.1, respectively; all P for trend < 0.05). The fat area ratio was higher in the participants with intermediate frailty (22.1%, SE = 0.4) and frailty (22.0%, SE = 0.9; P for trend = 0.003) than in the participants with no frailty criteria (20.3%, SE = 0.4). No significant interaction between body composition measures and sex on frailty syndrome was observed (all P values > 0.10).
At the univariate analyses, a lower cognitive performance was reported in frail participants than in participants without frailty. Therefore, to evaluate the potential confounding role played by poor cognitive performance on the relation between pQCT measures and frailty syndrome, restricted analyses of variance in a subset of 60 randomly selected participants (20 age- and MMSE score-matched participants for each frailty group) were performed. Consistent and significant (all P for trend < 0.001) results with previous findings were reported for all the studied associations.
To test whether inflammation explained part of the link between frailty syndrome and pQCT measures, we entered concentrations of IL-6, CRP, and TNF-α (log values) in the adjusted models. As shown in Figure 1, the findings of our analysis remained substantially similar.
Figure 1.
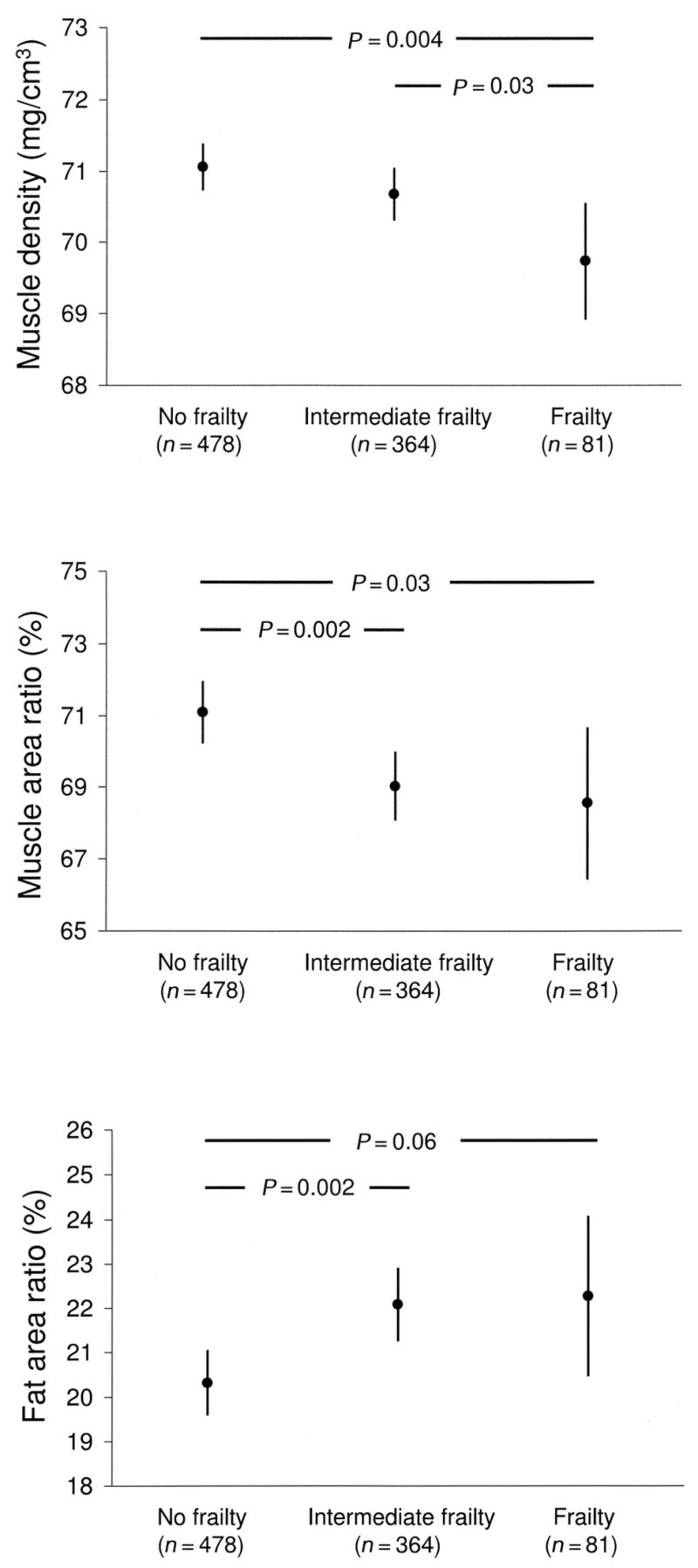
Differences in muscle density, muscle area ratio, and fat area ratio (obtained by peripheral quantitative computerized tomography scans) according frailty syndrome status (dependent variable). The differences were analyzed with an analysis of covariance adjusted for age, sex, Mini Mental State Examination score, myocardial infarction, congestive heart failure, hypertension, osteoarthritis, peripheral artery disease, stroke, and concentrations of albumin, hemoglobin, interleukin 6, C-reactive protein, and tumor necrosis factor α (log values). Intermediate frailty was defined as having 1 or 2 frailty criteria, and frailty was defined as having ≥3 frailty criteria. Values are expressed as adjusted means and 95% CIs. P for trend = 0.01 for muscle density, 0.004 for muscle area ratio, and 0.005 for fat area ratio.
Finally, we explored the relations between each single frailty criterion with pQCT measures (per SD increase) in unadjusted and adjusted logistic regression models (Table 3). Unadjusted analyses showed significant relations for the following: 1) muscle density with low hand grip strength, physical inactivity, and low walking speed; 2) percentage muscle area with exhaustion, physical inactivity, and low walking speed; and 3) percentage fat area with exhaustion and physical inactivity. Physical inactivity was the only frailty criterion that remained significantly associated with all the pQCT measures, even after full adjustment for covariates, including inflammatory markers. In particular, each SD reduction in muscle density (3.6 mg/cm3) and percentage muscle area (11.3%) and SD increase in percentage fat area (10.8%) were associated with a respective 27%, 39%, and 64% higher likelihood of being sedentary. We also found a strong significant association between muscle density and low walking speed in the fully adjusted model (OR: 0.67; 95% CI: 0.55, 0.82). Weight loss and pQCT measures were not statistically related.
TABLE 3.
Separate unadjusted and adjusted logistic regression analyses between frailty criteria (dependent variables) and body composition measures (per SD increase, independent variables)1
| Unadjusted | Model 12 | Model 23 | |
|---|---|---|---|
| Muscle density | OR (95% CI) | ||
| Exhaustion | 0.86 (0.72, 1.02) | 1.00 (0.83, 1.21) | 0.99 (0.81, 1.19) |
| Hand grip strength | 0.71 (0.61, 0.84)4 | 0.88 (0.73, 1.05) | 0.90 (0.75, 1.09) |
| Low physical activity | 0.55 (0.46, 0.65)4 | 0.74 (0.61, 0.90)4 | 0.73 (0.60, 0.90)4 |
| Weight loss | 0.96 (0.71, 1.29) | 1.09 (0.78, 1.51) | 1.09 (0.77, 1.53) |
| Low walking speed | 0.53 (0.45, 0.63)4 | 0.65 (0.54, 0.79)4 | 0.67 (0.55, 0.82)4 |
| Percentage muscle area | |||
| Exhaustion | 0.71 (0.60, 0.84)4 | 0.87 (0.70, 1.08) | 0.88 (0.70, 1.09) |
| Hand grip strength | 0.93 (0.80, 1.10) | 0.94 (0.76, 1.16) | 0.97 (0.78, 1.21) |
| Low physical activity | 0.55 (0.46, 0.66)4 | 0.63 (0.50, 0.80)4 | 0.61 (0.48, 0.77)4 |
| Weight loss | 1.06 (0.78, 1.45) | 1.06 (0.71, 1.59) | 1.03 (0.68, 1.55) |
| Low walking speed | 0.85 (0.73, 0.99)4 | 0.80 (0.64, 1.01) | 0.81 (0.64, 1.02) |
| Percentage fat area | |||
| Exhaustion | 1.41 (1.20, 1.66)4 | 1.15 (0.91, 1.45) | 1.15 (0.90, 1.46) |
| Grip strength | 0.96 (0.82, 1.13) | 1.02 (0.80, 1.31) | 1.01 (0.79, 1.29) |
| Low physical activity | 1.64 (1.39, 1.94)4 | 1.55 (1.20, 2.01)4 | 1.64 (1.26, 2.14)4 |
| Weight loss | 0.93 (0.69, 1.27) | 1.05 (0.67, 1.63) | 1.10 (0.70, 1.73) |
| Low walking speed | 1.01 (0.86, 1.18) | 1.16 (0.90, 1.49) | 1.17 (0.90, 1.52) |
SD for muscle density was 3.605 mg/cm3, SD for muscle area/total area was 11.342%, and SD for fat area/total area was 10.779%. n = 923.
Adjusted for age, sex, Mini Mental State Examination score, myocardial infarction, congestive heart failure, hypertension, osteoarthritis, peripheral artery disease, stroke, and concentrations of albumin and hemoglobin.
Adjusted for model 1 covariates plus concentrations of interleukin 6, C-reactive protein, and tumor necrosis factor α (log values).
P ≤ 0.05.
DISCUSSION
In the present study, we showed a strong association between the commonly used definition for the frailty syndrome developed by Fried et al (1) and lower extremity pQCT-derived indexes of body composition. Frail older persons have lower muscle density and muscle mass and higher fat mass. This relation appears to be similar in men and women and is independent of concentrations of IL-6, CRP, and TNF-α. In an analysis of the single criterion composing the frailty score, physical inactivity was the strongest correlate of body composition.
Skeletal muscle undergoes quality and quantity modifications with aging, which lead to a progressive decline in muscle mass and strength (15). The age-related loss of muscle mass may not be an isolated phenomenon, but rather it is strongly connected with a parallel increase in fat mass (16). The fat mass increase and the muscle mass decrease may act synergistically and lead to sarcopenic obesity (16), an important risk factor for physical disability (17–19).
Our findings are supportive of the recently proposed vicious cycle of frailty (20), which involves sarcopenia as one of the main contributors. According to this hypothesis, a decrease of metabolically active cell mass and the weakness resulting from the muscle loss are responsible for the reductions in resting metabolic rate and physical activity. Physical activity becomes progressively more difficult, and its habitual level declines further because of the body composition changes. The decreased physical activity and resting metabolic rate may then result in the dysregulation of energy input and output, causing undernutrition that may potentially further exacerbate the loss of muscle. Consistent with this hypothetical pathway is our finding that indicates physical inactivity as the frailty domain most strongly associated with body composition indexes. By stimulating muscle protein synthesis, physical activity may prevent the age-related decline in muscle energy efficiency and contractile functions (21) and through this mechanism may prevent the development of frailty. Moreover, physical activity mediates strong antiinflammatory mechanisms with sufficient power to reduce proinflammatory activity in vitro and in vivo (22). In a previous study, Walston et al (23) showed that the frailty syndrome is characterized by increased amounts of inflammation, which, in themselves, have shown a direct effect on skeletal muscle in animal (24–26) and human (27) models.
Our findings showed a strong relation between frailty and pQCT muscular indexes. It may be argued that this is a not surprising finding, given the inclusion of muscle strength as one of the defining criteria of frailty. Nevertheless, note that separate logistic regression models comparing the frailty criteria with pQCT measures showed that walking speed and physical activity, rather than hand grip strength, represented the strongest contributors to body composition changes. Even though hand grip strength has been shown to be an excellent predictor of health-related events (28, 29) and to provide a good approximation of total body muscle strength (30), it is still a measure of upper-body strength and may not entirely capture body composition in the lower extremities. Moreover, in a previous study (31), we showed that muscle power (ie, the ability to generate muscular work per unit of time) represents a more important determinant of physical function than does muscle strength (ie, the ability to exert maximal muscular force). Muscle power is not only associated with a steep decline with aging but is also related to skeletal muscle quality and quantity (32–34). Finally, walking speed and physical activity represent more complex indicators of well-being than the simple mechanical measure of muscle strength. In fact, walking and physical activity are the result of the multiple interactions of several different systems (eg, skeletal muscular apparatus, nervous system, respiratory system, etc). The harmonic integration of these systems allows for correct explication of the critical subcomponents of walking and physical activity (eg, motivation, motor programming, execution, energy production and delivery, etc). Therefore, the walking speed and physical activity criteria may better capture the extent of subclinical conditions potentially associated with frailty.
Interestingly, our analyses showed different thresholds for muscle and fat mass compared with muscle density when predicting frailty. In fact, participants without frailty had different muscle and fat areas compared with the participants with intermediate frailty or frailty. However, the participants with frailty had lower muscle density than did the participants with either intermediate frailty or without frailty. Even if our data does not provide definite conclusions, our findings still suggest that the intramuscular infiltration of fat may occur in a more advanced stage of the process leading to frailty. A previous study by Sipilä et al (35), in which fat mass and muscle area were shown to be more strongly associated with muscle power [an early indicator of mobility loss (31)] than with muscle density in early post-menopausal women, provide support for our findings.
In the present study, we also evaluated whether the association between frailty syndrome and body composition may have been driven by an underlying chronic inflammatory status. The additional analyses performed, including the use of IL-6, CRP, and TNF-α concentrations as covariates of the adjusted models, did not lead to substantial changes from previous findings, which suggests that the studied association is independent of systemic inflammation.
The need for reliable tools for the screening of age-associated frailty in clinical and research settings was discussed in many editorial and research articles (3). Showing that the easy and inexpensive frailty score proposed by Fried et al (1) has a biological validity may promote its implementation in the evaluation of older persons.
The study cross-sectional design, which does not allow the assessment of any cause-effect mechanism, represents its main limitation. We could not evaluate whether the body composition changes represented the initial step of the pathway leading to the frailty syndrome, the expression of a frailer health status of participants, or only the expression of physical inactivity. Longitudinal studies are needed to confirm our findings and clarify this issue.
In conclusion, our findings showed that the frailty syndrome was inversely associated with muscle mass and quality. Moreover, frail older subjects had higher amounts of fat mass. These associations appeared to be independent of concentrations of IL-6, CRP, and TNF-α. By providing a biological face validity to the definition of frailty syndrome provided by Fried et al (1), we should encourage the use of this scale in the screening of older persons who are at high risk of health-related events.
Footnotes
Supported by the Italian Ministry of Health as a _targeted project (ICS 110.1-RS97.71) for The InCHIANTI study and by the US National Institute on Aging (contracts 263 MD 9164 13, 263 MD 821336, and N01-AG-5-0002).
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Sarkisian CA, Lachs MS. Failure to thrive” in older adults. Ann Intern Med. 1996;124:1072–8. doi: 10.7326/0003-4819-124-12-199606150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–34. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved _targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 5.Council on Scientific Affairs. American Medical Association white paper on elderly health. Report of the Council on Scientific Affairs. Arch Intern Med. 1990;150:2459–72. [PubMed] [Google Scholar]
- 6.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 7.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–71. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57A:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 9.Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity and inflammation–results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–34. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 10.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 11.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 12.Rittweger J, Beller G, Ehrig J, et al. Bone-muscle strength indices for the human lower leg. Bone. 2000;27:319–26. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- 13.Neu CM, Rauch F, Rittweger J, Manz F, Schoenau E. Influence of puberty on muscle development at the forearm. Am J Physiol Endocrinol Metab. 2002;283:E103–7. doi: 10.1152/ajpendo.00445.2001. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women’s Health and Aging Study–health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. NIH Pub No. 95-4009, 1995. [Google Scholar]
- 15.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–7. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 17.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 18.Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change and risk of mobility disability in middle-aged and older women. The epidemiologic follow-up of NHANES I. JAMA. 1994;271:1093–8. [PubMed] [Google Scholar]
- 19.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;31:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 21.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–63. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 22.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78:819–35. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- 23.Walston J, McBurnie MA, Newman AB, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Martinez C, Lopez-Soriano FJ, Argiles JM. Acute treatment with tumor necrosis factor-alpha induces changes in protein metabolism in rat skeletal muscle. Mol Cell Biochem. 1993;125:11–8. doi: 10.1007/BF00926829. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino E, Pichard C, Greenwood CE, et al. Body composition and metabolic rate in rat during a continuous infusion of cachectin. Am J Physiol. 1991;260:E27–36. doi: 10.1152/ajpendo.1991.260.1.E27. [DOI] [PubMed] [Google Scholar]
- 26.Charters Y, Grimble RF. Effects of recombinant human tumor necrosis factor alpha on protein synthesis in liver, skeletal muscle and skin of rats. Biochem J. 1989;258:493–7. doi: 10.1042/bj2580493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anker SD, Ponikowski PP, Clark AL, et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20:683–93. doi: 10.1053/euhj.1998.1446. [DOI] [PubMed] [Google Scholar]
- 28.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 29.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–41. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 30.Rantanen T, Era P, Kauppinen M, Heikkinen E. Maximal isometric muscle strength and socio-economic status, health and physical activity in 75-year-old persons. J Aging Phys Activity. 1994;2:206–20. [Google Scholar]
- 31.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:M728–33. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 32.Lexell J, Taylor CC. Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J Anat. 1991;174:239–49. [PMC free article] [PubMed] [Google Scholar]
- 33.Frontera WR, Hughes VA, Krivickas LS, Roubenoff R. Contractile properties of aging skeletal muscle. Int J Sport Nutr Exerc Metab. 2001;11:S16–20. doi: 10.1123/ijsnem.11.s1.s16. [DOI] [PubMed] [Google Scholar]
- 34.Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80:447–55. doi: 10.1097/00002060-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Sipila S, Koskinen SOA, Taaffe DR, et al. Determinants of lower-body muscle power in early postmenopausal women. J Am Geriatr Soc. 2004;52:939–44. doi: 10.1111/j.1532-5415.2004.52261.x. [DOI] [PubMed] [Google Scholar]


