Abstract
Background
Alcohol dependence is characterized by excessive alcohol consumption, loss of control over intake, and the presence of a withdrawal syndrome, which includes both motivational and physical symptoms. Similar to human alcoholics, ethanol-dependent animals display enhanced anxiety-like behaviors and enhanced ethanol self-administration during withdrawal, effects hypothesized to result from a dysregulation of corticotropin-releasing factor (CRF) stress systems. Here, we used an animal model of ethanol dependence to test the effects of CRF1 receptor antagonists on excessive ethanol self-administration in dependent rats.
Methods
Wistar rats, trained to orally self-administer ethanol, were exposed intermittently to ethanol vapors to induce ethanol dependence. Nondependent animals were exposed to control air. Following a 2-hour period of withdrawal, dependent and nondependent animals were systemically administered antalarmin, MJL-1-109-2, or R121919 (CRF1 antagonists) and ethanol self-administration was measured.
Results
The nonpeptide, small molecule CRF1 antagonists selectively reduced excessive self-administration of ethanol in dependent animals during acute withdrawal. The antagonists had no effect on ethanol self-administration in nondependent rats.
Conclusions
These data demonstrate that CRF1 receptors play an important role in mediating excessive ethanol self-administration in dependent rats, with no effect in nondependent rats. CRF1 antagonists may be exciting new pharmacotherapeutic _targets for the treatment of alcoholism in humans.
Keywords: Alcohol, dependence, withdrawal, corticotropin-releasing factor, self-administration, rat
Alcoholism, defined as a chronic relapsing disorder characterized by compulsive use of alcohol and a loss of control over intake, is one of the leading causes of premature death in America (Stinson et al 1993). As dependence develops, there is a shift from controlled use to uncontrolled, excessive consumption of alcohol, which is paralleled by a shift from positive to negative reinforcement being the driving force mediating continued alcohol use (Koob 2003; Koob et al 2004). Initial alcohol use is driven mainly by the positive effects of alcohol, such as euphoria and tension reduction. However, with chronic alcohol consumption, cessation of use is often accompanied by negative withdrawal symptoms, such as increased anxiety and depression. Alleviation of these negative affect states (i.e., negative reinforcement) then becomes a major driving force for continued alcohol consumption (Hershon 1977; Koob 2003). Similar to human alcoholics, ethanol-dependent animals display enhanced anxiety-like behaviors and excessive ethanol self-administration during periods of withdrawal (Baldwin et al 1991; File et al 1989; O’Dell et al 2004; Roberts et al 2000; Valdez et al 2002b), providing a model system for studying the motivational changes associated with ethanol dependence. A better understanding of the neurobiological basis underlying ethanol reinforcement will be valuable for understanding the progression of ethanol dependence and developing novel pharmacotherapies for treatment.
Endogenous brain corticotropin-releasing factor (CRF) has been implicated in the motivational changes associated with ethanol dependence (Menzaghi et al 1994; Valdez and Koob 2004). CRF, a 41 amino-acid residue peptide, is distributed throughout the brain, with high concentrations of cell bodies in the paraventricular nucleus of the hypothalamus and in areas of the extended amygdala (Bloom et al 1982). CRF is involved in mediating the physiological and behavioral responses to stress (Dunn and Berridge 1990; Vale et al 1981). Central administration of CRF mimics the behavioral responses to stress in rodents (Britton et al 1985; Dunn and File 1987; Sutton et al 1982; Swerdlow et al 1986), while administration of CRF antagonists reverses these effects (Britton et al 1986; Swerdlow et al 1989; Zorrilla et al 2002). CRF exerts its physiological and behavioral effects via both the hypothalamic-pituitary-adrenal (HPA) system, as well as an extrahypothalamic system which includes regions of the extended amygdala (Alheid and Heimer 1995; Dunn and Berridge 1990). The cellular effects of CRF are mediated by two types of high-affinity receptors, CRF1 (Chang et al 1993; Chen et al 1993; Perrin et al 1993) and CRF2 (Lovenberg et al 1995). Both receptors belong to the B1 subgroup of G-protein coupled receptors and induce an increase in intracellular cyclic adenosine monophosphate (cAMP) upon ligand binding (Chen et al 1986; Giguere et al 1982). Genetic and pharmacological evidence indicates that the CRF1 receptor is involved in mediating anxiety-like behavior in animals (Heinrichs et al 1997; Liebsch et al 1995; McElroy et al 2002; Smith et al 1998; Timpl et al 1998). However, the role of the CRF2 receptor in mediating anxiety-related behaviors is less well understood. Indeed, some studies suggest that CRF2 is more associated with appetite regulation and antistress-like effects (Pelleymounter et al 2000; Spina et al 1996; Valdez et al 2002a, 2003a).
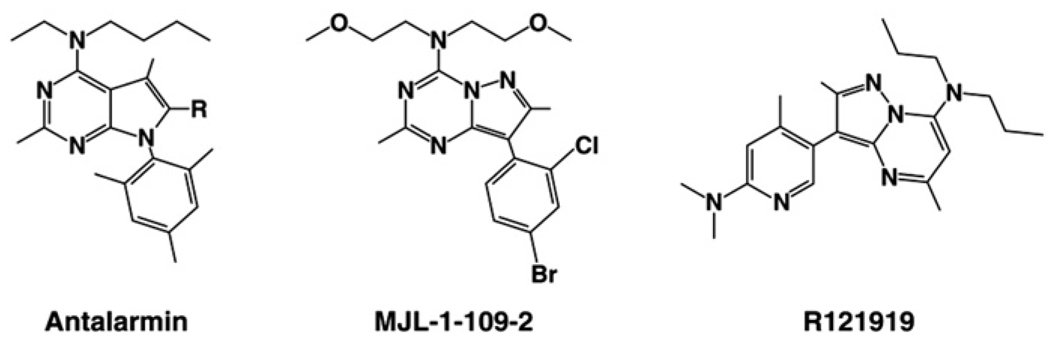
The increased anxiety-like behaviors during ethanol withdrawal are believed to result, in part, from increased levels of extracellular CRF in extrahypothalamic brain regions (Merlo Pich et al 1995; Olive et al 2002), and central administration of CRF antagonists can attenuate these behaviors (Baldwin et al 1991; Rassnick et al 1993; Valdez et al 2002b, 2003b). Ethanol-dependent animals also orally self-administer increased levels of ethanol during periods of withdrawal (O’Dell et al 2004; Roberts et al 2000; Valdez et al 2002b), effects which also likely result from enhanced CRF signaling in extrahypothalamic brain regions (Valdez et al 2002b). However, the specific receptor subtype of CRF involved in mediating these effects remains unknown. Because CRF1 receptors play an important role in mediating anxiety-like behaviors (Heinrichs et al 1997; Liebsch et al 1995; McElroy et al 2002; Smith et al 1998; Timpl et al 1998; Zorrilla and Koob 2004), it was hypothesized that CRF1 receptors also mediate the enhanced ethanol self-administration during withdrawal in dependent animals. Using an intermittent ethanol vapor exposure paradigm to induce ethanol dependence in male Wistar rats (O’Dell et al 2004), we show here that three separate, nonpeptide CRF1 receptor antagonists, antalarmin, MJL-1-109-2, and R121919 (Figure 1), selectively reduce ethanol self-administration in ethanol-dependent animals during acute withdrawal. Importantly, none of these antagonists altered ethanol intake in nondependent rats. Because these drugs selectively reduce ethanol intake in dependent animals but not nondependent animals, CRF1 antagonists may be a valuable new pharmacological treatment for alcoholism in humans.
Figure 1.
Chemical structures for antalarmin, MJL-1-109-2, and R121919.
Methods and Materials
Animals
Fifty-six adult male Wistar rats weighing 180 to 200 grams at the start of the experiment were obtained from Charles River Laboratory (Kingston, New York). Animals were housed two to three per cage with food and water available ad libitum. Lights were on a 12-hour light/dark cycle, lights on at 6:00 am. All procedures met the guidelines of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Drugs
Ethanol (10% wt/vol) was prepared using 95% ethyl alcohol and water. The CRF1 receptor antagonists antalarmin (N-butyl-N-ethyl-[2,5,6,-trimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo[2,3-d] pyrimidin4-yl]-amine; Ki = 1.0; cLogP = 7.0), R121919 (3-[6-(dimethylamino)-4-methyl-pyrid-3-yl]-2,5-dimethyl-N,N-dipropyl-pyrazolo[ 2,3-a]pyrimidin-7-amine, also referred to as NBI-30775; Ki = 3.5; cLogP = 4.8), and MJL-1-109-2 (pyrazolo[1,5-a]-1,3,5-triazin-4-amine,8-[4-(bromo)-2-chlorophenyl]-N,N-bis(2-methoxyethyl)-2,7-dimethyl-(9Cl); Ki = 1.9, cLogP = 3), were synthesized by Drs. Kenner Rice and Mei-Jing Lee at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Laboratory of Medicinal Chemistry) (Zorrilla and Koob 2004). Antalarmin was synthesized using modifications of the method of Chen (1994) and crystallized as described (Bornstein et al 1998). The CRF1 receptor antagonists R121919 (Chen et al 2004) and MJL-1-109-2 (Jagoda et al 2003) were synthesized as described previously. The drugs were administered either subcutaneously (R121919 at 2 mL/kg) or intraperitoneally (IP) (antalarmin at 4 mL/kg and MJL-1-109-2 at 4 mL/kg). As antalarmin and MJL-1-109-2 are not as soluble as R121919, larger volumes were administered and delivered intraperitoneally as opposed to subcutaneously. These drugs cross the blood-brain barrier and block both the peripheral and central effects of CRF (Zorrilla and Koob 2004). Pharmacologically significant brain and plasma levels of antalarmin (Zorrilla et al 2002), R121919 (Chen et al 2004), and DMP696, an analog of MJL-1-109-2, (Yu-Wen et al 2003) have been reported. Receptor occupancy data for MJL-1-109-2 (Jagoda et al 2003) and R121919 (Heinrichs et al 2002) have also been reported previously. Vehicle for MJL-1-109-2 and R121919 was 20% wt/vol hydroxypropyl-β-cyclodextrin (HBC) (pH = 4.5) (Cargill Inc, Cedar Rapids, Iowa). Antalarmin was administered in .5% wt/vol carboxymethylcellulose (CBC) (pH = 4.5) (Sigma Chemical, St. Louis, Missouri). Drugs were systemically administered 1 hour (80 minutes for antalarmin) prior to self-administration testing.
Operant Ethanol Self-Administration
Ethanol self-administration was established in standard operant chambers (Coulbourn Instruments, Allentown, Pennsylvania) that were housed in sound-attenuated ventilated cubicles. Animals were trained to orally self-administer ethanol or water in a concurrent, two-lever, free-choice contingency. Syringe pumps (Razel Scientific Instruments, Stamford, Connecticut) dispensed ethanol or water into two stainless steel drinking cups mounted 4.0 cm above the grid floor in the middle of one side panel. Two retractable levers were located 4.5 cm to either side of the drinking cups. Fluid delivery and recording of operant self-administration were controlled by a microcomputer. Lever presses were not recorded during the .5 seconds in which the pumps were active. A continuous reinforcement (fixed ratio 1) schedule was used such that each response resulted in delivery of 0.1 mL of fluid.
Rats were trained to press a lever for ethanol using a modification of the sweetened solution fading procedure (Samson 1986). No fluid or food restriction period was employed. This training method culminates in rats consuming sufficient unsweetened 10% ethanol to produce pharmacologically relevant blood alcohol levels (Roberts et al 1999). Rats were initially trained to press a lever for a sweetened solution containing glucose (3% wt/vol) and saccharin (.125% wt/vol) (Sigma Chemical). Ethanol self-administration was initiated by adding ethanol (10%) to the sweetened solution for 4 to 5 days, followed by 4 to 5 days of 10% ethanol + .125% saccharin only. Finally, the animals received the 10% ethanol solution alone. During all training sessions, rats were also allowed to press for water on the opposite lever. The lever that produced water or ethanol was altered daily to prevent selecting rats biased toward one lever. The animals received daily (5 days per week) 30-minute access to ethanol for 20 to 25 days until stable rates of intake were observed. The criterion for stable baseline intake was ±20% across three consecutive sessions. Testing was performed at 8:00 am (lights on at 6:00 am).
Ethanol Vapor Chamber Procedure
To induce dependence, two standard rat cages were housed in separate, sealed, clear plastic chambers into which ethanol vapor was intermittently introduced. Ethanol vapor was created by dripping 95% ethanol (Central Stores, San Diego, California) into 2000-mL Erlenmeyer vacuum flasks (Fisher Scientific) kept at 50°C on a warming tray. Air was blown over the bottom of the flask at 11 L/min to vaporize the ethanol. The concentration of ethanol vapor delivered was adjusted by varying the rate at which ethanol was pumped into the flasks and ranged from 22 to 27 mg/L. The chambers were connected to a timer that would turn the ethanol vapor on (4:00 pm) and off (6:00 am) every day, allowing animals to receive ethanol vapor for 14 hours and control air for 10 hours (O’Dell et al 2004). Blood samples were taken at 6:00 am for blood alcohol level (BAL) determination every 3 days during vapor exposure. Tail blood (.5 mL) was collected into heparinized Eppendorf tubes (Fisher Scientific). After centrifugation, the plasma was extracted with trichloroacetic acid and assayed for ethanol content using the nicotinamide adenine dinucleotide-alcohol dehydrogenase (NAD-ADH) enzyme spectrophotometric method (Sigma Chemical). _target BALs were 150 to 200 mg/dL across a 4-week exposure time. This paradigm has been shown to produce physical dependence, as shown by the appearance of somatic withdrawal signs on removal from the chambers (O’Dell et al 2004; Roberts et al 2000).
Experimental Procedure
Once baseline ethanol self-administration was attained, rats were subsequently transferred to ethanol (dependent) or control (nondependent) vapor chambers for 4 weeks of intermittent vapor exposure. At the end of the 4-week period of dependence induction, rats were retested for ethanol self-administration following a 2-hour withdrawal period from ethanol vapors. At this time point, dependent animals display a significant increase in ethanol lever pressing (Valdez et al 2002b). A Latin square design was used to test the effects of antalarmin (0, 10, or 20 mg/kg), R121919 (0, 5, 10, and 20 mg/kg), or MJL-1-109-2 (0, .6, 1.25, or 5.0 mg/kg) on ethanol self-administration in dependent and nondependent rats. Doses were based on previous studies demonstrating the anxiolytic properties of these drugs (Zorrilla and Koob 2004). Systemic injections were given 1 hour (80 minutes for antalarmin) prior to testing in the operant self-administration chambers. The test sessions were separated by 5 to 6 days, during which time the animals were returned to vapor chambers.
Statistical Analyses
Data were analyzed using a mixed two-way analysis of variance (ANOVA) with vapor treatment (ethanol or control) the between-subjects factor and antagonist dose the within-subjects factor. Tukey’s honestly significant difference (HSD) test was used for post hoc analysis of individual means.
Results
Effects of the CRF1 Specific Antagonist Antalarmin on Ethanol and Water Self-Administration in Dependent and Nondependent Rats
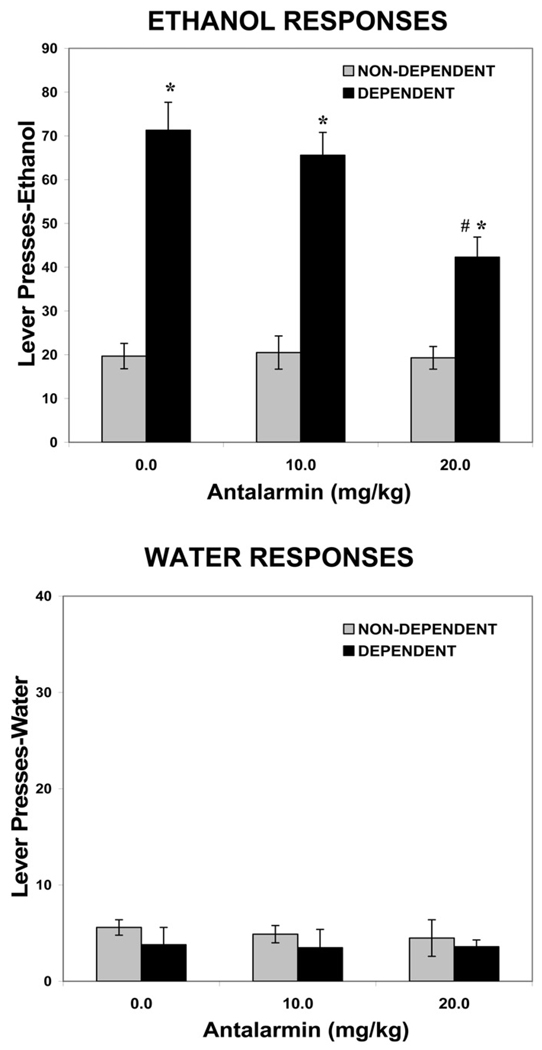
For the antalarmin group (n = 9), animal weights at the end of the experiment were 591.9 ± 53.6 grams for nondependent rats and 580 ± 14.9 grams for dependent rats. The mean blood alcohol level across the entire period of ethanol vapor exposure was 189.8 ± 24.7 mg/dL. Figure 2 shows the effects of antalarmin (0, 10.0, 20.0 mg/kg, IP) on ethanol and water self-administration in dependent versus nondependent animals.
Figure 2.
Effects of antalarmin on ethanol and water self-administration in dependent and nondependent rats. Ethanol dependence was induced by intermittent exposure to ethanol vapors for 4 weeks and animals were subsequently tested for ethanol and water self-administration following 2 hours of acute withdrawal. Withdrawn, ethanol-dependent animals display a significant increase in ethanol lever pressing compared with nondependent animals. Antalarmin significantly decreases ethanol self-administration in withdrawn, dependent but not nondependent animals. Neither ethanol vapor exposure nor antalarmin alters water responding. *p < .001 compared with same drug dose in nondependent animals. #p < .01 compared with vehicle treatment in dependent animals.
In the 30-minute test session, following vehicle injection (.5% carboxymethylcellulose), the dependent animals pressed approximately 71 times (1.2 g/kg) for 10% ethanol, compared with 20 ethanol presses (.34 g/kg) in nondependent animals. For ethanol self-administration, the two-way ANOVA revealed a significant effect of ethanol exposure [F(1,16) = 54.8, p < .001], a significant effect of antalarmin dose [F(2,32) = 9.8, p < .001], and a significant interaction between ethanol exposure and antalarmin dose [F(2,32) = 9.3, p < .001]. Further analysis revealed a significant reduction in ethanol self-administration in dependent animals at the 20 mg/kg dose of antalarmin compared with the 0 mg/kg dose (p < .01). However, no dose of antalarmin was effective in altering ethanol self-administration in nondependent animals (F < 1.0). For water self-administration, the two-way ANOVA revealed no effect of ethanol exposure [F(1,16) = 1.2, p < .29], no effect of antalarmin dose [F(2,32) < 1.0], and no interaction between ethanol exposure and antalarmin dose [F(2,32) < 1.0].
Effects of the CRF1 Specific Antagonist MJL-1-109-2 on Ethanol and Water Self-Administration in Dependent and Nondependent Rats
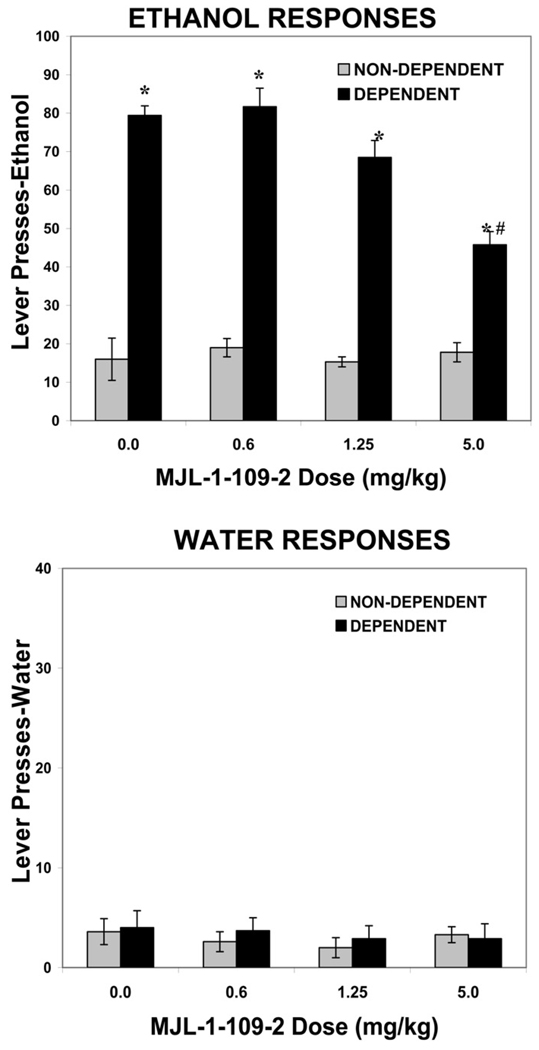
For the MJL-1-109-2 group (n = 10), animal weights at the end of the experiment were 605.4 ± 55.4 grams for nondependent rats and 587.2 ± 29.8 grams for dependent rats. The mean blood alcohol level across the entire period of ethanol vapor exposure was 173 ± 27.8 mg/dL. Figure 3 shows the effects of the CRF1 specific antagonist MJL-1-109-2 (0, .6, 1.25, 5.0 mg/kg, IP) on ethanol and water self-administration in dependent versus nondependent animals.
Figure 3.
Effects of MJL-1-109-2 on ethanol and water self-administration in dependent and nondependent rats. Ethanol dependence was induced by intermittent exposure to ethanol vapors for 4 weeks and animals were subsequently tested for ethanol and water self-administration following 2 hours of acute withdrawal. Withdrawn, ethanol-dependent animals display a significant increase in ethanol lever pressing compared with nondependent animals. MJL-1-109-2 significantly decreases ethanol self-administration in withdrawn, dependent but not nondependent animals. Neither ethanol vapor exposure nor MJL-1-109-2 alters water responding. *p < .0001 compared with same drug dose in nondependent animals. #p < .0001 compared with vehicle treatment in dependent animals.
In the 30-minute test session, following vehicle treatment (20% hydroxypropyl-β-cyclodextrin), the dependent animals pressed for 10% ethanol approximately 80 times (1.36 g/kg), compared with 18 ethanol presses (.31 g/kg) in nondependent animals. For ethanol self-administration, the two-way ANOVA revealed a significant effect of ethanol exposure [F(1,18) = 278.5, p < .0001], a significant effect of MJL-1-109-2 dose [F(3,54) = 12.7, p < .0001], and a significant interaction between ethanol exposure and MJL-1-109-2 dose [F(3,54) = 12.3, p < .0001]. There was a significant reduction in ethanol self-administration in dependent animals at the 5.0 mg/kg dose of MJL-1-109-2 compared with the 0 mg/kg dose (p < .0001). However, no dose of MJL-1-109-2 was effective in altering ethanol self-administration in nondependent animals (F < 1.0). For water self-administration, the two-way ANOVA revealed no effect of ethanol exposure [F(1,18) = 2.56, p = .127], no effect of MJL-1-109-2 dose [F(3,54) = 2.1, p = .113), and no interaction between ethanol exposure and MJL-1-109-2 dose [F(3,54) = 1.56, p = .209].
Effects of the CRF1 Specific Antagonist R121919 on Ethanol and Water Self-Administration in Dependent and Nondependent Rats
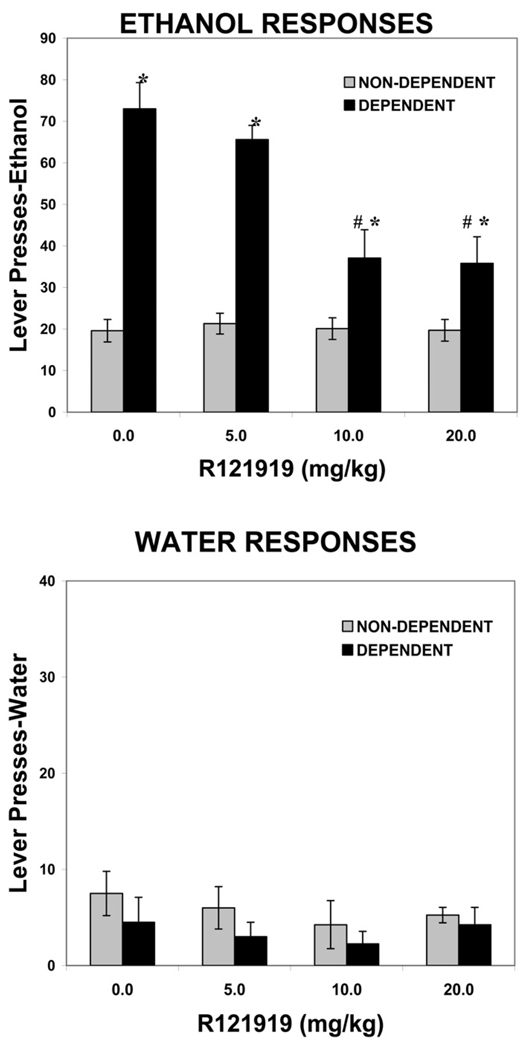
For the R121919 group (n = 9), animal weights at the end of the experiment were 608.7 ± 41.6 grams for nondependent rats and 581.1 ± 35.8 grams for dependent rats. The mean blood alcohol level across the entire period of ethanol vapor exposure was 178.8 ± 28.7 mg/dL. Figure 4 shows the effects of the CRF1 specific antagonist R121919 (0, 5.0, 10.0, and 20.0 mg/kg) on ethanol and water self-administration in dependent versus nondependent animals.
Figure 4.
Effects of R121919 on ethanol and water self-administration in dependent and nondependent rats. Ethanol dependence was induced by intermittent exposure to ethanol vapors for 4 weeks and animals were subsequently tested for ethanol and water self-administration following 2 hours of acute withdrawal. Withdrawn, ethanol-dependent animals display a significant increase in ethanol lever pressing compared with nondependent animals. R121919 significantly decreases ethanol self-administration in withdrawn, dependent but not nondependent animals. Neither ethanol vapor exposure nor R121919 alters water responding. *p < .001 compared with same drug dose in nondependent animals. #p < .0001 compared with vehicle treatment in dependent animals.
In the 30-minute test session, following vehicle treatment (20% hydroxypropyl-β-cyclodextrin), the dependent animals pressed approximately 73 times (1.32 g/kg) for 10% ethanol, compared with 20 ethanol presses (.33 g/kg) in nondependent animals. For ethanol self-administration, the two-way ANOVA revealed a significant effect of ethanol exposure [F(1,16) = 215.4, p < .0001], a significant effect of R121919 dose [F(3,48) = 20.4, p < .0001], and a significant interaction between ethanol exposure and R121919 dose [F(3,48) = 19.6, p < .0001]. There was a significant reduction in ethanol self-administration in dependent animals at both the 10.0 mg/kg (p < .0001) and 20 mg/kg dose of R121919 (p < .0001) compared with the 0 mg/kg dose. However, no dose of R121919 was effective in altering ethanol self-administration in nondependent animals (F < 1.0). For water self-administration, the two-way ANOVA revealed no effect of ethanol exposure [F(1,16) < 1.0], no effect of R121919 dose [F(3,48) < 1.0], and no interaction between ethanol exposure and R121919 dose [F(3,48) < 1.0].
Discussion
Excessive, uncontrolled drinking and the presence of a withdrawal syndrome following cessation of alcohol intake are two of the diagnostic criteria for dependence in humans (American Psychiatric Association 1994). Further, human alcoholics will report that the negative affect state, especially enhanced anxiety and stress, experienced during withdrawal is the main factor eliciting relapse and binge drinking during periods of abstinence (Hershon 1977). Some of these negative affect symptoms can persist months after chronic ethanol cessation (Begleiter and Porjesz 1979). Animal model systems for ethanol dependence have been developed which mimic excessive drinking and withdrawal (O’Dell et al 2004; Roberts et al 2000). Here, we have induced ethanol dependence in male Wistar rats by exposing the animals to intermittent ethanol vapors for 4 weeks. We report that three nonpeptide, small molecule CRF1 antagonists, antalarmin, MJL-1-109-2, and R121919, significantly and selectively reduce ethanol self-administration in ethanol-dependent rats. These data indicate that the CRF1 receptor subtype is important in mediating excessive ethanol intake in ethanol-dependent animals.
One potential issue with this study may be the specificity of the antagonists for CRF1 receptors. Importantly, the portion of the CRF1 receptor to which these antagonists bind is not contained in the CRF2 or CRF-binding peptide (Zorrilla and Koob 2004). Further, these nonpeptide antagonists have all been used in multiple studies demonstrating their specificity and effectiveness. Antalarmin has a Ki value of 1.0 nm and antagonizes both central and peripheral actions of CRF in vivo at a dose of 20 mg/kg, similar to the dose we found effective here (Webster et al 1996). In rats, antalarmin, at doses of 10 or 20 mg/kg, has also been shown to inhibit fear conditioning (Deak et al 1999), inhibit CRF- and novelty-induced anxiety-like behaviors (Zorrilla et al 2002), inhibit the locomotor activating effects of CRF (Zorrilla et al 2002), and inhibit naloxone-precipitated place aversion in morphine-dependent animals (Stinus et al 2005). Importantly, at the doses used in this study, antalarmin does not have nonspecific effects, as it does at higher doses (Zorrilla et al 2002). Here, we show that antalarmin dose-dependently and significantly reduced ethanol self-administration in dependent animals. Antalarmin had no effect in nondependent animals.
The CRF1 receptor antagonist R121919 also reduces anxiety-like behavior in animal models. In vitro studies have found that R121919 is highly selective for the CRF1 receptor (Heinrichs et al 2002) and has no reported pharmacological interactions (Zorrilla and Koob 2004). In rats, R121919, at doses similar to those used in this study (20–30 mg/kg), inhibits stress-induced plasma adrenocorticotropic hormone levels, inhibits anxiogenic-like behaviors, and inhibits CRF-induced locomotor activity (Chen et al 2004; Heinrichs et al 2002; Keck et al 2001). The CRF1 receptor antagonist R121919 has also been shown to be effective in reducing anxious and depressed symptoms in an open-label human clinical trial for treatment of depression (Kunzel et al 2003; Zobel et al 2000). Similar to antalarmin, we show here that R121919 dose-dependently and significantly reduced ethanol self-administration in dependent animals. Our data indicate a plateau in the level of reduction of ethanol responding in dependent animals, which may result from receptor occupancy of CRF1 receptors by R121919. Heinrichs et al (2002) have previously reported receptor occupancy levels for R121919, demonstrating nearly 50% occupancy at 2.5 mg/kg and 100% occupancy at 20 mg/kg. Thus, R121919 may be occupying the majority of CRF1 receptors at 10 mg/kg, producing a plateau effect at the highest dose (20 mg/kg). Alternatively, other receptors may also be involved in mediating the enhanced intake of ethanol in dependent animals, such as the CRF2 receptor (discussed below). R121919 had no effect in nondependent rats.
The CRF1 receptor antagonist MJL-1-109-2 has been the least studied drug of the three used here but is an analog of the well-studied compound DMP696 (He et al 2000; Jagoda et al 2003). However, MJL-1-109-2, similar to antalarmin and R121919, also is able to penetrate the blood brain barrier and has a Ki value of 1.9 nm (Jagoda et al 2003). Here, MJL-1-109-2 significantly attenuated ethanol self-administration in dependent animals. MJL-1-109-2 was without effect in nondependent animals. Furthermore, studies in our group have shown that MJL-1-109-2 does not reduce food intake at the doses used here (Sabino et al, unpublished data, 2005), further suggesting a behaviorally selective action of MJL-1-109-2 on excessive ethanol drinking and not consummatory behavior in general. The use of three CRF1 specific antagonists strengthens our conclusion that the CRF1 receptor subtype is important for mediating excessive drinking in ethanol-dependent animals.
CRF mediates the neuroendocrine (Rivier et al 1982; Vale et al 1981), autonomic (Vale et al 1983), and behavioral responses to stress and anxiety (Britton et al 1982; Koob and Heinrichs 1999; Sutton et al 1982). During the development of ethanol dependence, both hypothalamic as well as extrahypothalamic CRF systems become dysregulated. In rats, acute ethanol activates the HPA axis (Rivier et al 1984), while chronic exposure to ethanol, as well as ethanol withdrawal, leads to an attenuation of HPA axis activity (Dave et al 1986; Lee et al 2000; Rivier et al 1990; Zorrilla et al 2001). In contrast, extrahypothalamic CRF systems become hyperactive during withdrawal from chronic ethanol exposure. There is an increase in extracellular CRF release in both the central nucleus of the amygdala (CeA) (Merlo Pich et al 1995) and bed nucleus of the stria terminalis (BNST) (Olive et al 2002). A decrease in CRF-like immunoreactivity in the amygdala during acute withdrawal has also been reported, which likely reflects an increase in CRF release in these brain regions (Zorrilla et al 2001). Ethanol-dependent animals also display enhanced sensitivity to an intracerebroventricular (ICV) infusion of CRF, as measured by electrophysiology (Slawecki et al 1999) and locomotor activity (Ehlers and Chaplin 1987). The authors suggest that this increased sensitivity to CRF may be the result of an increase in CRF receptor expression. Thus, while CRF activity within the HPA axis appears to become blunted with ethanol dependence, extrahypothalmic CRF systems become hyperactive.
It was hypothesized that this dysregulation of brain CRF “stress” systems underlies both the enhanced anxiety-like behaviors as well as contributes to the enhanced ethanol self-administration associated with withdrawal. Ethanol-dependent rodents display enhanced anxiety-like behaviors during acute and protracted withdrawal as measured by the elevated plus maze test (Baldwin et al 1991; Valdez et al 2002b), enhanced acoustic startle (Macey et al 1996), social interaction test (Overstreet et al 2002), and light/dark box (Kliethermes et al 2004). In rats, the subtype nonselective, peptide CRF receptor antagonists α-helical CRF9–41 and D-Phe-CRF12–41 (intracerebroventricular administration) reduce ethanol withdrawal-induced anxiety-like behavior (Baldwin et al 1991; Valdez et al 2002b). However, α-helical CRF9–41 was found to be ineffective in reducing other physical symptoms of withdrawal, such as body tremor and tail stiffness (Baldwin et al 1991). This same antagonist, α-helical CRF9–41, also decreased ethanol withdrawal-induced anxiety-like behavior when injected directly into the CeA (Rassnick et al 1993). Using a model involving multiple ethanol withdrawals, Overstreet et al (2004) have also shown an enhancement of anxiety-like behaviors in ethanol-dependent rats, an effect reversed by systemic administration of CRF1 specific antagonists. Thus, CRF1 receptors appear to mediate the enhanced anxiety associated with withdrawal.
Ethanol-dependent animals also display enhanced ethanol self-administration during acute and protracted periods of withdrawal (O’Dell et al 2004; Roberts et al 2000; Valdez et al 2002b). We suggest that dependent animals self-administer excessive amounts of ethanol during withdrawal to alleviate the CRF-mediated increase in negative affect, such as enhanced anxiety or hyperarousal. Indeed, data from a study by Olive et al (2002) suggest that animals will self-administer ethanol during periods of withdrawal to attenuate the increased CRF release in the extended amygdala. Further, the enhanced ethanol self-administration during withdrawal can be reversed by intracerebroventricular administration of subtype nonselective, peptide CRF receptor antagonists (Valdez et al 2002b) and, as we show here, systemic administration of small molecule CRF1 antagonists. The subtype nonselective, peptide CRF receptor antagonist D-Phe-CRF12–41 reduces ethanol self-administration during acute withdrawal (2 hours) as well as protracted withdrawal (3 to 5 weeks) in dependent rats (Valdez et al 2002b). However, the antagonist had no effect in nondependent animals. Here, we have found that three CRF1 specific antagonists reduce ethanol self-administration in dependent rats during acute withdrawal, suggesting that the ICV effects of D-Phe-CRF12–41 (Valdez et al 2002b, 2003b) are likely mediated by a CRF1 receptor mechanism. It will be important for future studies to also address the effectiveness of these CRF1 receptor antagonists in reducing ethanol self-administration during periods of protracted abstinence as well.
The data presented in this study support a role for CRF1 receptors in mediating excessive ethanol drinking during withdrawal in dependent animals. CRF1 receptors are located throughout the brain, primarily in the pituitary gland, cerebral cortex, cerebellum, hippocampus, hindbrain, and olfactory bulb (Van Pett et al 2000). CRF1 receptors are also expressed in areas of the extended amygdala, such as the BNST and central and basolateral nuclei of the amygdala (Chen et al 2000; Van Pett et al 2000), regions which also have increased CRF release during ethanol withdrawal (Merlo Pich et al 1995; Olive et al 2002). Several lines of evidence indicate that the CRF1 receptor plays an important role in mediating anxiety-like behavior. CRF1 receptor deficient mice display reduced basal (Muller et al 2003; Smith et al 1998; Timpl et al 1998) and ethanol withdrawal-induced anxiety-like behavior (Timpl et al 1998). CRF1 antisense oligodeoxynucleotides reduce anxiety-like behavior in rats when injected intracerebroventricularly (Heinrichs et al 1997; Liebsch et al 1999) or directly into the CeA (Liebsch et al 1995). Further, multiple studies have found that selective CRF1 receptor antagonists inhibit anxiety-like behaviors in animals (Zorrilla and Koob 2004). Although not directly addressed in this study, the important role of CRF1 receptors in mediating anxiety-like behaviors provides strong support for the hypothesis that CRF1 antagonists reduce excessive drinking during withdrawal by reducing withdrawal-associated anxiety. In addition, studies from our lab have shown that ethanol-dependent rats display heightened anxiety-like behaviors following a 2-hour withdrawal period, which were reversed by IVC administration of subtype nonselective CRF receptor antagonists (Valdez et al 2002b), further suggesting that CRF1 receptors reduce ethanol intake by reducing heightened anxiety-like behaviors.
The specific brain regions and pathways involved in mediating CRF1 antagonist-induced decreases in ethanol self-administration in dependent animals remain unknown. Several studies suggest that regions of the extended amygdala may be involved in mediating CRF-induced anxiety (Merlo Pich et al 1995; Muller et al 2003; Olive et al 2002; Rassnick et al 1993), and the same regions may mediate excessive ethanol consumption during withdrawal as well. Specifically, the CeA, a region mediating anxiogenic-like behaviors (Liang et al 1992; Walker and Davis 1997), is involved in mediating both acute effects of ethanol as well as the anxiogenic effects of chronic ethanol exposure (Pandey et al 2003; Rassnick et al 1993; Roberto et al 2004; Yoshimoto et al 2000). Thus, this brain region may be involved in mediating the increased ethanol self-administration during withdrawal. The basolateral nucleus of the amygdala may also play a role, as CRF released from the CeA can activate basolateral CRF1 neurons by volume transmission (Roozendaal et al 2002). Previous studies using in vivo and in vitro autoradiography have demonstrated that the CRF1 antagonists used here bind to CRF1 receptors within these amygdala structures (Heinrichs et al 2002; Jagoda et al 2003). Future studies will be important to determine which brains regions, primarily within the extended amygdala, are important in mediating excessive drinking during withdrawal.
None of the CRF1 receptor antagonists altered ethanol self-administration in nondependent animals. However, one study (Lodge and Lawrence 2003a) has previously shown that antalarmin reduces volitional ethanol consumption in isolation-reared, Fawn-Hooded rats. Isolation rearing produces an anxious, high-alcohol preferring phenotype, similar to other alcohol-preferring strains of rats (Lodge and Lawrence 2003b). These alcohol-preferring animals have been shown to have dysregulated CRF systems (Ehlers et al 1992; Hwang et al 2004), dopaminergic systems (Lodge and Lawrence 2003b), and serotonin systems (Hensler et al 2004). Thus, alcohol-preferring animals, such as those used in the Lodge and Lawrence (2003a) study, likely do not represent a true ethanol-nondependent phenotype. These alcohol-preferring strains more closely resemble ethanol-dependent animals than nondependent animals in our model system.
The present study suggests an important role for CRF1 receptors in mediating ethanol self-administration in dependent animals. These data do not rule out a potential role for CRF2 receptors in modulating this response as well. Urocortin 3, a specific CRF2 agonist, injected intracerebroventricularly, can reduce enhanced ethanol self-administration and anxiety-like behaviors associated with acute ethanol withdrawal in ethanol-dependent rats (Valdez et al 2003a). In rats, CRF2 agonists produce anxiolytic-like behaviors (Valdez et al 2002a, 2003a), and CRF2 deficient mice display enhanced anxiety-like behavior (Bale et al 2000; Kishimoto et al 2000). These data suggest that CRF1 and CRF2 receptors may have opposing roles in controlling ethanol withdrawal-induced anxiety and ethanol self-administration, consistent with the opposing electrophysiological actions of CRF1 and CRF2 ligands on glutamatergic transmission (Liu et al 2004).
We have studied the acute effects of CRF1 antagonists on ethanol self-administration in ethanol-dependent versus nondependent animals. However, the chronic effects of these antagonists will also have important clinical implications. For example, some studies have shown alterations in the dopaminergic system following chronic exposure to antalarmin (Lawrence et al 2005). Several other studies have reported no dysregulation of the HPA axis following chronic administration of CRF1 antagonists (Oshima et al 2003; Wong et al 1999). In future studies, we will more thoroughly examine different durations of dosing and dosing patterns of the antagonists used here.
These data have important clinical implications for the treatment of alcohol dependence in humans. Dysregulation of CRF stress systems may represent a long-lasting change within the brain resulting from chronic ethanol consumption. Upon cessation of ethanol use, stress systems become overactive, likely causing negative affect states, such as heightened anxiety and depression. Novel pharmacotherapies aimed at treating alcohol-induced anxiety and depression may prove to be successful in treating relapse and excessive drinking during withdrawal. Thus, the development of these CRF1 antagonists, antalarmin, MJL-1-109-2, and R121919, as a novel class of pharmacotherapy may be promising for the treatment of alcoholism.
Acknowledgments
This research was supported by the Pearson Center for Alcoholism and Addiction Research and National Institutes of Health Grants AA12602 (GFK) and AA015239 (CKF) from the National Institute on Alcohol Abuse and Alcoholism. This research was also supported in part by the Intramural Research Program of the National Institutes of Health (NIH), the National Institute of Diabetes and Digestive and Kidney Diseases (KCR).
We thank Mike Arends for his editorial assistance.
Footnotes
This is publication number 17702-NP from The Scripps Research Institute.
References
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amgdaloid, and corticopetal components of substantia innominata. Neuroscience. 1995;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press, Inc; 1994. [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Persistence of a “subacute withdrawal syndrome” following chronic ethanol intake. Drug Alcohol Depend. 1979;4:353–357. doi: 10.1016/0376-8716(79)90019-x. [DOI] [PubMed] [Google Scholar]
- Bloom FE, Battenberg EL, Rivier J, Vale W. Corticotropin-releasing factor (CRF): Immunoreactive neurons and fibers in rat hypothalamus. Regul Pept. 1982;4:43–48. doi: 10.1016/0167-0115(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Webster EL, Torpy DJ, Richman SJ, Mitsiades N, Igel M, et al. Chronic effects of a nonpeptide corticotropin-releasing hormone type I-receptor antagonist on pituitary-adrenal function, and body weight and metabolic regulation. Endocrinology. 1998;139:1546–1555. doi: 10.1210/endo.139.4.5938. [DOI] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Britton KT, Lee G, Vale W, Rivier J, Koob GF. Corticotropin releasing factor receptor (CRF) antagonist blocks activating and ’anxiogenic’ actions of CRF in the rat. Brain Res. 1986;369:303–306. doi: 10.1016/0006-8993(86)90539-1. [DOI] [PubMed] [Google Scholar]
- Britton KT, Morgan J, Rivier J, Vale W, Koob GF. Chlordiazepoxide attenuates response suppression induced by corticotropin-releasing factor in the conflict test. Psychopharmacology (Berl) 1985;86:170–174. doi: 10.1007/BF00431704. [DOI] [PubMed] [Google Scholar]
- Chang CP, Pearse RVII, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Chen C, Wilcoxen KM, Huang CQ, Xie YF, McCarthy JR, Webb TR, et al. Design of 2,5-dimethyl-3-(6-dimethyl-4-methylpyridin-3-yl)-7-dipropylamino pyrazolo [1,5-a] pyrimidine (NBI 30775/R121919) and structure-activity relationships of a series of potent and orally active corticotropin-releasing factor antagonists. J Med Chem. 2004;47:4787–4798. doi: 10.1021/jm040058e. [DOI] [PubMed] [Google Scholar]
- Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W. Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in rat brain. Brain Res. 1986;381:49–57. doi: 10.1016/0006-8993(86)90688-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF1) -like immunoreactivity in the mouse brain: Light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL. Pyrrolopyrimidines as CRF antagonists. WO 94/13676. International Patent. 1994
- Dave JR, Eiden LE, Karanian JW, Eskay RL. Ethanol exposure decreases pituitary corticotropin-releasing factor binding, adenylate cyclase activity, proopiomelanocortin biosynthesis, and plasma beta-endorphin levels in the rat. Endocrinology. 1986;118:280–286. doi: 10.1210/endo-118-1-280. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm Behav. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. Chronic ethanol exposure potentiates the locomotor-activating efects of corticotropin-releasing factor (CRF) in rats. Regul Pept. 1987;19:345–353. doi: 10.1016/0167-0115(87)90176-5. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, Lumeng L, Li TK, Owens MJ, et al. Corticotropin releasing factor (CRF): Studies in alcohol preferring and non-preferring rats. Psychopharmacology (Berl) 1992;106:359–364. doi: 10.1007/BF02245418. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcott PK. Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psychopharmacology (Berl) 1989;98:262–264. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- Giguere V, Labrie F, Cote J, Coy DH, Sueiras-Diaz J, Schally AV. Stimulation of cyclic AMP accumulation and corticotropin release by synthetic ovine corticotropin-releasing factor in rat anterior pituitary cells: Site of glucocorticoid action. Proc Natl Acad Sci U S A. 1982;79:3466–3469. doi: 10.1073/pnas.79.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Gilligan PJ, Zaczek R, Fitzgerald LW, McElroy JP, Shen HS, et al. 4-(1,3-Dimethoxyprop-2-ylamino)-2,7-dimethyl-8-(2, 4-dichlorophenyl) pyrazolo[1,5-a]-1,3,5-triazine: A potent, orally bioavailable CRF(1) receptor antagonist. J Med Chem. 2000;43:449–456. doi: 10.1021/jm9904351. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Hodge CW, Overstreet DH. Reduced 5-HT3 receptor binding and lower baseline plus maze anxiety in the alcohol-preferring inbred fawn-hooded rat. Pharmacol Biochem Behav. 2004;77:281–289. doi: 10.1016/j.pbb.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38:953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Stewart R, Zhang JK, Lumeng L, Li TK. Corticotropin-releasing factor gene expression is down-regulated in the central nucleus of the amygdala of alcohol-preferring rats which exhibit high anxiety: A comparison between rat lines selectively bred for high and low alcohol preference. Brain Res. 2004;1026:143–150. doi: 10.1016/j.brainres.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Jagoda E, Contoreggi C, Lee MJ, Kao CH, Szajek LP, Listwak S, et al. Autoradiographic visualization of corticotropin releasing hormone type I receptors with a nonpeptide ligand: Synthesis of [76Br]MJL-1-109-2. J Med Chem. 2003;46:3559–3562. doi: 10.1021/jm034077k. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F, et al. The anxiolytic effect of the CRH1 receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci. 2001;13:373–380. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, et al. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: Studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Kunzel HE, Zobel AW, Nickel T, Ackl N, Uhr M, Sonntag A, et al. Treatment of depression with the CRH-1 receptor antagonist R121919: Endocrine changes and side effects. J Psychiatr Res. 2003;37:525–533. doi: 10.1016/s0022-3956(03)00070-0. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Parish CL, Chen F, Lodge DJ, Krstew EV, Card K, et al. Chronic corticotropin-releasing factor type 1 receptor antagonism with antalarmin regulates the dopaminergic system of Fawn-Hooded rats. J Neurochem. 2005;94:1523–1534. doi: 10.1111/j.1471-4159.2005.03300.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcohol Clin Exp Res. 2000;24:110–122. [PubMed] [Google Scholar]
- Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJD, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. J Neurosci. 1992;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Landgraf R, Engelmann M, Lorscher P, Holsboer F. Differential behavioural effects of chronic infusion of CRH 1 and CRH 2 receptor antisense oligonucleotides into the rat brain. J Psychiatr Res. 1999;33:153–163. doi: 10.1016/s0022-3956(98)80047-2. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, et al. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 1995;59:229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, et al. Corticotropin-releasing factor and urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience. 2003a;117:243–247. doi: 10.1016/s0306-4522(02)00793-5. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The effect of isolation rearing on volitional ethanol consumption and central CCK/dopamine systems in Fawn-Hooded rats. Behav Brain Res. 2003b;15:113–122. doi: 10.1016/s0166-4328(02)00328-5. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: Effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- McElroy JF, Ward KA, Zeller KL, Jones KW, Gilligan PJ, He L, et al. The CRF(1) receptor antagonist DMP696 produces anxiolytic effects and inhibits the stress-induced hypothalamic-pituitary-adrenal axis activation without sedation or ataxia in rats. Psychopharmacology (Berl) 2002;165:86–92. doi: 10.1007/s00213-002-1239-3. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, et al. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann N Y Acad Sci. 1994;739:176–184. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriquez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MB, Zimmerman S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 7th ed. Washington, DC: National Academy Press; 1996. [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A, Flachskamm C, Reul JM, Holsboer F, Linthorst AC. Altered serotonergic neurotransmission but normal hypothalamic-pituitary-adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology. 2003;28:2148–2159. doi: 10.1038/sj.npp.1300267. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, et al. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamicpituitary-adrenal axis in the rat: Role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: Effect on CRF mRNAlevels, and CRF and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivier J, Vale W. Inhibition of adrenocorticotropic hormone secretion in the rat by immunoneutralization of corticotropin-releasing factor. Science. 1982;218:377–379. doi: 10.1126/science.6289439. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: Animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Koob GF. Operant self-administration of sweetened versus unsweetened ethanol: Effects on blood alcohol levels. Alcohol Clin Exp Res. 1999;23:1151–1157. [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Somes C, Ehlers CL. Effects of chronic ethanol exposure on neurophysiological responses to corticotropin-releasing factor and neuropeptide Y. Alcohol Alcohol. 1999;34:289–299. doi: 10.1093/alcalc/34.3.289. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjiam LM, Gold LH, et al. Corticotropin-releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RKW, Basso AM, Rivier J, Vale W, et al. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Dufour MC, Steffens RA, DeBakey SF. Alcohol-related mortality in the United States, 1979–1989. Alcohol Health Res World. 1993;17:251–260. [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place preference. Neuropsychopharmacology. 2005;30:90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9–41) Neuropsychopharmacology. 1989;2:285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: Blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JMHM, Stalla GK, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: Mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002a;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: Implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002b;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003a;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003b;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, et al. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;39:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: Suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Wong ML, Webster EL, Spokes H, Phu P, Ehrhart-Bornstein M, Bornstein S, et al. Chronic administration of the non-peptide CRH type 1 receptor antagonist antalarmin does not blunt hypothalamic-pituitary-adrenal axis response to acute immobilization stress. Life Sci. 1999;65:PL53–PL58. doi: 10.1016/s0024-3205(99)00268-4. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Ueda S, Kato B, Takeuchi Y, Kawai Y, Noritake K, et al. Alcohol enhances characteristic releases of dopamine and serotonin in the central nucleus of the amygdala. Neurochem Int. 2000;37:369–376. doi: 10.1016/s0197-0186(00)00037-1. [DOI] [PubMed] [Google Scholar]
- Yu-Wen L, Hill G, Wong H, Kelly N, Ward K, Pierdomenico M, et al. Receptor occupancy of nonpeptide corticotropin-releasing factor 1 antagonist DMP696: Correlation with drug exposure and anxiolytic efficacy. J Pharmacol Exp Ther. 2003;305:86–96. doi: 10.1124/jpet.102.045914. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: The first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]