Abstract
Angiotensin-(1-12) [ANG-(1-12)] is a newly identified peptide detected in a variety of rat tissues, including the brain. To determine whether brain ANG-(1-12) participates in blood pressure regulation, we treated male adult (mRen2)27 hypertensive rats (24–28 wk of age) with Anti-ANG-(1-12) IgG or Preimmune IgG via an intracerebroventricular cannula for 14 days. Immunoneutralization of brain ANG-(1-12) lowered systolic blood pressure (−43 ± 8 mmHg on day 3 and −26 ± 7 mmHg on day 10 from baseline, P < 0.05). Water intake was lower on intracereroventricular day 6 in the Anti-ANG-(1-12) IgG group, accompanied by higher plasma osmolality on day 13, but there were no differences in urine volume, food intake, or body weight during the 2-wk treatment. In Preimmune IgG-treated animals, there were no significant changes in these variables over the 2-wk period. The antihypertensive effects produced by endogenous neutralization of brain ANG-(1-12) suggest that ANG-(1-12) is functionally active in brain pathways regulating blood pressure.
Keywords: hypertension, transgenic rats, renin-angiotensin system, brain, sympathetic nerve, antibody
recently, a novel angiotensin (ANG) peptide, ANG-(1-12), was reported in plasma and tissues (18). The 12 amino acid peptide represents a C-terminally extended sequence longer than the 10-amino acid ANG I [ANG-(1-10)]. Although low in plasma, ANG-(1-12) is detected in heart, kidney, and brain in equal or higher amounts relative to other angiotensin peptides (18). ANG-(1-12) is a vasoconstrictor in isolated perfused rat aorta, and intravenous administration increases blood pressure (BP). Both the effects in isolated vessels and on BP are abolished by angiotensin-converting enzyme (ACE) inhibition or AT1 receptor blockade (18). These findings imply that ANG-(1-12) is processed to ANG II for the pressor and vasoconstrictive effect. The enzyme that forms ANG-(1-12) is not known, but recent studies (4, 7, 24) demonstrate that renin is not involved in the metabolism of ANG-(1-12). In addition, ANG-(1-12), which is localized in cardiac myocytes, is expressed in higher concentrations in the hypertrophied heart of spontaneously hypertensive rats (14).
ANG II plays a crucial role in the hypertensive phenotype of the (mRen2)27 rat, since cerebral or systemic administration of an AT1 receptor blocker or ANG II antisera reduces blood pressure (10, 16). Indeed, the mRen2 gene is expressed at high levels in brain, and ANG II levels are very high in hypothalamus and medulla in these animals (15, 23). While there is no doubt that brain ANG II plays a role in hypertension in the (mRen2)27 rats, as well as other models of hypertension, controversy over whether all of it derives from renin arises as the cellular localization (neuronal vs. glial) of brain renin-angiotensin system (RAS) components and their independence from the circulating system continues (9). This is largely because angiotensinogen (Aogen) has a wider distribution than renin (20). Thus, while a transmitter-like intracellular system may exist in neurons in which both renin and Aogen have been localized, a system involving extracellular components possibly independent of renin likely exists as well. Our hypothesis is that a nonrenin-dependent pathway involving ANG-(1-12) derived from Aogen may yield ANG II in the brain ventricles, whereas an intracellular neuronal system exists involving renin and Aogen in brain tissue. Accordingly, the present study was designed to specifically test the hypothesis that immunoneutralization of brain ANG-(1-12) with a highly specific antisera would lower systolic blood pressure (SBP) in (mRen2)27 rats.
MATERIALS AND METHODS
Animals.
Experiments were performed in 22- to 28-wk-old male hypertensive (mRen2)27 hemizygote rats weighing 547 ± 10 g (Hypertension and Vascular Research Center Colony, Wake Forest University School of Medicine) (2, 15, 16, 24) group-housed in a temperature- and humidity-controlled room (12:12-h light-dark cycle) with free access to standard rat chow and water. They were housed overnight (1630–0830) in metabolic cages (Allentown, Inc., Allentown, NJ) for collection of urine on dry ice and assessment of food and water intake 1 wk before intracerebroventricular cannula placement and on days 6 and 11 of the intracerebroventricular infusion. The experimental protocol conformed to guidelines of and was approved by the Institution Animal Care and Use Committee at Wake Forest University School of Medicine.
Anti-ANG-(1-12) antisera.
The antisera, prepared for us by AnaSpec (San José, CA), was generated in rabbits against the free C-terminal region of rat ANG-(1-12). The peptide was covalently coupled at the N-terminus via an additional cysteine residue [Cys]-Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Leu11-Tyr12) to keyhole limpet hemocyanin (KLH). We used the IgG fraction purified on a Protein A column, as previously described (19), yielding an antisera that could be preabsorbed by ANG-(1-12) (14). To determine antibody specificity and to verify stability in the osmotic infusion pumps, rat ANG-(1-12) was iodinated using chloramine T, purified by HPLC, and competition curves were performed with ANG-(1-12) and other angiotensins. The IC50 for ANG-(1-12) was ∼5 × 10−14 moles (50 fmol), while cross reactivity was less than 0.01% with ANG I and 0.001% with ANG II or ANG-(1-7). These data demonstrate high specificity of the Anti-ANG-(1-12) antibody for the unique C-terminal portion (Leu11-Tyr12 sequence) of the peptide. Preimmune IgG, purified from serum of the same rabbit taken before immunization with the KLH-N terminal-ANG-(1-12) and artificial cerebrospinal fluid (aCSF) were used as controls. The composition of aCSF was (in mmol/l): 150 sodium, 3.0 potassium, 1.4 calcium, 0.8 magnesium, 1.0 phosphate, and 155 chloride.
Tail-cuff monitoring.
SBP and heart rate (HR) were obtained by the tail cuff method (Narco Biosystems, Houston, TX) on three consecutive baseline days averaged to obtain a single baseline value and intracerebroventricular infusion days 2–5 and 9–11 in animals trained to the procedure (14). At least five consecutive cycles (inflation/deflation) were performed on each rat and the mean of the last five recordings was recorded as the daily SBP.
Intracerebroventricular cannulation and osmotic minipump implantation.
Animals were anesthetized with 2.5–3.5% isoflurane inhalation. A 28-gauge stainless-steel cannula (brain infusion kit2; Alzet, Palo Alto, CA), was implanted stereotaxically (David Kopf Instruments, Tujunga, CA) into the lateral cerebral ventricle (0.4 mm posterior, 0.21 mm lateral to bregma, and 3.5 mm with depth from skull surface), as previously described (16). The brain was frozen, sectioned, and examined microscopically to visualize correct placement of the cannula into a lateral cerebral ventricle at the end of each study. The cannula was connected via polycarbonate tube to an osmotic minipump (5.0 μl/h; model 2ML2; Alzet, Palo Alto, CA) placed under the skin in the lateral abdomen for infusion for 14 days. There were three groups of (mRen2)27 rats: group 1 received the Anti-ANG-(1-12) IgG (n = 6, body weight: 547 ± 20 g; age: 24.5 ± 0.3 wk); group 2 was exposed to the Preimmune IgG (n = 6, body weight: 545 ± 18 g, age: 27.7 ± 2.0 wk); and group 3 received aCSF (n = 4, body weight: 560 ± 23 g, age: 22.5 ± 3.4 wk). There were no significant differences in age and body weight in aCSF, Preimmune IgG, or Anti-ANG-(1-12) IgG groups.
Anti-ANG-(1-12) IgG and Preimmune IgG were prepared at the same concentration (0.6 mg/μl, each group) diluted with aCSF. Activity of Anti-ANG-(1-12) IgG in residual pump contents (n = 4) was determined by binding of [125I]-ANG-(1-12) as described previously (12, 13) and compared with the original sample.
Analysis of plasma angiotensin peptides and osmolarity.
On ICV day 13, plasma samples were taken following decapitation for ANG I, ANG II, and ANG-(1-7) analysis using radioimmunoassay (12, 13). Osmolarity was measured in the plasma samples using a μ OSMOMETTE (Precision Systems, Inc., Natic, MA).
Statistical analysis.
Values are presented as means ± SE. Comparisons over multiple time points and among the three treatment groups were made by two-way ANOVA for repeated measures with post hoc Bonferroni's analysis. Comparison of each intracerebroventricular time point from pre-ICV baseline or among aCSF, Preimmune IgG and Anti-ANG-(1-12) IgG groups was assessed by one-way ANOVA with Dunnett's analysis. For plasma peptides and osmolality, unpaired t-tests were used for the two-group comparison at one time point. The criterion for statistical significance was P < 0.05. Tests were performed using Prism 4.0 and Instat 3 (GraphPad Software, San Diego, CA).
RESULTS
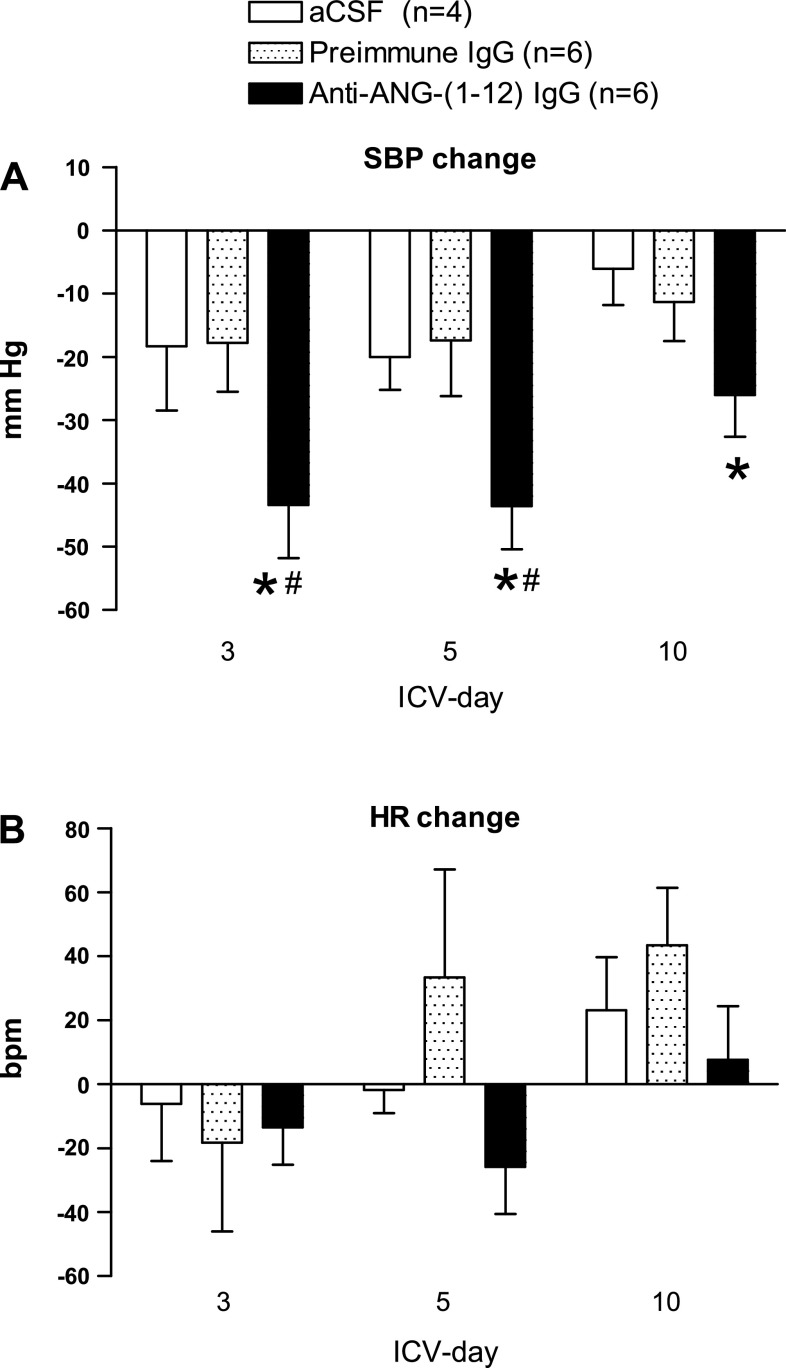
Baseline SBP and HR were similar between aCSF, Preimmune IgG, and Anti-ANG-(1-12) IgG treatment groups: 186 ± 8 mmHg, 171 ± 6 mmHg, and 173 ± 7 mmHg; and 310 ± 20 beats per minute (bpm), 260 ± 13 bpm, 256 ± 13 bpm, respectively. Continuous intracerebroventricular administration of Anti-ANG-(1-12) IgG lowered SBP from baseline (Fig. 1A) with a decrease of −43 ± 8 mmHg (P < 0.05 vs. baseline) on day 3 and a −26 ± 7 mmHg (P < 0.05) reduction on day 10. There were no significant changes in SBP in the Preimmune IgG or aCSF treatment groups when compared with baseline. The reduction in SBP in the Anti-ANG-(1-12) IgG treatment group was significantly greater than the Preimmune IgG on days 3 and 5, but not on day 10. There were no significant changes in HR in the Anti-ANG-(1-12) IgG group in the face of the decrease in SBP, and no consistent changes in HR in the two control groups (Fig. 1B).
Fig. 1.
Changes from pre-ICV baseline systolic blood pressure (SBP: A) and heart rate (HR: B) in male (mRen2)27 transgenic rats administered artificial cerebrospinal fluid (aCSF), Preimmune IgG or Anti-ANG-(1-12) IgG. A: although aCSF and Preimmune IgG did not lower SBP significantly from pre-ICV baseline values, Anti-ANG-(1-12) IgG treatment lowered SBP throughout the treatment period. B: no consistent changes in HR were observed in the three treatment groups. Values are expressed as means ± SE. pre, preintracerebroventricular (ICV) baseline.*P < 0.05 vs. pre-ICV baseline SBP. #P < 0.05 vs. aCSF and Preimmune IgG on same ICV day.
Water intake was lower on ICV day 6 in the Anti-ANG-(1-12) IgG-treated group (Fig. 2A). There were no significant differences in urine volume, food intake, or body weight during the 2-wk treatment across time or between groups (Fig. 2, B–D). Plasma osmolality on ICV day 13 was higher in Anti-ANG-(1-12) IgG group than Preimmune IgG (Fig. 2E). Plasma ANG I, II, and ANG-(1-7) concentrations were not significantly different on ICV day 13 between Preimmune IgG and Anti-ANG-(1-12) IgG (Table 1).
Fig. 2.
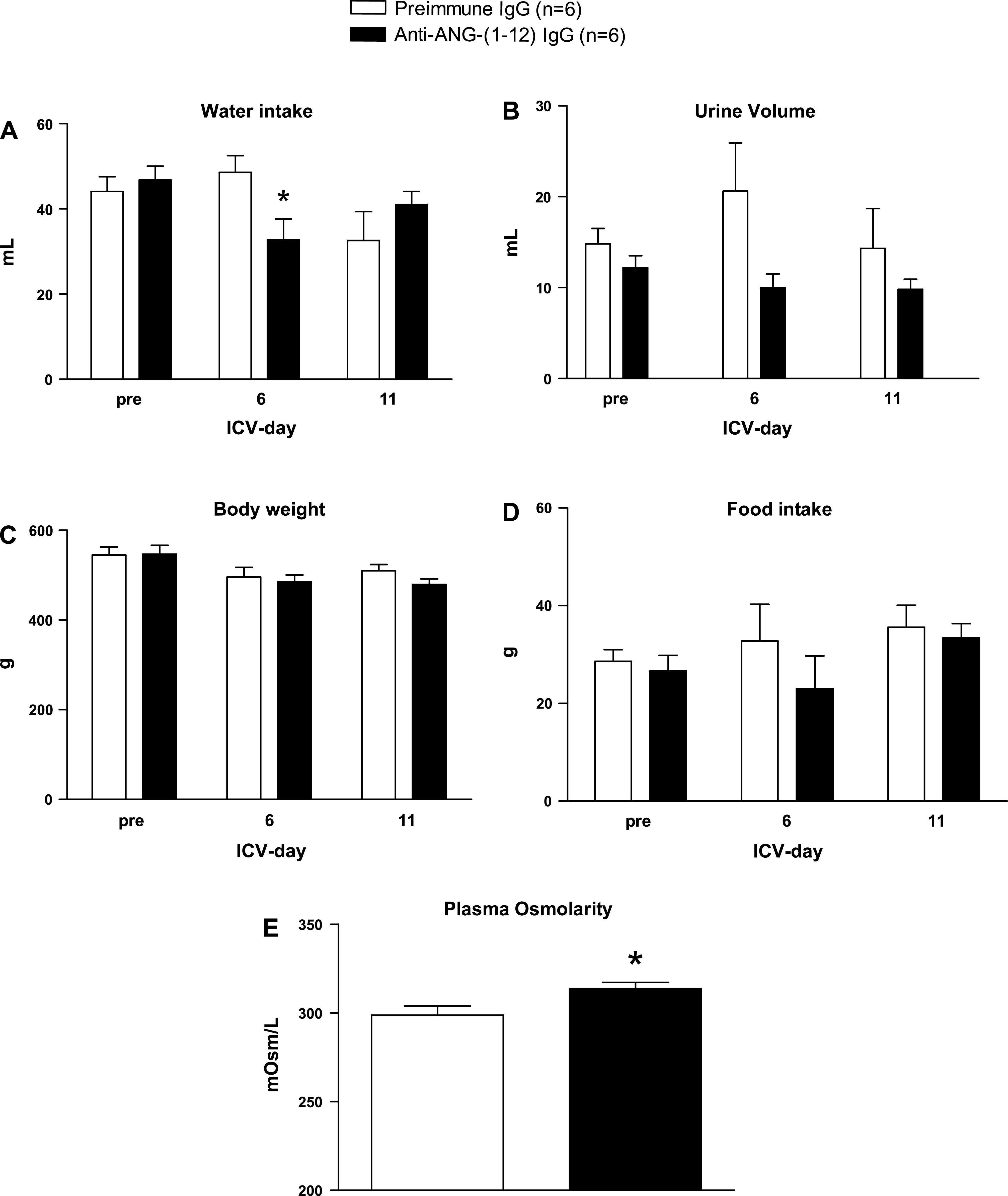
Fluid balance and body weight for rats with chronic intracerebroventricular administration of Anti-ANG-(1-12) IgG or Preimmune IgG. A: water intake. The water intake was lower on ICV day 6 in Anti-ANG-(1-12) IgG group. B: urine volume. C: body weight. D: food intake. Urine volume tended to be lower in rats receiving the Anti-ANG-(1-12) IgG, but this did not attain statistical significance. There were no differences in food intake or body weight during the 2-wk-treatment. E: plasma osmolarity on ICV day 13 was higher in anti-ANG-(1-12) IgG group than Preimmune IgG. Values are expressed as means ± SE. pre, pre-ICV baseline. *P < 0.05 vs Preimmune IgG group.
Table 1.
Plasma angiotensin peptides on ICV day 13
| n | ANG I pg/ml | ANG II, pg/ml | ANG-(1-7), pg/ml | |
|---|---|---|---|---|
| Preimmune IgG | 6 | 14±2 | 39±9 | 22±4 |
| Anti-Ang-(1-12) IgG | 6 | 15±1 | 32±10 | 16±4 |
Values are expressed as means ± SE.
The activity of the Anti-ANG-(1-12) IgG to bind I125-ANG-(1-12) was 91–98% of control (94 ± 2%, n = 4) in residual fluid removed from the osmotic pump on ICV day 13.
DISCUSSION
Chronic intracerebroventricular immunoneutralization of ANG-(1-12) in (mRen2)27 transgenic hypertensive rats reduces SBP without a significant change in HR accompanied by a transient reduction in water intake and increased plasma osmolality. The specificity of the response to Anti-ANG-(1-12) IgG is underscored by the lack of effect on SBP of either the Preimmune IgG or aCSF. The fall in SBP was evident on the third day of treatment, and the reduction waned but persisted for 10 days. Intracerebroventricular administration of a specific antibody to ANG II causes an immediate decrease in BP (∼80 mmHg) accompanied by bradycardia (∼100 bpm) in acute studies in conscious homozygote-female transgenic (mRen2)27 rats (12 wk of age) (16). Chronic ICV AT1 receptor blockade in homozygous male (mRen2)27 rats at a similar age to the present study lowers SBP to normotensive levels within the 1st wk (∼100 mmHg), and the effect persists for 4 wk of treatment (10). Blockade of ANG II with antiserum against ANG II also reduces water intake, even in a dehydrated condition (8, 22). Thus, the early effects of central immunoneutralization of endogenous ANG-(1-12) in brain are similar to that of acute endogenous ANG II blockade with antiserum, but less than that seen with AT1 receptor blockade. Although higher doses of the anti-ANG-(1-12) IgG may be required to achieve a maximal effect, differences in the distribution of antisera for either ANG II or ANG-(1-12) vs. the more lipophilic AT1 receptor antagonists may explain differences in magnitude of responses. Alternately, incomplete blockade of the system in the absence of concomitant blockade of the renin pathway may also occur. In fact, in preliminary studies (11), treatment with either the Anti-ANG-(1-12) IgG or a renin inhibitor resulted in a reduction in SBP of 39–43 mmHg on day 3 that waned by day 10. In contrast, intracerebroventricular treatment with a combination of the Anti-ANG-(1-12) IgG and a renin inhibitor resulted in a reduction in SBP that was sustained at ∼63 mmHg over 10 days of treatment.
In this study, there were no significant differences in circulating ANG peptides on day 13 of intracerebroventricular treatment between Preimmune IgG and Anti-ANG-(1-12) IgG groups. Circulating angiotensin peptides can alter central sympathetic nerve activity via circumventricular organs devoid of the blood-brain barrier (26). This result suggests that chronic immunoneutralization of brain ANG-(1-12) and the lower SBP resulting from the central administration of the antisera were not accompanied by reductions in circulating ANG II that might contribute to the effect. Furthermore, there was no compensatory increase in ANG II as a result of the lower SBP at the end of the study. In addition, the increase in plasma osmolality is not accompanied by changes in plasma concentrations of ANG II. The fact that the decrease in water intake was transient may reflect an effect of the increased plasma osmolality to increase drinking by the latter time points of the study. However, a contribution of a reduction in the effectiveness of the immunoneutralization, as reflected by the waning effects on the blood pressure at day 10, to the restoration of normal water intake by day 11 cannot be ruled out.
Central immunoneutralization of ANG-(1-12) lowered SBP without any change in HR. ANG II is a known participant in resetting of the baroreceptor reflex to higher pressures, in addition to attenuating the sensitivity of reflex for control of HR in many forms of hypertension, including the (mRen2)27 rats (5, 6). The absence of an increase in HR or an increase in plasma ANG II in conjunction with the fall in SBP in the Anti-ANG-(1-12) IgG-treated animals could result from a resetting of the reflex to the lower pressure as a result of the sequestration of ANG-(1-12). In addition, the lack of changes in HR or ANG II could also result from the existing impairment in baroreflex control in the (mRen2)27 rats. It is not possible to distinguish these possibilities since we did not assess baroreflex sensitivity in the present study. However, microinjections of ANG-(1-12) into the nucleus of the solitary tract of Sprague-Dawley rats reduce baroreflex sensitivity (1), which is similar to what occurs with ANG II (3). Thus, there is a potential contribution of endogenous ANG-(1-12) to the impaired reflex in the (mRen2)27, either directly or through generation of ANG II.
There is no agreement as to whether all components of the RAS exist in brain tissue as an intracellular, neurotransmitter-like system. Aogen expression is widespread in the brain, and likewise, neuronal circuits involved in the regulation of autonomic function contain ACE, ANG II, ANG-(1-7), as well as ACE2 and neprilysin (5, 26). A volume transmission system, perhaps derived from Aogen in glial or other non-neuronal sources is proposed (25, 26), and actions of circulating peptides on cerebral vasculature or at the blood-brain barrier are also likely. Antibodies normally do not pass easily through intact cellular or subcellular membranes in living cells (17). Therefore, we propose that blockade of endogenous ANG-(1-12) circulating in CSF, or in the extracellular fluid, are responsible for the reduction in SBP in the (mRen2)27 rats.
In the circulation and isolated vessels, ANG-(1-12) produces pressor and vasoconstrictor actions that, as reported by Nagata et al. (18), are blocked by an ACE inhibitor or AT1 receptor blocker. In our studies, there was no renin participation in ANG-(1-12) processing to ANG II or ANG-(1-7) in plasma, kidney (4), or the perfused heart (24). The enzyme responsible for generation of ANG-(1-12) from Aogen in brain or other tissues is unknown at present, but our current studies support processing of the peptide into ANG II, since SBP was reduced by the ANG-(1-12) antisera and ANG-(1-12) injections into the nucleus tractus solitarii suppress the BRS in SD rats (1). This interpretation is in keeping with previous studies showing that in the heart and plasma, ANG II is the predominant peptide generated from ANG-(1-12) by ACE (4, 24), and the actions are blocked with AT1 antagonist (21). Ferrario et al. (7) have provided more definitive evidence for the processing of ANG-(1-12) by a nonrenin pathway in recent experiments in bilaterally nephrectomized rats. In addition, cardiac chymase is also implicated in the processing of ANG-(1-12) into ANG II (21).
Perspectives and Significance
In the present study, we demonstrated that chronic intracerebroventricular administration of ANG-(1-12) antisera in (mRen2)27 transgenic hypertensive rats reduces SBP without a significant change in HR accompanied by a transient reduction in water intake and increased in plasma osmolality. We propose that in hypertensive rats, the novel peptide ANG-(1-12) may serve as a precursor to ANG II in brain pathways regulating blood pressure. The uncertainty over the cellular localization (neuronal vs. glial) of brain RAS components and their independence from the circulating system continues to be a major issue (9). A transmitter-like intracellular system may exist in neurons where both renin and Aogen have been localized, as well as a system involving extracellular components. ANG-(1-12) was processed to ANG II via ACE without renin involvement in other tissues and in serum (4, 7, 24). Thus, there is a possibility that ANG-(1-12) endogenous to the brain arises via renin-independent mechanisms to yield ANG II, which may explain the mismatch in the localization of renin and Aogen.
GRANTS
This work was supported by the National Institutes of Health Grants HL-51952 and HL-56973. Maria A. Garcia-Espinosa was supported in part by a Consortium for Southeastern Hypertension Control Warren Trust Fellowship during these studies. The support of Unifi, Greensboro, NC, and Farley-Hudson Foundation, Jacksonville, NC, is also acknowledged.
Present address: D. Ganten, Charite-University Medicine Berlin, Berlin, Germany.
REFERENCES
- 1.Arnold AC, Chappell MC, Ferrario CM, Diz DI. Exogenous angiotensin-(1-12) impairs baroreflex sensitivity in the solitary tract nucleus in anesthetized Sprague-Dawley rats. FASEB J 22: 1171, 2008. [Google Scholar]
- 2.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull 51: 119–128, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Casto R, Hilbig J, Schroeder G, Stock G. Atrial natriuretic factor inhibits central angiotensin II pressor responses. Hypertension 9: 473–477, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Chappell MC, Westwood BM, Pedengrass KD, Jessup JA, Ferrario CM. Distinct processing pathways for the novel peptide angiotensin-(1-12) in the serum and kidney of the hypertensive mRen2 Lewis rat. Hypertension 50: E139, 2007. [Google Scholar]
- 5.Diz DI, Jessup JA, Westwood BM, Bosch SM, Vinsant S, Gallagher PE, Averill DB. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin Exp Pharmacol Physiol 29: 473–482, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Diz DI, Kasper SO, Sakima A, Ferrario CM. Aging and the brain renin-angiotensin system: insights from studies in transgenic rats. Cleve Clin J Med 74 Suppl 1: S95–S98, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell AT, Sowers JR. Differential regulation of angiotensin-(1-12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol 296: H2242–H2247, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franci CR, Kozlowski GP, McCann SM. Water intake in rats subjected to hypothalamic immunoneutralization of angiotensin II, atrial natriuretic peptide, vasopressin, or oxytocin. Proc Natl Acad Sci USA 86: 2952–2956, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganong WF The brain and the renin-angiotensin system. In: Central Nervous System Mechanisms in Hypertension, edited by Buckley JP and Ferrario CM. New York: Raven, 1981, p. 283–291.
- 10.Garcia-Espinosa MA, Chappell MC, Ganten D, Ferrario CM, Diz DI. Chronic intracerebroventricular AT1 receptor blockade, but not renin inhibition, normalizes blood pressure in (mRen2)27 transgenic rats [Online]. FASEB J 22: 953.10, 2008. [Google Scholar]
- 11.Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Chappell MC, Ferrario CM, Diz DI. Angiotensin-(1-12) contributes to renin-independent angiotensin II activity in brain [Online]. Hypertension 52: e81, 2008. [Google Scholar]
- 12.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension 31: 699–705, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Iyer SN, Yamada K, Diz DI, Ferrario CM, Chappell MC. Evidence that prostaglandins mediate the antihypertensive actions of angiotensin-(1-7) during chronic blockade of the renin-angiotensin system. J Cardiovasc Pharmacol 36: 109–117, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, Angiotensin-12 [Ang-(1-12)], in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 294: H2614–H2618, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriguchi A, Brosnihan KB, Kumagai H, Ganten D, Ferrario CM. Mechanisms of hypertension in transgenic rats expressing the mouse Ren-2 gene. Am J Physiol Regul Integr Comp Physiol 266: R1273–R1279, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Moriguchi A, Tallant EA, Matsumura K, Reilly TM, Walton H, Ganten D, Ferrario CM. Opposing actions of angiotensin-(1-7) and angiotensin II in the brain of transgenic hypertensive rats. Hypertension 25: 1260–1265, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Muller S, Zhao Y, Brown TL, Morgan AC, Kohler H. TransMabs: cell-penetrating antibodies, the next generation. Expert Opin Biol Ther 5: 237–241, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 350: 1026–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S, Averill DB, Chappell MC, Diz DI, Brosnihan KB, Ferrario CM. Angiotensin receptors contribute to blood pressure homeostasis in salt-depleted SHR. Am J Physiol Regul Integr Comp Physiol 284: R164–R173, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept 43: 1–20, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res 82: 40–50, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest 117: 1088–1095, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senanayake PD, Moriguchi A, Kumagai H, Ganten D, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides 15: 919–926, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol 294: H2242–H2247, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 139: 191–202, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Bohlen HO, Albrecht D. The CNS renin-angiotensin system. Cell Tissue Res 326: 599–616, 2006. [DOI] [PubMed] [Google Scholar]