Abstract
Cellular dependence on growth factors for survival is developmentally programmed and continues in adult metazoans. Antigen-activated T cell apoptosis in the waning phase of the immune response is thought to be triggered by depletion of cytokines from the microenvironment. T cell apoptosis resulting from cytokine deprivation is mediated by reactive oxygen species (ROS), but their source and position in the apoptotic cascade is poorly understood. RNA interference approaches implicated the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in neglect-induced apoptosis in T cells. Using mice deficient for the catalytic subunit gp91phox to characterize the molecular link to activated T cell apoptosis, we show that gp91phox-deficient T (T−/−) cells generated mitochondrial superoxide but had diminished hydrogen peroxide production in response to neglect, which, in turn, regulated Jun N-terminal kinase–dependent Bax activation and apoptosis. Activated T−/− cells were distinguished by improved survival after activation by superantigens in vivo, adoptive transfers into congenic hosts, and higher recall responses after immunization. Thus, the NADPH oxidase may regulate adaptive immunity in addition to its previously well-characterized role in the innate response.
The regulated deletion of T cells is critical for the maintenance of homeostasis in the mammalian immune system (Goldrath and Bevan, 1999; Plas et al., 2002). Two pathways of cell death have been well characterized in mammalian cells (Hengartner, 2000): the extrinsic pathway, which is triggered by death receptors of the tumor necrosis factor receptor superfamily (Ashkenazi and Dixit, 1998), and the intrinsic pathway, which culminates in the release of apoptotic intermediates from mitochondria (Wang, 2001). The Bcl-2 family proteins are key intermediates in the latter because their activities principally converge on the regulation of mitochondrial integrity (Cheng et al., 2001; Youle and Strasser, 2008).
T cell apoptosis not only shapes the immune repertoire but is essential for immune responses to new and repeated antigenic challenges (Goldrath and Bevan, 1999; Plas et al., 2002). Apoptosis of excess T cells after antigen clearance provides the immune system functionality to manage multiple encounters with infectious organisms (Goldrath and Bevan, 1999). The undesired activation of autoreactive T cells accompanying this event is curtailed by Fas–Fas ligand-mediated activation-induced cell death (AICD; Lenardo et al., 1999). Although emerging evidence suggests that aspects of initial antigenic encounter may regulate the timing of T cell contraction, activated T cell death in many systems correlates with the depletion of cytokines after antigenic clearance (Vella et al., 1998; Strasser and Pellegrini, 2004). Activated T cell apoptosis during contraction is independent of both caspases and death receptor signaling and is rescued by exogenous cytokines (Vella et al., 1998; Nussbaum and Whitton, 2004; Yajima et al., 2006). However, only some effectors of T cell apoptosis are well characterized, and molecular interactions linking cytokine signaling to the regulation of Bax/Bak-BIM–mediated mitochondrial damage remain unresolved. In this context, the identification of reactive oxygen species (ROS) as the effectors of activated T cell apoptosis acquired considerable significance (Hildeman et al., 1999), but the absence of identified ROS sources precluded a detailed understanding of this process. In this paper, we address this gap in the understanding of the molecular cascade that regulates activated T cell persistence. We present evidence for the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase as a critical regulator of T cell function.
RESULTS AND DISCUSSION
Activated T cell neglect-induced death in vitro
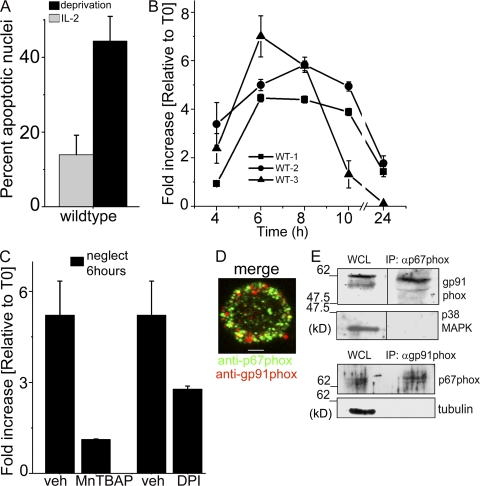
T cells activated in vitro using antibodies to CD3-CD28 as surrogate antigen were maintained in culture with the cytokine IL-2. IL-2 withdrawal (neglect) triggered cell death, which was characterized by apoptotic nuclear damage (Fig. 1 A), was inhibited by the broad-spectrum antioxidant Mn(III)tetrakis (4-benzoic acid)porphyrin chloride (MnTBAP), and was FasL independent (Fig. S1, A and B). Several derivatives of superoxide accumulate in T cells undergoing neglect-induced death (Hildeman, 2004). The H2O2-sensitive reagent CM-H2DCFDA (referred to as DCFDA) revealed a temporally regulated increase in levels of H2O2 in T cells cultured without IL-2 (Fig. 1 B). ROS accumulation peaked between 6 and 8 h (with some variation between mice), before eventual decline by 10 h, preceding nuclear damage by several hours (unpublished data). The surge was suppressed by MnTBAP or diphenyliodonium, a noncompetitive inhibitor of NADPH oxidase (Fig. 1 C). NADPH oxidase is a multisubunit complex that includes catalytic and regulatory subunits forming core elements of a complex that also includes adaptor proteins (Lambeth, 2004). Confocal imaging verified that the catalytic (gp91phox) and regulatory (p67phox) subunits are expressed (Fig. 1 D) and can be immunoprecipitated (Fig. 1 E) from activated T cells, confirming a recent result showing this complex in T cells (Jackson et al., 2004). Subsequently, NADPH oxidase function in T cell apoptosis was probed using RNA interference approaches.
Figure 1.
ROS regulate activated T cell neglect-induced death. (A) Activated T cells were cultured with or without (deprivation) IL-2 for 18 h before analysis of nuclear damage (mean ± SD of five experiments; P < 0.001). (B) T cells were cultured as in A and change in DCFDA fluorescence relative to T0 (onset of assay) assessed at the time points indicated. (C) Change in DCFDA fluorescence (relative to T0) in cells cultured with MnTBAP (P < 0.005) or diphenyliodonium (P < 0.05) or the vehicle control (veh), without IL-2 for 6 h (neglect). B and C show mean ± SD of three experiments. (D) Merged confocal image of an activated T cell (representative of 60 cells) stained for p67phox (green) and gp91phox (red). Bar, 2 µm. (E) Representative immunoblot of two immunoprecipitation analyses of gp91phox or p67phox in T cells. WCL, whole cell lysate. p38MAPK and tubulin established specificity. Black lines indicate that intervening lanes were spliced out.
NADPH oxidase regulates activated T cell death
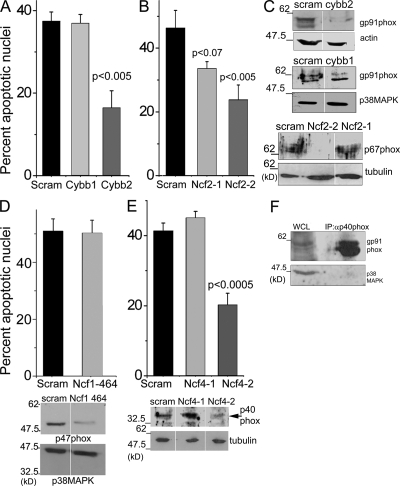
Retroviral infection with Cybb2 short hairpin RNA (shRNA), to deplete gp91phox protein in activated T cells, inhibited neglect-induced death (Fig. 2, A and C). Comparable inhibition was obtained with one other shRNA construct, Cybb3 (unpublished data). Depletion of p67phox by the shRNA Ncf 2-2 also attenuated the apoptotic response (Fig. 2, B and C). shRNA Cybb1 or Ncf2-1 to gp91phox or p67phox, respectively, did not deplete protein (Fig. 2 C) or protect from apoptosis (Fig. 2, A and B). p47phox/Ncf1 and p40phox/Ncf4 are adaptor proteins that can translocate p67phox to the membrane for a functional NADPH oxidase complex (Heyworth et al., 1991; Suh et al., 2006). Although shRNA to p47phox did not inhibit apoptosis (Fig. 2 D), the depletion of p40phox protein blunted cell death (Fig. 2 E). Furthermore, p40phox and gp91phox immunoprecipitated in a complex (Fig. 2 F), which also included the p67phox protein from T cells (Fig. 1 E).
Figure 2.
Apoptosis after shRNA-mediated disruption of NADPH oxidase. (A and B) Apoptotic damage in T cells infected with shRNA to gp91phox/Cybb (A) or p67phox/Ncf2 (B), or a scrambled control. (C) Immunoblots in T cells infected with the shRNA shown. (D and E) Apoptotic damage in cells infected with shRNA to p47phox (Ncf1) or scrambled control (D) or p40phox (Ncf4) and a scrambled control (E). Immunoblots are shown of T cells infected with the different shRNA. (F) In activated T cells, p40phox immunoprecipitated gp91phox. p38MAPK is the specificity control. White lines indicate that intervening lanes were spliced out. Data in all graphs are normalized to nuclear damage at T0 and are the mean ± SD of three independent experiments. P-values above the bars were calculated relative to the scrambled group.
Thus, perturbations of gp91phox, p67phox, or p40phox inhibited apoptosis, implicating this complex in the apoptotic response to cytokine deprivation. Consequently, events regulated by NADPH oxidase in activated T cell apoptosis were characterized in mice with a _targeted deletion of the catalytic subunit gp91phox.
gp91phox regulates premitochondrial events in the apoptotic cascade
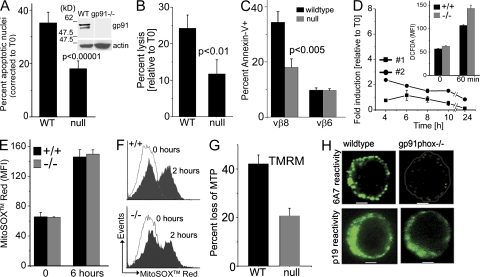
In T cells derived from mice with a null allele for gp91phox (T−/− cells), apoptotic nuclear damage and cell lysis were substantially reduced compared with WT T cells (T+/+) from age-matched controls (Fig. 3, A and B). Both CD4+ and CD8+ gp91phox−/− subsets were protected from apoptosis (Fig. S2, A and B). Immunoblot analysis confirmed that the gp91phox protein was not detected in mutant T cells (Fig. 3 A, inset). To assess if protection was restricted to cells activated in vitro, the apoptotic responses of T cells activated in response to staphylococcal enterotoxin B (SEB) in vivo was tested, as apoptosis of superantigen-activated T cells is ROS dependent (Hildeman et al., 1999). 2 d after intravenous injection of SEB, LN cells were harvested and cultured without cytokine. Apoptosis in the Vβ8+ T cells was substantially lower in cells derived from gp91phox−/− as compared with WT mice (Fig. 3 C). There were no differences in the nonreactive Vβ6+ subset (Fig. 3 C).
Figure 3.
Activated T−/− cells are protected from neglect-induced death. (A and B) T+/+ and T−/− day-2 activated cells were analyzed for nuclear damage (A) or lysis (B) after culture without IL-2 for 18 h. Data are normalized to values at T0 and mean ± SD of five experiments is shown. Inset shows immunoblot for gp91phox in lysates of WT and mutant (gp91−/−) T cells. White lines indicate that intervening lanes were spliced out. (C) T cells were harvested from mice injected 48 h before with SEB, cultured overnight in vitro, and stained with annexin V and antibodies to CD4, Vβ8, and Vβ6. Data are from four mice tested in two separate experiments. Error bars indicate the mean ± SD of four mice tested separately. (D) DCFDA oxidation in T−/− cells cultured without IL-2. Inset shows DCFDA oxidation in CD3-CD28–stimulated primary T cells. Data shown are representative of three mice. Error bars indicate the mean ± SD of replicates (triplicate wells). (E) MitoSOX red oxidation in cells cultured without IL-2 plotted as mean ± SD of three experiments. (F) Representative (n = 3) flow cytometry profile of MitoSOX red oxidation in CD3-CD28–stimulated activated T cells. (G) MTP loss assessed by TMRM in activated T cells cultured as in A. MTP loss in cells cultured without IL-2 relative to cells cultured with cytokine is shown as the mean ± SD of four experiments (P < 0.0005). (H) Confocal images (central plane) of activated T cells (representative of 50 cells from three independent experiments) from WT or mutant mice cultured without IL-2 for 6–8 h and stained with clone 6A7 (top) or clone p19 (bottom). Bars, 2 µm.
DCFDA oxidation was minimal in T−/− cells even after prolonged culture without cytokine (Fig. 3 D). However, gp91phox-independent DCFDA oxidation in response to CD3-CD28 stimulation was comparable in primary T+/+ and T−/− cells (Fig. 3 D, inset). The dye MitoSOX red was used to detect mitochondrial superoxide. Oxidation of MitoSOX red in freshly activated T cells (0 h) or after neglect (6 h) was comparable in both genotypes (Fig. 3 E). Similarly, MitoSOX red oxidation was independent of gp91phox in response to CD3-CD28 restimulation of activated T cells (Fig. 3 F). Thus, T−/− cells present a specific defect in neglect-induced NADPH oxidase-dependent ROS.
Loss of mitochondrial outer membrane integrity, an irreversible event in dying cells is characterized by a drop in mitochondrial transmembrane potential (MTP). Partitioning of the potentiometric dye TMRM into mitochondria is dependent on MTP, with reduced dye uptake indicating compromised organelle integrity. Both TMRM (Fig. 3 G) and Mitotracker red (Fig. S3 A) show that T+/+ cells, but not T−/− cells, undergo a substantial loss in MTP after cytokine deprivation.
Mitochondrial damage is regulated by proteins of the Bcl-2 family (Rathmell et al., 2002; Pellegrini et al., 2003). After receipt of an apoptotic stimulus, proapoptotic Bax undergoes a change in conformation, which can be monitored by an epitope-specific antibody (clone 6A7; Hsu and Youle, 1997). In live activated T cells from either background, reactivity to clone 6A7 was undetectable (not depicted), but 6A7 reactivity was revealed in T+/+ cells, but not T−/− cells, cultured without IL-2 (Fig. 3 H, top). An antibody that is not conformation specific (p19) gave comparable staining in both T+/+ and T−/− cells (Fig. 3 H, bottom), indicating that gp91phox did not suppress Bax expression.
Thus, neglect-induced Bax activation, loss of mitochondrial integrity, and caspase-3 processing (Fig. S3 B) were inhibited in activated T−/− cells, positioning gp91phox at an early step of the apoptotic cascade. Subsequent experiments investigated the mechanism by which gp91phox activity impinged on Bax activation.
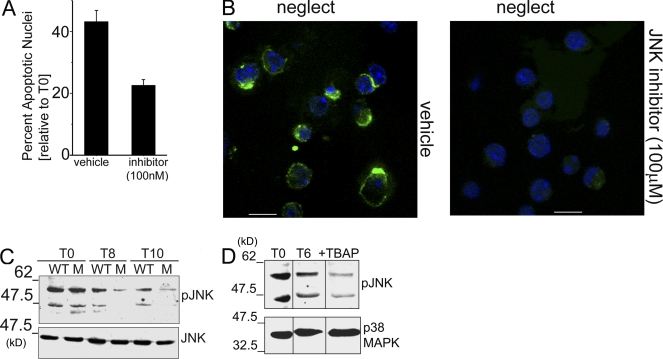
Sustained Jun N-terminal kinase (JNK) activation is ROS dependent in T cells
JNK can be activated by the inactivating oxidation of thiol protein tyrosine phosphatases (Kamata et al., 2005). T cell death was substantially blocked by the JNK inhibitor SP600125 (Fig. 4 A), which is indicative of a role for this kinase. JNK is reported to regulate Bcl-2 family proteins (Lei et al., 2002; Putcha et al., 2003). Consistently, Bax activation (6A7 reactivity) was suppressed by SP600125 at drug concentrations that inhibited apoptosis (Fig. 4 B). To ascertain modulation of JNK activity, its phosphorylation was assessed in activated T cells undergoing neglect. An antibody recognizing JNK phosphorylated on Thr183/Tyr185 showed that JNK was phosphorylated for an extended duration in T+/+ cells but not in T−/− cells (Fig. 4 C). Furthermore, MnTBAP prevented sustained JNK phosphorylation (Fig. 4 D). High levels of JNK phosphorylation at T0 in freshly activated cells may reflect their recent experience of cytokine-enriched environments. However, in the absence of exogenous cytokine, reduced JNK phosphorylation correlated with survival. These findings are consistent with ROS modulation of Bax function via the regulation of JNK activation and position gp91phox as a key source of ROS in this context.
Figure 4.
JNK is an intermediate in gp91phox-mediated apoptosis. (A) Nuclear damage in activated T cells cultured without IL-2 for 12–15 h with or without 100 nM SP600125 (P < 0.0001; mean ± SD of three independent experiments plotted). (B) Cells treated as in A at 6–8 h were stained with clone 6A7 (green) and Hoechst 33342 to mark nuclei (blue). Bars, 5 µm. Representative field views of three independent experiments are shown. (C) Whole cell lysates (WCL) of WT or gp91phox−/− mutant (M) T cells cultured in the presence or absence of IL-2 for 8 or 10 h, probed with an antibody to pJNK-Thr183/Tyr185 and reprobed for JNK. Immunoblot is representative of three separate experiments (D) Representative blot of two independent experiments of WCL of activated WT T cells (T0), cultured without IL-2 (T6) or with MnTBAP for 6 h, probed for pJNK-Thr183/Tyr185 and p38MAPK. Black lines indicate that intervening lanes were spliced out.
Activated T−/− cells were susceptible to AICD, dexamethasone, hydrogen peroxide, or etoposide (Fig S4), indicating that gp91phox specifically regulated the apoptotic response triggered by cytokine deprivation. To confirm these results, subsequent experiments assessed activated T cell survival in vivo.
T−/− cells display improved survival in vivo
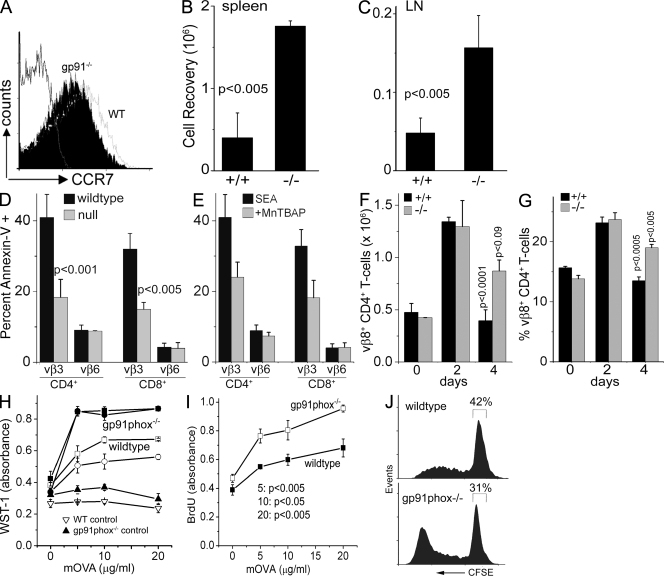
We assessed the recovery of in vitro activated T cells after adoptive transfer into congenic hosts. Expression of the homing antigens CD62L (not depicted) or CCR7, which regulate migration in vivo, was comparable (Fig. 5 A). T+/+ or T−/− cells activated in vitro (donors) were injected into the tail veins of immune-competent WT hosts and recoveries assessed after an interval of 7 d. Donors were distinguished from host lymphocytes (isolated from LNs or spleens) by the expression of a congenic (CD45.2+) cell surface marker. Compared with adoptively transferred T+/+ cells, the number of T−/− cells retrieved was significantly higher from both host spleen (Fig. 5 B) and LN (Fig. 5 C), indicating improved survival. The proliferative responses of WT and mutant T cells were comparable (not depicted).
Figure 5.
T−/− cells show improved survival in vivo and elevated recall responses to antigen. (A) Cell surface CCR7 on day 2 activated T+/+ and T−/− cells. (B and C) Total numbers of WT or gp91phox−/− T cells recovered, from host spleen or LN, 7 d after adoptive transfer. (D) LN from mice injected with SEA 48 h before harvest were stained with annexin V and antibodies to CD4, CD8, Vβ3, and Vβ6. (E) annexin V staining in LN T cells from mice injected with SEA under the cover of MnTBAP. (F and G) CD4+Vβ8+ subsets in LN of SEB-injected WT and null mice. P-values shown above the bars were calculated relative to day 2 and are derived from a minimum of three independent experiments. Error bars in B–G indicate the mean ± SD of three experiments. (H–J) Responses to mOVA in LN cells from WT or gp91phox null mice injected with mOVA or PBS (control) in vivo using WST-1 (H), BrdU uptake (I), or CFSE dye dilution (J). In H, p-values comparing responses in WT and null mice (four mice in each group) were the following: 5 µg/ml, P < 0.01; 10 µg/ml, P < 0.001; 20 µg/ml, P < 0.005. In I and J, the profiles represent three experiments. Error bars in H and I indicate the mean ± SD of replicates (triplicate wells).
Apoptosis of T cells activated in response to bacterial superantigens in vivo was assessed subsequently. Both WT and null mice injected with SEA on day 2 after injection present a comparable increase in Vβ3+ T cell number and low annexin V reactivity (unpublished data). Responses diverge on day 3, with increased annexin V+ Vβ3+ T cells detected in WT LNs as compared with mutant mice (Fig. 5 D). In both backgrounds, apoptosis was low in the Vβ6+ subset (Fig. 5 D). Consistently, apoptosis of Vβ3+ was inhibited in WT mice injected with SEA and MnTBAP (Fig. 5 E). Similarly, in both genotypes, CD4+Vβ8+ cells showed a comparable increase in response to SEB injection (Fig. 5, F and G). However, this subset persisted in greater numbers in gp91phox−/− mice on day 4 at a time when numbers had begun to show a decline in WT mice (Fig. 5, F and G). Again, there were no accompanying changes in the Vβ6+ subset in either genotype (unpublished data).
T−/− cells have improved recall response to antigen
After antigen clearance, the majority of antigen-reactive T cells are deleted with a small proportion set aside to form T cell memory. Although challenged by recent studies (Kaech et al., 2003; Badovinac et al., 2004), contraction of activated T cells is thought to be triggered by competition for limiting amounts of cytokine in the waning phase of the immune response. Because gp91phox regulated activated T cell apoptosis, its absence should manifest in the persistence of antigen-reactive cells, as T−/− cells would progress in greater numbers to making memory. To test this, T cell recall responses were assessed after immunization.
gp91phox−/− and WT mice were immunized with maleylated ovalbumin (mOVA) and recall responses assessed 12–15 d later using different assays of proliferation. Basal response to antigen was negligible in unimmunized animals of either genotype (Fig. 5 H, triangles). Antigen-specific proliferative responses, using WST-1 or BrdU uptake, were consistently elevated in T cells from draining LN of gp91phox−/− mice compared with WT animals (Fig. 5, H and I). Tracking dilution of the CFSE label in T cells loaded with dye before culture with antigen revealed higher numbers of CFSE+ T−/− cells in every division cycle. We also noted a small but consistent increase in the number of T cells entering division in null mice (Fig. 5 J). These data are in concordance with the survival of a higher number of mOVA responder T cells in mutant mice. The initial representation of effector-memory cells (CD44high CD62Llow CD25neg) in input populations was comparable in WT and mutant mice (unpublished data).
NADPH oxidase is a central well characterized component of the innate immune response against fungal and bacterial infections (Bjorgvinsdottir et al., 1997; Blanchard et al., 2003). In this paper, we characterize an apoptotic cascade triggered by NADPH oxidase in activated T cells. Cytokine deprivation–induced activated T cell apoptosis in vitro mimics several features of activated T cell death in vivo, permitting analysis of the molecular mechanisms underlying this process (Hildeman et al., 1999; Vig et al., 2004). Using this approach, we describe a mechanism linking ROS and Bcl-2 family proteins. We propose that NADPH oxidase is the interacting redox partner responsive to cytokine-dependent survival cues in activated T cells because cytokine withdrawal triggered a gp91phox-associated ROS surge. Investigations into the spatiotemporal organization of gp91phox and its regulation by cytokines in T cells are ongoing. We report a specific defect in the generation of hydrogen peroxide (indicated by DCFDA oxidation) in response to cytokine deprivation, although mitochondrial superoxide production was not inhibited in T−/− cells. Although it may be argued that mitochondrial superoxide is not required in this pathway, it is also possible that the two oxidative systems interact functionally, with mitochondrial superoxide serving to propagate the apoptotic cascade. However, selective perturbation of the mitochondrial oxidative system and detailed dissection of the underlying mechanism is linked to the development of more specific ROS indicators and inhibitors. Comparable TCR stimulation-induced oxidation of DCFDA in primary T cells and DHE and MitoSOX red in activated T cells established that T−/− cells do not have a generalized defect in ROS production.
shRNA-mediated disruptions supported a role for NADPH oxidase in this death pathway and allayed concerns that resistance of gp91phox−/− cells to apoptosis resulted from the altered development of these cells. Thus, shRNA-mediated depletion of gp91phox, the activator p67phox, or the adaptor p40phox inhibited neglect-induced death in activated T cells. Activated T−/− cells displayed improved survival after adoptive transfer into immune-competent hosts or after in vivo activation by bacterial superantigens in assays where cells were not manipulated in culture. Furthermore, increased viability and proliferation of T−/− cells relative to WT cells, in the recall response to antigen, was consistent with compromised contraction of T−/− cells in vivo. Notably, primary responses to immunization were not elevated in mutant mice. Ongoing experiments are designed to assess if antigen reactivity is maintained in mutant animals at extended intervals after the first immunization.
Activated T cell death requires Bax-Bak and BH3-only proteins to disrupt mitochondrial function (Rathmell et al., 2002; Fischer et al., 2008; Youle and Strasser, 2008). Bax activation and loss of MTP were inhibited in T−/− cells. We propose that JNK is the redox-sensitive intermediate, as its sustained activation was prevented by the synthetic antioxidant MnTBAP and blunted in T−/− cells, and inhibiting JNK activity blocked Bax activation and apoptosis.
Although the role of the NADPH oxidase in the regulation of the innate response is well characterized, these data offer new insight into a novel role for this oxidase in the coordination of the adaptive immune response. A more comprehensive analysis of the organization of this complex in T cells is needed to understand its response to cytokine-dependent cues and recruitment for activated T cell attrition at the end of the immune response.
MATERIALS AND METHODS
Animals.
C57BL/6J, B6/SJL, gp91phox−/− (B6.129S6-Cybbtm1Din/J), p47phox−/− (B6(Cg)-Ncf1m1J/J), and B6Smn.C3-Faslgld/J mice strains were obtained from The Jackson Laboratory. Experiments were approved by the Institutional Animal Ethics Committees of the National Center for Biological Sciences (Bangalore, India).
Reagents.
Antibodies were procured from the following sources: gp91phox, BD; gp91phox, p40phox, p67phox, p38MAPK, and p19-Bax, Santa Cruz Biotechnology, Inc.; caspase-3 and phospho-JNK, Cell Signaling Technology; and 6A7, tubulin (NeoMarkers), and CCR7, CD25, and CD45.2 (eBioscience). All shRNA sets were obtained from OriGene Technologies. All other reagents were obtained from Sigma-Aldrich, EMD, or Invitrogen.
T cell activation.
T cell subsets purified from mouse spleens using Mag Cellect isolation kits (R&D Systems) were stimulated with α-CD3–α-CD28–coated beads (Invitrogen). Cells were separated from beads after 48 h and either continued in culture with 1 µg/ml IL-2 (R & D Systems) or used in assays of neglect wherein cells were cultured (0.3 × 106/ml) with or without IL-2 for 16–18 h.
Retroviral delivery of shRNA.
Retroviruses were generated and cells infected according to standard procedures. Infected T cells were switched to media supplemented with 1 µg/ml IL-2, 2 ng/ml IL-7, and 1 µg/ml puromycin for 3 d (day 6 from onset of culture). Live cells were selected on day 7, continued in culture with IL-2 for another 18–24 h, and were then used in assays of neglect. Loss of protein expression was assessed on day 7 by Western blot analysis of cell lysates.
Assays of apoptotic damage and intracellular ROS.
Apoptotic damage was assessed after 18–24 h using 1 µg/ml Hoechst 33342, annexin V–FITC, or the uptake of propidium iodide. Cells were stained with annexin V–FITC for 15 min at room temperature, washed in excess of buffer, and analyzed. MTP was measured by staining cells with 150 nM TMRM in medium for 30 min at 37°C. Cells were washed once in excess medium and analyzed by flow cytometry.
T cells were incubated with 2 µM CM-H2DCFDA in RPMI at 37°C for 30 min or 5 µM MitoSOX red for 10 min at 37°C. At the end of incubation, cells were washed twice and immediately analyzed by flow cytometry. Data are presented as the percentage of change in mean fluorescence intensity for each time point with respect to the initial fluorescence intensity (time = 0 h).
Immunoprecipitation.
6–8 × 106 cells were lysed for 30 min at 4°C in 1% CHAPS buffer supplemented with protease inhibitors (Sigma-Aldrich). gp91phox was immunoprecipitated using 1% NP-40. Immune complexes were precipitated for 2 h at 4°C using Sepharose G plus beads on a rotational cell mixer. Beads bound to complexes were washed five times with ice-cold PBS at 1,700 rpm. Finally, beads were boiled in SDS lysis buffer for 10 min before Western blot analysis.
Confocal imaging.
T cells were fixed with 1% paraformaldehyde and permeabilized with 0.2% NP-40 for 5 min at room temperature. 5% BSA for 1 h was used for blocking and antibody incubations were performed for 1 h on ice. For 6A7 staining, 0.2% CHAPS for 15 min on ice was used for permeabilization, followed by 5% BSA for 1 h at room temperature. Cells were imaged on an FV1000 (Olympus) at 60× with a 1.4 NA objective lens and Z-stacks were acquired at a step size of 0.8 µm.
In vivo responses.
WT (C57BL/6J) and gp91phox−/− mice were immunized subcutaneously with 100 µg mOVA (Abraham et al., 1995) in CFA. 10–12 d later, 106 cells/ml from draining LNs were cultured in 0.2 ml in 96-well plates with or without mOVA for 72 h. Mice were injected with 40 ng SEA intravenously. Cells from draining LN were harvested on day 3 and stained for Vβ subsets, CD4 and CD8 subsets, and annexin V–FITC. In other assays, LN were harvested on day 2 from mice injected with 100 µg SEB, cultured overnight in complete medium without cytokine, and apoptosis was assessed using annexin V–FITC in the Vβ subsets of CD4+ and CD8+ T cells. Proliferation was measured using WST-1 cell proliferation kit or the BrdU colorimetric kit (Roche) as per the manufacturer’s instructions. Cells were loaded with 0.5 µM CFSE in PBS at ambient temperature for 10 min, followed by three washes in chilled medium to remove excess dye. Dilution of dye after culture in vitro was assessed by flow cytometry.
For adoptive transfers, 10 × 106 activated T cells (CD45.2+) were injected into the tail vein of congenic strains (CD45.1+) of mice. 7 d after injection, spleen and LNs of host were analyzed for the donor and host cell populations. The number of donor CD45.2+ T cells recovered from each spleen and LN preparation estimated and the data plotted relative to the number injected (10 × 106).
Statistical analysis and data presentation.
In immunoblots, the spacer indicates a splice line between separate sections of the same membrane. All data are presented as mean ± SD derived from a minimum of three to five independent experiments unless stated otherwise. Statistical significance was calculated using an f-test and a two-population Student’s t test.
Online supplemental material.
Fig. S1 shows that activated T cell neglect-induced death is dependent on ROS but independent of Fas-FasL. Fig. S2 shows that CD4+ and CD8+ T cell subsets from gp91phox−/− mice are protected from apoptosis. Fig. S3 shows that mitochondrial integrity is maintained in gp91phox−/− T cells as compared with WT T cells and that processing of caspase3 is diminished in T−/− cells. Fig. S4 shows the susceptibility of T−/− cells to apoptosis triggered by a variety of stimuli. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082851/DC1.
Acknowledgments
We are grateful to Sankar Ghosh (Columbia University, NY, NY), Veronica Rodrigues (National Centre for Biological Sciences [NCBS] Bangalore and Department of Biological Sciences, Mumbai, India), G.V. Shivashankar (NCBS Bangalore), and Satyajit Rath (National Institute of Immunology, New Delhi, India) for helpful suggestions and discussion. We thank G. Mohan (Animal Facility at NCBS) for assistance with experiments in this study.
We acknowledge the NCBS Central Imaging and Flow Cytometry Facility, funded by the Department of Science and Technology, India (grant SR/S5/NM-36/2005 to the Centre of Nanotechnology and grant 43/2003-SF), and the Wellcome Trust, UK. The study is funded by an International Senior Research Fellowship in Biomedical Sciences in India, by the Wellcome Trust, UK, and by a grant from DST, India to A. Sarin. D. Purushothaman acknowledges financial support from the Council of Scientific and Industrial Research, India.
The authors have no competing financial interests.
Footnotes
Abbreviations used: AICD, activation-induced cell death; JNK, Jun N-terminal kinase; MnTBAP, Mn(III)tetrakis (4-benzoic acid)porphyrin chloride; mOVA, maleylated ovalbumin; MTP, mitochondrial transmembrane potential; NADPH, nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; SEB, staphylococcal enterotoxin B; shRNA, short hairpin RNA.
References
- Abraham R., Singh N., Mukhopadhyay A., Basu S., Bal V., Rath S. 1995. Modulation of immunogenicity and antigenicity of proteins by maleylation to _target scavenger receptors on macrophages.J. Immunol. 154:1–8 [PubMed] [Google Scholar]
- Ashkenazi A., Dixit V.M. 1998. Death receptors: signaling and modulation.Science. 281:1305–1308 [DOI] [PubMed] [Google Scholar]
- Badovinac V.P., Porter B.B., Harty J.T. 2004. CD8+ T cell contraction is controlled by early inflammation.Nat. Immunol. 5:809–817 [DOI] [PubMed] [Google Scholar]
- Bjorgvinsdottir H., Ding C., Pech N., Gifford M.A., Li L.L., Dinauer M.C. 1997. Retroviral-mediated gene transfer of gp91phox into bone marrow cells rescues defect in host defense against aspergillus fumigatus in murine X-linked chronic granulomatous disease.Blood. 89:41–48 [PubMed] [Google Scholar]
- Blanchard T.G., Yu F., Hsieh C.-L., Redline R.W. 2003. Severe inflammation and reduced bacteria load in murine Helicobacter infection caused by lack of phagocyte oxidase activity.J. Infect. Dis. 187:1609–1615 [DOI] [PubMed] [Google Scholar]
- Cheng E.H.-Y.A., Wei M.C., Weiler S., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J. 2001. BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis.Mol. Cell. 8:705–711 [DOI] [PubMed] [Google Scholar]
- Fischer S.F., Belz G.T., Strasser A. 2008. BH3-only protein Puma contributes to death of antigen-specific T cells during shutdown of an immune response to acute viral infection.Proc. Natl. Acad. Sci. USA. 105:3035–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A.W., Bevan M.J. 1999. Selecting and maintaining a diverse T-cell repertoire.Nature. 402:255–262 [DOI] [PubMed] [Google Scholar]
- Hengartner M.O. 2000. The biochemistry of apoptosis.Nature. 407:770–776 [DOI] [PubMed] [Google Scholar]
- Heyworth P.G., Curnutte J.T., Nauseef W.M., Volpp B.D., Pearson D.W., Rosen H., Clark R.A. 1991. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558.J. Clin. Invest. 87:352–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman D.A. 2004. Regulation of T-cell apoptosis by reactive oxygen species.Free Radic. Biol. Med. 36:1496–1504 [DOI] [PubMed] [Google Scholar]
- Hildeman D.A., Mitchell T., Teague T.K., Henson P., Day B.J., Kappler J., Marrack P.C. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis.Immunity. 10:735–744 [DOI] [PubMed] [Google Scholar]
- Hsu Y.-T., Youle R.J. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family.J. Biol. Chem. 272:13829–13834 [DOI] [PubMed] [Google Scholar]
- Jackson S.H., Devadas S., Kwon J., Pinto L.A., Williams M.S. 2004. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation.Nat. Immunol. 5:818–827 [DOI] [PubMed] [Google Scholar]
- Kaech S.M., Tan J.T., Wherry E.J., Konieczny B.T., Surh C.D., Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells.Nat. Immunol. 4:1191–1198 [DOI] [PubMed] [Google Scholar]
- Kamata H., Honda S.-i., Maeda S., Chang L., Hirata H., Karin M. 2005. Reactive oxygen species promote TNF[alpha]-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases.Cell. 120:649–661 [DOI] [PubMed] [Google Scholar]
- Lambeth J.D. 2004. NOX enzymes and the biology of reactive oxygen.Nat. Rev. Immunol. 4:181–189 [DOI] [PubMed] [Google Scholar]
- Lei K., Nimnual A., Zong W.-X., Kennedy N.J., Flavell R.A., Thompson C.B., Bar-Sagi D., Davis R.J. 2002. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-Terminal Kinase.Mol. Cell. Biol. 22:4929–4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M., Chan F.K.-M., Hornung F., McFarland H., Siegel R., Wang J., Zheng L. 1999. Mature T lymphocyte apoptosis; immune regulation in a dynamic and unpredictable antigenic environment.Annu. Rev. Immunol. 17:221–253 [DOI] [PubMed] [Google Scholar]
- Nussbaum A.K., Whitton J.L. 2004. The contraction phase of virus-specific CD8+ T cells is unaffected by a pan-caspase inhibitor.J. Immunol. 173:6611–6618 [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Belz G., Bouillet P., Strasser A. 2003. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim.Proc. Natl. Acad. Sci. USA. 100:14175–14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas D.R., Rathmell J.C., Thompson C.B. 2002. Homeostatic control of lymphocyte survival: potential origins and implications.Nat. Immunol. 3:515–521 [DOI] [PubMed] [Google Scholar]
- Putcha G.V., Le S., Frank S., Besirli C.G., Clark K., Chu B., Alix S., Youle R.J., LaMarche A., Maroney A.C., Johnson E.M. 2003. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis.Neuron. 38:899–914 [DOI] [PubMed] [Google Scholar]
- Rathmell J.C., Lindsten T., Zong W.X., Cinalli R.M., Thompson C.B. 2002. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis.Nat. Immunol. 3:932–939 [DOI] [PubMed] [Google Scholar]
- Strasser A., Pellegrini M. 2004. T-lymphocyte death during shutdown of an immune response.Trends Immunol. 25:610–615 [DOI] [PubMed] [Google Scholar]
- Suh C.-I., Stull N.D., Li X.J., Tian W., Price M.O., Grinstein S., Yaffe M.B., Atkinson S., Dinauer M.C. 2006. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcγIIA receptor-induced phagocytosis.J. Exp. Med. 203:1915–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella A.T., Dow S., Potter T.A., Kappler J., Marrack P. 1998. Cytokine-induced survival of activated T cells in vitro and in vivo.Proc. Natl. Acad. Sci. USA. 95:3810–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M., Srivastava S., Kandpal U., Sade H., Lewis V., Sarin A., George A., Bal V., Durdik J.M., Rath S. 2004. Inducible nitric oxide synthase in T cells regulates T cell death and immune memory.J. Clin. Invest. 113:1734–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. 2001. The expanding role of mitochondria in apoptosis.Genes Dev. 15:2922–2933 [PubMed] [Google Scholar]
- Yajima T., Yoshihara K., Nakazato K., Kumabe S., Koyasu S., Sad S., Shen H., Kuwano H., Yoshikai Y. 2006. IL-15 regulates CD8+ T cell contraction during primary infection.J. Immunol. 176:507–515 [DOI] [PubMed] [Google Scholar]
- Youle R.J., Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death.Nat. Rev. Mol. Cell Biol. 9:47–59 [DOI] [PubMed] [Google Scholar]