Abstract
The tumor suppressor phosphatase and tensin homolog (PTEN) is frequently involved in human prostate carcinoma. PTEN is therefore an attractive _target for the development of preclinical animal models. Prostate intraepithelial neoplasia lesions develop in mice with Pten heterozygosity, but disease progression has been reported only in combination with either other tumor suppressor gene alterations or the conditional inactivation of both Pten alleles in prostate epithelial cells. We report that on a C57BL/6 background, in contrast to previous studies on mixed 129 genetic backgrounds, Pten locus heterozygosity is fully penetrant for the development of prostate adenocarcinoma. Grossly observable tumors were detected at 6 months of age, and, by 10 to 12 months, 100% of examined mice developed adenocarcinoma of the anterior prostate. Furthermore, double heterozygotes carrying both Pten and Tsc2-null alleles showed no increase relative to Pten+/− heterozygotes in either lesion development or progression. Lesions in both Pten+/−; Tsc2+/−, and Pten+/− mice exhibited loss of PTEN expression and activation of PI3K signaling. PI3K activation occurred early in prostate intraepithelial neoplasia lesion formation in these animals, consistent with loss of PTEN function, and contributed to the etiology of tumors that developed in Pten+/− mice. Furthermore, prostate lesion growth in Pten+/− mice was dependent on mTOR, as evidenced by a reduction in both phospho-S6 levels and proliferative index after rapamycin treatment.
The tumor suppressor gene phosphatase and tensin homolog (PTEN), also known as MMAC1 (for mutated in multiple advanced cancers),1 functions as a lipid and protein phosphatase, inhibiting the ability of PDK1 to activate AKT.2,3,4 Loss of PTEN function results in constitutive AKT activation and phosphorylation of downstream _targets, including the tuberous sclerosis complex 2 (TSC2) tumor suppressor.5,6,7,8 TSC2 inhibits mTOR signaling by functioning as a GTPase activating protein for the small GTPase Rheb, which activates mTOR.9,10,11 Phosphorylation of TSC2 by AKT at S939 and S981 causes it to partition into the cytosol away from its _target Rheb and its activation partner TSC1 in the membrane, relieving repression of mTOR.12 Loss of PTEN function therefore results in functional inactivation of the TSC2 protein via AKT phosphorylation, resulting in activation of mTOR signaling.9
Loss of one PTEN allele is a high frequency event in prostate cancer, occurring in as many as 70 to 80% of primary tumors13,14,15,16 and homozygous inactivation of PTEN is associated with advanced disease and metastasis.15,17,18,19 Pten knockout mice (heterozygous Pten+/−) develop prostate intraepithelial neoplasia (PIN) with a variable penetrance ranging from 40 to 50%20,21,22 to 90%.23 Lesions develop primarily in the dorsolateral prostate and anterior prostate but appear to spare the ventral prostate. Importantly, progression to adenocarcinoma generally is not observed in Pten+/− mice, possibly due to age-dependent morbidity associated with the high incidence of thyroid lymphomas that occur in these animals. It has recently been suggested that genetic background and/or modifier genes may influence the development of lesions in Pten-haploinsufficient animals.23 In that study, both onset and spectrum of lesions at several anatomical sites observed with a Pten null allele placed on a 129;BALB/c mixed background differed from that observed with Pten null alleles on either a 129;C57BL/6 or 129;CD-1 mixed backgrounds.
In contrast to Pten heterozygotes, conditional Pten knockout mice with complete loss of Pten in the prostate develop invasive prostate carcinoma, with variable latency.24,25,26,27,28 Inactivation of Pten in combination with other mutations can also promote cancer progression. Double heterozygous mice that carry p27 (now Cdkn1b) and Pten defects (Pten+/−, p27+/−) develop invasive carcinoma.29 Similarly, when crossed with Nkx3–1 knockout mice (a homeobox gene expressed in prostate epithelium) Nkx3–1+/−, Pten+/− mice also develop metastatic prostate carcinoma.30,31 Heterozygosity at the Pten locus also promotes prostate cancer progression in the transgenic murine prostate cancer model (TRAMP)32 and conditional Pten knockouts crossed with p53 (Trp53) knockouts develop highly lethal invasive prostate carcinoma at a young age.33 Recently, other alterations that regulate PTEN/PI3K signaling at the level of mTOR have also been shown to contribute to prostate carcinogenesis: Pten haploinsufficiency cooperates with Rheb overexpression to promote prostate tumorigenesis34 and Lkb1 deficiency, a tumor suppressor and upstream kinase for AMP-activated kinase signaling to TSC2, leads to the development of PIN lesions.35
TSC2 has not been previously implicated in prostate tumorigenesis, but alterations in this tumor suppressor gene do predispose to genitourinary tumors, primarily renal cell carcinoma.36 Tsc2 knockout mice develop renal cell carcinoma and vascular lesions, primarily liver hemangiomas, but do not develop prostate lesions, even at older ages.37,38 Interestingly, it was recently reported that Pten+/−;Tsc2+/− double heterozygous mice develop invasive prostate carcinoma with 100% penetrance.39 In this study, tumors were reported to arise as early as 5 months of age and were found in all lobes of the prostate (dorsolateral prostate, anterior prostate, and ventral prostate). Pten expression from the normal allele was reported to be retained in these tumors, suggesting that the Tsc2 defect was responsible for progression of Pten-dependent prostate cancer in these animals. However, a similar study using Pten+/−;Tsc2+/− mice reported no progression of PIN lesions in double heterozygotes.40 Thus the potential for Tsc2 defects to contribute to the development of Pten-dependent prostate carcinoma requires further study.
We report here that in contrast to previous studies in which Pten null alleles were placed on mixed 129 genetic backgrounds, Pten haploinsufficiency is fully penetrant for development of prostate carcinoma on a C57BL/6 background. 100% of Pten+/− mice developed prostate adenocarcinoma by 10–12 months of age. Furthermore, double heterozygotes carrying both Pten and Tsc2 null alleles did not exhibit any acceleration in lesion development or progression. Activated mTOR signaling that could be reversed with rapamycin was observed in PIN lesions and adenocarcinomas that developed in Pten+/− animals, with adenocarcinomas from both Pten+/−;Tsc2+/− and Pten+/− mice exhibiting loss of PTEN expression.
Materials and Methods
In Vivo Studies
Mice were housed in suspended polycarbonate cages or individually ventilated cages (Lab Products, Maywood, NJ) on autoclaved hardwood bedding (PJ Murphy Forest Products Corp., Montville, NJ) in an AAALAC-accredited facility (M. D. Anderson Cancer Center, Science Park–Research Division). Room conditions included temperature (20–22°C), humidity (60–70%), and light (14/10 hours; light/dark). Commercial rodent pelleted food (Harlan Teklad, Madison, WI) and autoclaved water were available ad libitum. All procedures were in compliance with the Public Health Service Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The protocol involving use of these mice was approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee. Male mice were euthanized at various ages from 7 to 12 months by CO2 asphyxiation, and tissues were harvested and either snap-frozen in liquid N2 and stored at −80°C or fixed in 10% neutral buffered formalin and paraffin embedded. The intact male reproductive system was transversely sectioned and then paraffin-embedded for histopathological and immunohistological analysis. The Pten+/− mice were a kind gift from Dr. Ramone Parsons (Columbia University)20 and the Tsc2+/− mice were obtained from Dr. David Kwiatkowski (Brigham and Women’s Hospital).38
Pten+/− Mice Treated with Vehicle and Rapamycin
Pten+/− mice 12 to 14 months old were treated with rapamycin (LC Laboratories, Woburn, MA) (0.15 mg/kg) (n = 4) or vehicle (n = 3) for 14 days daily (i.p) and sacrificed at the end of the study. The vehicle was Tween 80, polyethylene glycol, and ethanol). Tissues were harvested and fixed as described above.
Genetic Background Characterization of C57BL/6-Pten Mice
We performed a genetic background characterization of our C57BL/6.129S1/v-Pten congenic strain containing a Pten _targeted mutation (Ptentm1Rps).20 For this, we selected 92 microsatellite markers (simple sequence length polymorphism) evenly distributed over all of the 19 autosomal chromosomes (genome scan) and polymorphic between C57BL/6J and 129S1/Sv inbred strains. The average marker spacing was 15 cM. D19Mit88 on chromosome 19 was the only marker showing 129S1/C57BL/6 heterozygosity for all Pten+/− mice. This is expected since the Pten gene is localized in the same region on chromosome 19 as D19Mit88, and the _targeted allele is 129S1/Sv (W9.5 ES cells) in origin. In agreement with the number of backcross generations performed in our Pten null colony (>N6), the background strain characterization showed that 91 of 92 markers (98.9%) were homozygous C57BL/6.
Histological Analysis
Tissues were stained with hematoxylin and eosin, and prostates were examined microscopically by two study pathologists (J.B. and C.J.C.) blinded as to age and genotype of study animals. Hematoxylin and eosin sections were evaluated for precursor lesions identified as hyperplastic, low-grade PIN, or high-grade PIN and adenocarcinoma whose severity was described by Shapell et al.41 To detect downstream _targets of the PTEN signaling pathway, immunohistochemistry was performed on paraffin-embedded prostate tissue sections using primary antibodies against AKT (1:100; Santa Cruz no. sc-1619, Santa Cruz, CA), phospho-AKT (Ser 473) (1:50; Cell Signaling Technologies, Beverly, MA, no. 3787), S6 (1:50; Cell Signaling Technologies no. 2217), and phospho-S6 (S235/236) (1:50; Cell Signaling Technologies no. 2211). Ki-67 (1:50 Santa Cruz no.15402) was used to determine proliferative index in vehicle and rapamycin treated mice. Primary antibodies were detected with biotinylated secondary antibodies, including anti-goat IgG for AKT, and an Envision plus labeled polymer, anti-rabbit-horseradish peroxidase (Dako Laboratories, Carpinteria, CA), for phospho-AKT, S6 and phospho-S6. This was followed by peroxidase-conjugated avidin/biotin (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA) and DAB substrate (Dako Laboratories). Intensity of immunohistochemistry staining was graded on a scale as follows: “-“ indicating no apparent staining, “+” indicating weak staining, “++” indicating moderate staining and “+++” indicating strong staining. The four point scale for grading immunohistochemistry was used as described previously.42,43
For detection of PTEN, after deparaffinization, sections were pretreated with microwave irradiation in 0.01 mol/L citrate buffer (pH 6.0), followed by blocking 20 minutes with 10% normal donkey serum. Sections were incubated with anti-PTEN antibody (Neomarkers, 1:50 dilution, Freemont, CA) overnight at 4°C. After washing with phosphate-buffered saline, the sections were incubated with the secondary fluorescein isothiocyanate-conjugated donkey anti-mouse IgG (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA). The slides were examined using a Fluoroview laser confocal microscope (Olympus America, Melville, NY).
Western Analysis
Normal and tumorigenic anterior prostate tissue lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat dry milk and separately incubated with phospho-AKT (Thr 308) (1:1000, Cell Signaling Technologies), phospho-AKT (Ser 473) AKT (1:1000, Cell Signaling Technologies), (1:1000, Cell Signaling Technologies), phospho-S6 (1:2000, Cell Signaling Technologies), S6 (1:1000, Cell Signaling Technologies), phospho-TSC2 (Ser 939) (1:1000, Cell Signaling Technologies), and TSC2 (1:1000, Epitomics; Burlingame, CA) for 2 hours, followed by streptavidin horseradish peroxidase-conjugated goat anti-rabbit secondary antibody for one hour at room temperature. Lumiglo (KPL) was used for detection on X-ray film (BioMax, Eastman Kodak, Rochester, NY). As a loading control, blots were stripped and reprobed with an antibody to γ-tubulin (1:5000; Sigma, St. Louis, MO).
Results
C57BL/6-Pten+/− Mice Develop Prostate Adenocarcinoma
In-house colonies of Pten+/−20 and Tsc2+/−38 mice maintained on a C57BL/6 genetic background and confirmed by microsatellite analysis to be homozygous C57BL/6 (see Materials and Methods) were used to generate double heterozygous mice (Pten+/−; Tsc2+/−) for analysis. Prostate lesions were evaluated in wild-type, Pten+/− single heterozygotes, Pten+/−; Tsc2+/− double heterozygotes and Tsc2+/− single heterozygotes with groups of male mice examined at 7–9 and 10–12 months of age. Whole prostates were removed from mice and processed using conventional histology techniques. At necropsy, grossly observable prostate lesions were often noted, in one case in a moribund animal at 6 months of age. As shown in Supplemental Figure 1 (found at http://ajp.amjpathol.org), enlargement and changes in the coloration and texture of the anterior prostrate were the most common observations in these animals. Large, solid masses were also observed, often adjacent to the bladder, which were later confirmed to be tumors arising from the ventral prostate. Although at a lower frequency, grossly observable lesions were also identified in the dorsolateral prostate and seminal vesicle.
Histological analysis confirmed the neoplastic nature of grossly observable lesions and allowed the identification of a variety of microscopic lesions in all three lobes of the prostate. The adenocarcinomas observed in both the 7–9 and 10–12 month groups were moderately to well differentiated, forming in some cases large neoplastic masses with ill-defined glandular structures. Tumors with neuroendocrine differentiation or poorly differentiated histology were not observed.
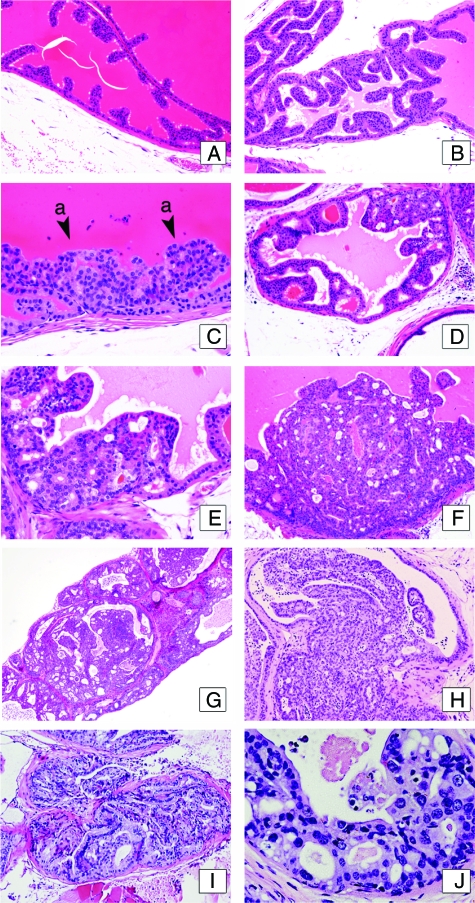
As shown in Table 1 and Figure 1, adenocarcinomas were observed in the anterior prostates of 100% of the Pten+/− male mice by 12 months of age, whereas no wild-type or Tsc2+/− mice developed these lesions. While complete penetrance for adenocarcinoma was observed in the anterior prostate of Pten+/− mice, adenocarcinomas also arose in the ventral and dorsolateral prostates of 33% and 67% of heterozygous mice, respectively, and in seminal vesicles of 14% of Pten+/− and 33% of Pten+/−;Tsc2+/− mice (Figure 1, A–F). Seminal vesicles with invasive adenocarcinoma and intraepithelial neoplasia exhibited similar features and cytological characteristics as lesions arising in other lobes of the prostate (Figure 1, G–J). Double heterozygous Pten+/−;Tsc2+/− animals failed to exhibit any increased incidence of adenocarcinoma relative to Pten+/− mice. Thus, in the anterior prostate the Pten null allele was fully penetrant for development of prostate adenocarcinoma, and the presence of a Tsc2 null allele did not accelerate lesion development throughout the prostate.
Table 1.
Adenocarcinoma Incidence in the Prostate
| wt | Pten+/− | Pten Tsc2 | Tsc2+/− | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | No. of lesions | n | No. of lesions | n | No. of lesions | n | No. of lesion | |||||
| 7–9 months | ||||||||||||
| AP | 4 | 0 | 0% | 11 | 7 | 64% | 9 | 3 | 33% | 10 | 0 | 0.0% |
| VP | 5 | 0 | 0% | 10 | 2 | 20% | 9 | 3 | 33% | 10 | 0 | 0.0% |
| DLP | 2 | 0 | 0% | 10 | 3 | 30% | 9 | 3 | 33% | 10 | 0 | 0.0% |
| SV | 5 | 0 | 0% | 11 | 0 | 0% | 9 | 0 | 0 | 10 | 0 | 0.0% |
| 10–12 months | ||||||||||||
| AP | 5 | 0 | 0% | 8 | 8 | 100% | 9 | 8 | 89% | 14 | 0 | 0.0% |
| VP | 6 | 0 | 0% | 6 | 2 | 33% | 9 | 2 | 20% | 14 | 0 | 0.0% |
| DLP | 6 | 0 | 0% | 6 | 4 | 67% | 9 | 3 | 33% | 14 | 0 | 0.0% |
| SV | 6 | 0 | 0% | 7 | 1 | 14% | 9 | 3 | 33% | 14 | 0 | 0.0% |
In two age groups, 7–9 months and 10–12 months, the number of lesions was counted in four regions of the male reproductive system. AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral prostate; SV, seminal vesicle. n = number of prostates examined that contained the region of interest.
Figure 1.
Histology of lesions of the anterior prostate. Sections of 6- to 12-month-old mice. A: Anterior prostate from wild-type mouse showing a single stratum of luminal epithelial cells with normal recurrent mucosal folds projecting into the gland. B and C: Anterior prostate from an 8-month-old Pten;Tsc2 double heterozygous mouse showing luminal epithelial hyperplasia (C, a; arrowheads) without cellular atypia. D and E: Anterior prostate from 12-month-old Pten heterozygous mouse. Low-grade PIN lesions presenting stratification of the luminal epithelia with cribriform pattern and moderate cellular atypia. F: High-grade PIN from a 12-month-old Pten;Tsc2 double heterozygous mouse. This lesion displays a highly dysplastic luminal epithelium and the presence of nuclear atypia. G–I: Invasive adenocarcinoma from the anterior prostate of a 12-month-old Pten heterozygous mouse. Widespread local invasion of moderate to well differentiated tumor cells. J: Higher magnification of PIN lesion in the anterior prostate. Note the difference in the sizes of the nucleolus among cells, chromatin condensation, nuclear atypia, and formation of small intraluminal glands. H& E; magnifications: A, B, D, F, G, ×10; C, E, H, I, ×20; J, ×40.
Prostate Cancer Progression in C57BL/6-Pten+/− Mice
Precursor lesions for prostate adenocarcinoma were also evaluated microscopically. As shown in Table 2 the anterior prostates of wild-type, Pten+/− and Pten+/−;Tsc2+/−, and Tsc2+/− mice presented with hyperplasia, characterized by increased number of acini and increased epithelial tufting (Figure 1). In addition, a significant number of animals carrying the Pten-null allele showed atypical glandular structures with epithelial stratification and a cribriform pattern, essentially identical to the PIN lesions described previously in Pten+/− mice and other murine models of prostate cancer. These lesions were then further classified as low grade or high-grade PIN (Figure 1, D–F) according to the severity as described by Shapell et al (Bar Harbor Consensus Report).41
Table 2.
Summary of Precursor Lesions in the Prostate
| 7–9 months
|
10–12 months
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AP | VP | DLP | SV | AP | VP | DLP | SV | ||
| WT | Hyperplasia | 1/4 (25%) | 2/5 (40%) | 0/2 | 0/5 | 2/5 (40%) | 0/6 | 0/6 | 0/6 |
| LGPIN | 0/4 | 0/5 | 0/2 | 0/5 | 0/5 | 0/6 | 0/6 | 0/6 | |
| HGPIN | 0/4 | 0/5 | 0/2 | 0/5 | 0/5 | 0/6 | 0/6 | 0/6 | |
| Pten+/− | Hyperplasia | 3/11 (27%) | 3/10 (30%) | 2/10 (20%) | 1/11 (9%) | 0/8 | 1/6 (17%) | 0/6 | 1/7 (14%) |
| LGPIN | 6/11 (55%) | 1/10 (10%) | 4/10 (40%) | 0/11 | 3/8 (38%) | 1/6 (17%) | 2/6 (33%) | 3/7 | |
| HGPIN | 1/11 (9%) | 4/10 (40%) | 0/10 | 0/11 | 1/8 (13%) | 0/6 | 1/6 (17%) | 0/7 | |
| Pten/Tsc2 | Hyperplasia | 1/9 (11%) | 0/9 | 1/9 (11%) | 0/9 | 0/9 | 3/10 (30%) | 0/9 | 0/9 |
| LGPIN | 5/9 (55%) | 0/9 | 1/9 (11%) | 0/9 | 5/9 (56%) | 0/10 | 0/9 | 1/9 (11%) | |
| HGPIN | 1/9 (11%) | 0/9 | 1/9 (11%) | 0/9 | 1/8 (13%) | 0/10 | 1/9 (11%) | 3/9 (33%) | |
| Tsc2+/− | Hyperplasia | 2/10 (20%) | 1/10 (10%) | 0/10 | 0/10 | 1/14 (7%) | 2/14 (14%) | 2/14 (14%) | 0/14 |
| LGPIN | 0/10 | 0/10 | 0/10 | 0/10 | 0/14 | 0/14 | 0/14 | 0/14 | |
| HGPIN | 0/10 | 0/10 | 0/10 | 0/10 | 0/14 | 0/11 | 0/14 | 0/14 | |
Prostates from animals in two age groups, 7–9 months and 10–12 months, were examined for hyperplasia (HP), low-grade PIN (LGPIN), and high-grade PIN (HGPIN). AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral prostate; SV, seminal vesicle.
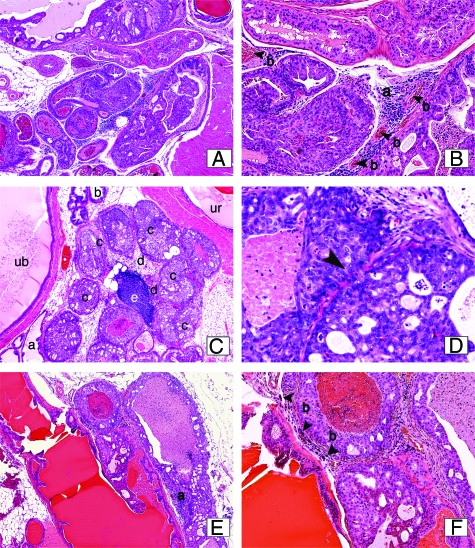
As shown in Table 2, hyperplasias were noted in wild-type and in Tsc2+/− mice, but progression to PIN lesions was not observed in these animals. Pten+/− mice also developed hyperplasia by 7–9 months, but in contrast to wild-type and Tsc2+/− animals, these lesions progressed from hyperplasia to more advanced lesions, which also occurred in Pten+/−; Tsc2+/− mice. As shown in Figure 2A–F, in both Pten+/− and Pten+/−; Tsc2+/− mice, other than differing in their anatomical location, the histology of hyperplasias and PIN lesions observed in the dorsolateral prostate, ventral prostate and intraepithelial neoplasia in seminal vesicles were similar to the cognate lesions observed in the anterior prostate.
Figure 2.
Histology of prostate lesions. A and B: High-grade PIN and intraepithelial adenocarcinoma derived from the dorsal prostate in an 8-month-old Pten;Tsc2 double heterozygous mouse. Note the presence of an inflammatory infiltrate (B, a) as well as increased vascularization (B, b arrowheads). C and D: Ventral prostate from a 12-month-old Pten heterozygous mouse showing normal glands (C, a) glands with atypical hyperplasia (C, b), and a mixed high-grade PIN/adenocarcinoma areas (C, c) with stromal invasion (C, d). A lymphocytic infiltration is also present (C, e) ub, urinary bladder; ur, urethra. D shows a higher magnification (×20) of adenocarcinoma with a notable membrane rupture and stromal invasion (arrowhead). E and F: Seminal vesicle from a 12-month-old Pten heterozygous mouse with low- and high- grade intraepithelial neoplasia and focal areas of invasive adenocarcinoma (E, a). The presence of an inflammatory infiltrate is indicated (F, b arrowheads). H&E; magnifications: ×4 (A, C, E), ×4; B, D, F, ×10.
Loss of Pten Function in Prostate Adenocarcinomas
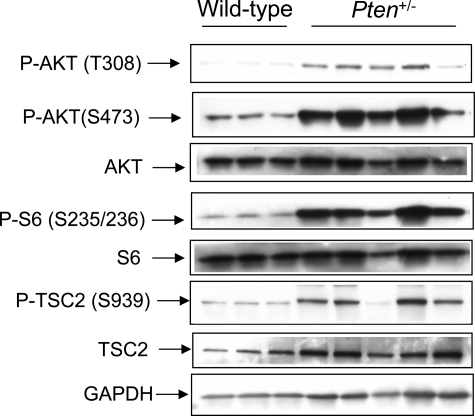
The PTEN tumor suppressor protein functions to inhibit PI3K/AKT activation. On loss of PTEN function, AKT becomes activated, phosphorylating and inactivating TSC2 to activate other signaling pathways such the mTOR pathway and its effectors, including ribosomal S6. Thus, activation of AKT is a reliable indicator of loss of PTEN function in the prostate.26 Anterior prostates obtained from animals histologically confirmed to have adenocarcinoma were used to determine AKT activation and mTOR signaling by Western analysis. As shown in Figure 3 and Supplemental Table 1 (found at http://ajp.amjpathol.org), all prostates from Pten+/− and Pten+/−;Tsc2+/− mice exhibited elevated Akt activity, as assessed by phosphorylation of Akt at S473 and T308, and/or phosphorylation of S6 and Tsc2. In approximately 50% of the anterior prostate lesions examined, Pten expression was absent or barely detectable (summarized in Supplemental Table 1 at http://ajp.amjpathol.org). Importantly, Tsc2 expression was retained in both Pten+/− and Pten+/−; Tsc2+/− double heterozygotes, and consistent with loss of PTEN and activation of AKT, was phosphorylated at S939 (Figure 3), indicating functional inactivation of Tsc2 occurred in these lesions rather than loss of heterozygosity.
Figure 3.
Prostates from tumor-bearing mice exhibit constitutive activation of PI3K/Akt signaling and functional inactivation of Tsc2. Tissue lysates of normal anterior prostate from wild-type mice and prostates of mice with histologically confirmed tumors from Pten and Pten;Tsc2 double heterozygous mice were examined by Western analysis with antibodies for phosphorylated Akt (S473 and T308), S6 (S235/236), Tsc2 (S939), and the corresponding total proteins. After stripping, membranes were probed with GTPase activating proteinDH antibody as a loading control.
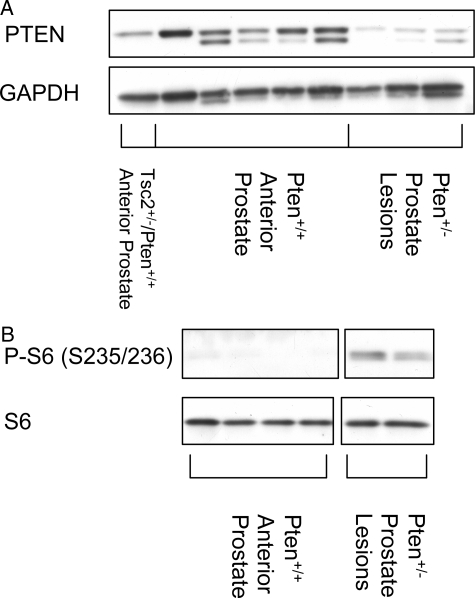
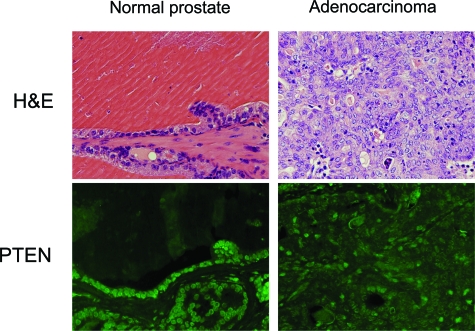
Because prostates contain both tumor and normal tissues, to determine whether the wild-type Pten allele was lost in prostate adenocarcinomas, we isolated macroscopic tumors from Pten+/− mice and Western analysis was performed to assess Pten expression in these lesions. In addition, we performed immunohistochemistry on anterior prostates from Pten+/− mice confirmed to have microscopic adenocarcinomas. Pten expression was lost or barely detectable in adenocarcinomas from Pten+/− mice (Figure 4a and Figure 5), consistent with elevated S6 phosphorylation observed in these tumors (Figure 4b) and activation of AKT signaling observed in the prostates of Pten+/− animals (Figure 6). Similarly, while normal epithelial cells in the anterior prostate exhibited strong immunoreactivity with an antibody directed against Pten, this was not observed in adenocarcinomas of Pten+/− mice (Figure 5).
Figure 4.
Loss of Pten expression of adenocarcinomas of Pten+/− mice. Anterior prostates and macroscopic tumors were dissected from Pten+/−, Pten+/+, and Pten+/+;Tsc2+/− mice. Tissues were examined by Western analysis for expression of Pten (A) and phosphorylation of S6 (S235/236) (B).
Figure 5.
H&E and immunofluorescence detection of PTEN. Adenocarcinoma (right column) exhibits little immunoreactivity for PTEN in the nucleus of the neoplastic cells compared with cells in normal glands (left column), which have with intense immunoreactivity. Upper panels, H&E; lower panels, immunofluorescence demonstrates immunoreactivity for PTEN.
Figure 6.
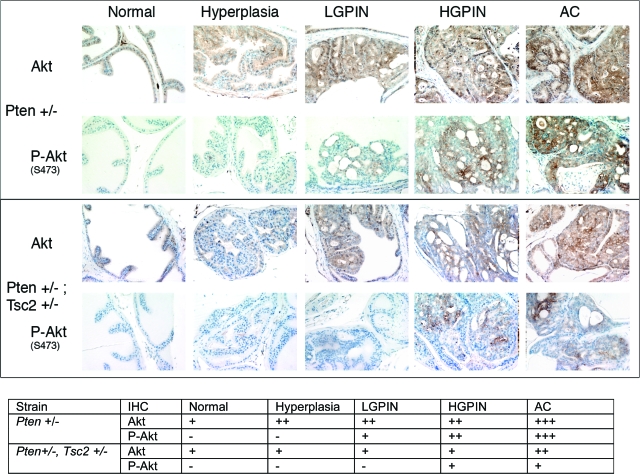
Activation of Akt during prostate tumorigenesis. Sections from Pten and Pten;Tsc2 double heterozygous mice bearing lesions at different stages during tumor development were stained with antibodies to total Akt and phosphorylated Akt (S473). Sections were quantified by the following criteria: −, no staining; +, weak staining; ++, moderate staining; +++, strong staining.
Progression of Prostate Lesions in Pten-Haploinsufficient Mice Correlates with Elevated AKT and mTOR Signaling
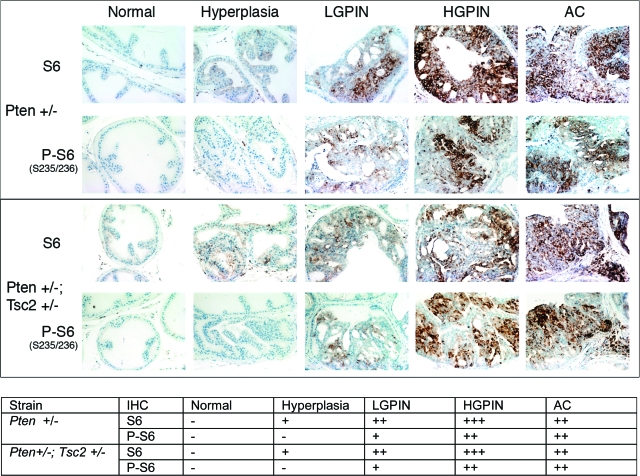
To explore when during the progression of prostate lesions loss of PTEN function and activation of AKT and mTOR signaling occurred, normal prostate and hyperplasias, PIN lesions and adenocarcinomas from Pten+/− and Pten+/−; Tsc2+/− mice were stained for phospho-AKT and phospho-S6, markers for loss of PTEN function and mTOR activation, respectively. As shown in Figures 6 and 7, all adenocarcinomas stained positively for phospho-AKT (Ser 473) and phospho-S6 (Ser 235/236), whereas normal anterior prostate was negative for both phospho-AKT and phospho-S6 immunoreactivity. Hyperplasias were also negative for phospho-AKT and phospho-S6, indicating that development of these lesions was not associated with loss of PTEN function, which was also consistent with the presence of hyperplasias as background lesions in this strain. However, PIN lesions that developed in both Pten+/− and Pten+/−; Tsc2+/− mice contained detectable levels of phospho-AKT and phospho-S6, indicating that loss of PTEN function had occurred during this stage of progression. Interestingly in concordance with a previous study,40 in Pten+/−; Tsc2+/− mice, both high-grade PIN and adenocarcinoma had levels of phospho-AKT that were lower than comparable lesions arising in Pten+/− mice. Thus heterozygosity at the Tsc2 locus appeared to have a dampening effect on AKT activation following loss of PTEN function.
Figure 7.
Activation of mTOR during prostate tumorigenesis. Sections from Pten and Pten;Tsc2 double heterozygous mice bearing lesions at different stages during tumor development were stained with antibodies to total S6 and phospho-S6 (S235/236). Sections were quantified by the following criteria: −, no staining; +, weak staining; ++, moderate staining; +++, strong staining.
Growth of Prostate Lesions Is mTOR-Dependent
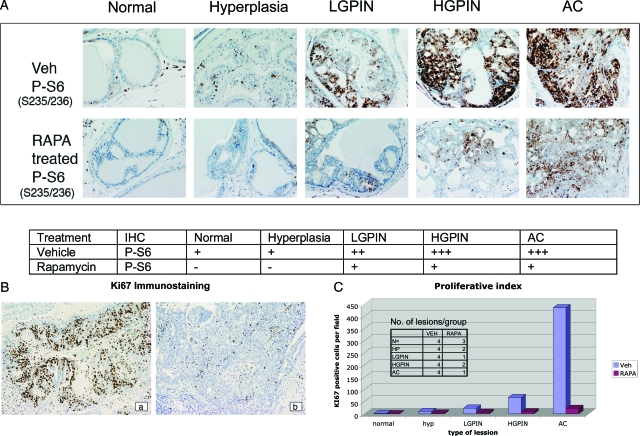
To further understand the implications of elevated mTOR signaling in prostate lesions that develop as a result of loss of PTEN function, we treated Pten+/− mice for 14 days with 0.15 mg/kg of rapamycin and stained the prostate for phospho-S6 to monitor mTOR signaling (Figure 8A). We observed that rapamycin given for even a short duration (14 days) was sufficient to down-regulate mTOR signaling as evidenced by reduction of S6 phosphorylation in PIN lesions and adenocarcinomas. Also, the total number of lesions was reduced in rapamycin-treated mice (Figure 8), although the number of animals examined were too small to evaluate statistically. However, we further investigated the functional significance of inhibition of mTOR signaling by examining cell proliferation (Ki-67 levels) in these lesions (Figure 8, B and C). Dramatic reductions in cell proliferation in response to rapamycin confirmed that these lesions were dependent on mTOR for growth.
Figure 8.
Rapamycin treatment of prostate lesions. Prostate sections from Pten +/− mice were stained with antibodies to phosphorylated S6 (P-S6: S235/236) (A) and Ki-67 (B). Representative staining pattern in mice treated with vehicle versus rapamycin (RAPA) for phospho-S6 (S235/236) (A) and Ki-67 (B) in vehicle (a) versus rapamycin (b) treated mice. Phospho-S6 positivity (A) was quantified by the following criteria: −, no staining; +, weak staining; ++, moderate staining; +++, strong staining. Sections stained with Ki-67 (C) were quantified as number of positive cells per field (magnification, ×10; n = 2–5 fields examined).
Discussion
Loss of the tumor suppressor PTEN is observed in a high percentage of human prostate cancers, making it an important _target for the development of preclinical mouse models of this human disease. We found that Pten deficiency (Pten+/−) was fully penetrant for prostate adenocarcinoma in C57BL/6 mice, in contrast to the previous studies on mixed 129 backgrounds, indicating that the genetic background is important for susceptibility to tumorigenesis. Furthermore, the presence of the Tsc2-null allele in double heterozygous Pten+/−; Tsc2+/− mice did not alter lesion development or progression. The prostate lesions that progressed beyond hyperplasia had activated PI3K signaling in both Pten+/− single and Pten+/−;Tsc2+/− double heterozygotes, as evidenced by downstream activation of AKT and mTOR, indicating that loss of PTEN function was associated with disease progression. Prostate lesions that developed in the Pten-deficient mice were dependent on mTOR signaling for growth as demonstrated by inhibition of phospho-S6 levels and reduction in proliferative index in mice treated with rapamycin.
mTOR-dependent growth of lesions in Pten+/− mice is consistent with loss of PTEN function driving tumor progression in this model. In this regard, the phenotype of lesions that develop in Pten+/− mice on a pure C57BL/6 background compares favorably to models where Pten is conditionally inactivated in prostate epithelial cells on 129 mixed backgrounds (129/C57BL/6,24 129;C57BL/6;DBA;BALB/c,25,26 and 129;FVB27). Similar to what we observed, in these models bi-allelic loss of Pten was fully penetrant for neoplasia (high-grade PIN, prostate carcinoma in situ, and invasive prostatic carcinoma), with focal invasion observed with variable latency from 2 to 7 months. The longer latency observed in our study with Pten+/− C57BL/6 mice (complete penetrance by 10–12 months) is consistent with the need for acquisition of a “second hit” at the wild-type Pten allele in Pten+/− heterozygotes. In the present report, the underlying mechanism responsible for loss of Pten function (loss of heterozygosity, mutation, etc) was not examined. However, loss of heterozygosity at the Pten locus has been demonstrated to occur in prostate lesions that develop in Pten+/− mice combined with other genetic defects such as overexpression of Fgf844 or loss of NKx3.1.30
Previous studies in human prostate cancer cells and xenografts in immunocompromised mice demonstrated that the rapamycin analog CCI-779 was effective at suppressing mTOR signaling as evidenced by decreased phospho-S6 levels determined by immunostaining and Western analysis.45 Further, a reduction in proliferation was observed in xenografts of mice bearing PTEN-negative PC3 cells on treatment with CCI-779, linking mTOR activation on loss of PTEN function to cell growth.46 Together with our data, these studies demonstrate that Pten-deficient preclinical models have utility for evaluating mTOR as a therapeutic _target for prostate cancer. Moreover, constitutive activation of AKT in mouse prostatic epithelial cells causes intraepithelial neoplasia, which is reduced on treatment with the mTOR inhibitor RAD001.47 While cell growth in prostate lesions in Pten+/− mice was dependent on mTOR, the role of other growth factors, or steroid hormones in the development of lesions in Pten+/− mice on the C57BL/6 background remains to be determined. As reviewed by Roy-Burman et al,48 other signaling pathways including those activated by androgens, fibroblast growth factors and retinoids likely contribute to the growth of tumors in this model.
In mouse models, it is well documented that phenotype can be affected by genetic background. For example, tumor type and onset is very different in BALB/c versus C57BL/6 Trp53 knockout mice.49 Recently, background strain has been reported to modify the latency and spectrum of tumors that develop in Pten+/− mice.23 Further, genetic background may also influence prostate gene expression in mice.50 In the report from Freeman et al,23 a higher incidence of prostate abnormalities was observed on 129;BALB/c compared with 129;C57BL/6 and 129;CD-1 mixed backgrounds, although the onset of tumor development was delayed. In another prostate cancer model, Kim and collaborators showed that the prostatic phenotype of Nkx3–1 mutant mice was similar in three congenic lines involving 129S1/Sv, FVB/N, and C57BL/6 backgrounds; however, the incidence of PIN-like lesions was higher in FVB/N and C57BL/6 mice.51 In this regard, it is interesting to note that, working with a mouse model with prostate-specific expression of human AKT1, Xu and colleagues have showed that C57BL/6-Pbsn-Akt1 transgenic mice have approximately fivefold higher rates of proliferation on the ventral prostate when compared with FVB/N-Pbsn-Akt1.52
Of interest is our observation that Pten+/−;Tsc2+/− double heterozygous mice do not exhibit any increase in lesion progression relative to Pten+/− mice, which differs from the recent report that Pten+/−;Tsc2+/− double heterozygous mice have a higher incidence of prostatic adenocarcinoma than Pten+/− mice.39 However, our study is consistent with the work of others40 where Pten+/−; Tsc2+/− double heterozygous mice exhibited no increase in prostate lesions relative to Pten+/− mice. Likely, part of the difference between these studies is due to the difference in the genetic background of the animals used in our study (C57BL/6J) compared with that of the Pandolfi group (129 mixed background).39
Importantly, while existing mouse models in which a germline Pten defect is combined with other defined genetic alterations in genes such as p53, Rb, p27, Nkx3–1, Rarg, and Stat5a are valuable for studying disease pathogenesis and prostate cancer progression, they have the inherent property of directing tumor development down specific molecular pathways reflective of subsets of the human disease. In this regard, the availability of Pten+/− mice in which the pathways to tumor progression following loss of PTEN function are not constrained may be advantageous as a preclinical model for evaluating therapeutic and preventative strategies for human prostate cancer. Future studies in C57BL/6 Pten+/− mice to characterize the spectrum of additional molecular alterations that contribute to disease progression will prove valuable, as will studies aimed at identifying modifier genes present in C57BL/6 and other strains of mice that can modulate prostate cancer progression.
Footnotes
Address reprint requests to Cheryl L. Walker, University of Texas M. D. Anderson Cancer Center, P.O. Box 389, Smithville, Texas 78957. E-mail: chwalker@mdanderson.org.
This study made use of the Research Animal Support Facility - Smithville, Genetic Services, which is supported by P30 CA16672-30 DHHS/NCI Cancer Center Support Grant to M. D. Anderson Cancer Center, the Histology and Tissue Processing Facility Core of the Center for Research on Environmental Disease, supported by grant ES007784, and was supported in part by prostate cancer SPORE P50 CA90270.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene. MMAC1, at chromosome 10q233 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- Sulis ML, Parsons R. PTEN: from pathology to biology. Trends Cell Biol. 2003;13:478–483. doi: 10.1016/s0962-8924(03)00175-2. [DOI] [PubMed] [Google Scholar]
- Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras. PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, Said JW, Isaacs WB, Sawyers CL. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray IC, Phillips SM, Lee SJ, Neoptolemos JP, Weissenbach J, Spurr NK. Loss of the chromosomal region 10q23–25 in prostate cancer. Cancer Res. 1995;55:4800–4803. [PubMed] [Google Scholar]
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–209. [PubMed] [Google Scholar]
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- Freeman D, Lesche R, Kertesz N, Wang S, Li G, Gao J, Groszer M, Martinez-Diaz H, Rozengurt N, Thomas G, Liu X, Wu H. Genetic background controls tumor development in PTEN-deficient mice. Cancer Res. 2006;66:6492–6496. doi: 10.1158/0008-5472.CAN-05-4143. [DOI] [PubMed] [Google Scholar]
- Backman SA, Ghazarian D, So K, Sanchez O, Wagner KU, Hennighausen L, Suzuki A, Tsao MS, Chapman WB, Stambolic V, Mak TW. Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc Natl Acad Sci USA. 2004;101:1725–1730. doi: 10.1073/pnas.0308217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, Cordon-Cardo C, Pandolfi PP. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Ma X, Ziel-van der Made AC, Autar B, van der Korput HA, Vermeij M, van Duijn P, Cleutjens KB, de Krijger R, Krimpenfort P, Berns A, van der Kwast TH, Trapman J. _targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res. 2005;65:5730–5739. doi: 10.1158/0008-5472.CAN-04-4519. [DOI] [PubMed] [Google Scholar]
- Ratnacaram CK, Teletin M, Jiang M, Meng X, Chambon P, Metzger D. Temporally controlled ablation of PTEN in adult mouse prostate epithelium generates a model of invasive prostatic adenocarcinoma. Proc Natl Acad Sci USA. 2008;105:2521–2526. doi: 10.1073/pnas.0712021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Cardiff RD, Desai N, Banach-Petrosky WA, Parsons R, Shen MM, Abate-Shen C. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA. 2002;99:2884–2889. doi: 10.1073/pnas.042688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, Gregg JP, Borowsky AD, Cardiff RD, Shen MM. Nkx3.1;Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 2003;63:3886–3890. [PubMed] [Google Scholar]
- Kwabi-Addo B, Giri D, Schmidt K, Podsypanina K, Parsons R, Greenberg N, Ittmann M. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci USA. 2001;98:11563–11568. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardella C, Chen Z, Salmena L, Carracedo A, Alimonti A, Egia A, Carver B, Gerald W, Cordon-Cardo C, Pandolfi PP. Aberrant Rheb-mediated mTORC1 activation and Pten haploinsufficiency are cooperative oncogenic events. Genes Dev. 2008;22:2172–2177. doi: 10.1101/gad.1699608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HB, McCarthy A, Collins CM, Ashworth A, Clarke AR. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 2008;68:2223–2232. doi: 10.1158/0008-5472.CAN-07-5169. [DOI] [PubMed] [Google Scholar]
- Gomez MR, Sampson JR, Whittemore VH. Harris JC, editor. Oxford: Oxford University Press; Tuberous Sclerosis Complex. 1999:pp 3–46. [Google Scholar]
- Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Behrendt N, Chen Z, Noda T, Hino O, Cordon-Cardo C, Pandolfi PP. Genetic analysis of Pten and Tsc2 functional interactions in the mouse reveals asymmetrical haploinsufficiency in tumor suppression. Genes Dev. 2005;19:1779–1786. doi: 10.1101/gad.1314405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- Maxwell P, McCluggage WG. Audit and internal quality control in immunohistochemistry. J Clin Pathol. 2000;53:929–932. doi: 10.1136/jcp.53.12.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams EJ, Green JA, Clark AH, Youngson JH. Comparison of different scoring systems for immunohistochemical staining. J Clin Pathol. 1999;52:75–77. doi: 10.1136/jcp.52.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Saribekyan G, Liao CP, Cohen MB, Roy-Burman P. Cooperation between FGF8b overexpression and PTEN deficiency in prostate tumorigenesis. Cancer Res. 2006;66:2188–2194. doi: 10.1158/0008-5472.CAN-05-3440. [DOI] [PubMed] [Google Scholar]
- Wu L, Birle DC, Tannock IF. Effects of the mammalian _target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–6145. [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer. 2004;11:225–254. doi: 10.1677/erc.0.0110225. [DOI] [PubMed] [Google Scholar]
- Kuperwasser C, Hurlbut GD, Kittrell FS, Dickinson ES, Laucirica R, Medina D, Naber SP, Jerry DJ. Development of spontaneous mammary tumors in BALB/c p53 heterozygous mice. A model for Li-Fraumeni syndrome. Am J Pathol. 2000;157:2151–2159. doi: 10.1016/S0002-9440(10)64853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi-Frias D, Pritchard C, Mecham BH, Coleman IM, Nelson PS. Genetic background influences murine prostate gene expression: implications for cancer phenotypes. Genome Biol. 2007;8:R117. doi: 10.1186/gb-2007-8-6-r117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, Cunha GR, Cardiff RD, Shen MM, Abate-Shen C. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- Xu Q, Majumder PK, Ross K, Shim Y, Golub TR, Loda M, Sellers WR. Identification of prostate cancer modifier pathways using parental strain expression mapping. Proc Natl Acad Sci USA. 2007;104:17771–17776. doi: 10.1073/pnas.0708476104. [DOI] [PMC free article] [PubMed] [Google Scholar]