Abstract
Background: Carotid intima-media thickness (IMT) provides a mechanism for detecting early atherosclerosis. Little information is available concerning carotid IMT and the progression of atherosclerosis in adolescents and young adults with type 2 diabetes mellitus.
Objective: We sought to determine the factors that contribute to early changes in carotid IMT in youth with type 2 diabetes mellitus and to identify any predictors of increased carotid IMT.
Methods: Demographic, anthropometric, laboratory data and carotid imaging were obtained in 129 youth of mixed ethnicity, ages 10–23 yr. Associations of carotid IMT outcomes and risk variables were analyzed by regression analysis. Logistic regression was performed to elucidate independent determinants that predict a worse carotid IMT.
Results: Carotid IMT increased with higher glycosylated hemoglobin (HbA1c) levels and longer duration of diabetes. Regression modeling showed that HbA1c and duration of diabetes in the presence of traditional cardiovascular risk factors (male sex, LDL cholesterol, and blood pressure) were independent determinants of carotid IMT. Logistic regression analysis demonstrated that each 1% increase in HbA1c or each year increase in duration of type 2 diabetes mellitus is associated with approximately 30% increased odds of a thicker carotid IMT.
Conclusions: Poorer glycemic control and longer disease duration have independent adverse effects on carotid IMT in youth with type 2 diabetes mellitus. These adverse effects appear to be more prominent in males. Developing effective strategies to improve blood glucose control in youth with type 2 diabetes mellitus is essential to prevent or limit the development and progression of atherosclerotic cardiovascular disease.
Poor glucose control, longer duration of diabetes, and traditional cardiovascular risk factors are associated with increased carotid intimal wall thickness in adolescents with type 2 diabetes mellitus.
Cardiovascular disease is a major cause of morbidity and mortality in adults with type 2 diabetes mellitus. Recent reports document that almost 70% of adults with type 2 diabetes mellitus will die from cardiovascular disease (1). Although adolescents and young adults have experienced a marked increase in the frequency of type 2 diabetes mellitus in the past two decades, little is known about the early development of cardiovascular disease and the atherosclerotic processes that occur in these youth.
Most of the information regarding the development and progression of atherosclerosis in adolescents and young adults is derived from autopsy studies performed on individuals who have died traumatic deaths (2). However, the development of noninvasive imaging techniques such as carotid intima-media thickness (IMT) provides a mechanism for studying the evolution of atherosclerosis. In adults, investigators have used carotid IMT to document the extent and progression of atherosclerosis (3). Studies have shown that increased carotid IMT is associated with known cardiovascular risk factors and is efficacious in predicting future coronary artery disease and stroke (4,5). Carotid IMT has also been used to study atherosclerosis in adults with type 2 diabetes mellitus. These studies have shown that type 2 diabetes mellitus is associated with an increase in carotid IMT and a 40% higher risk of myocardial infarction and stroke (5). Despite these findings, little information is available concerning carotid IMT and the progression of atherosclerosis in adolescents with type 2 diabetes mellitus.
To address this issue, we sought to determine the factors that contribute to early changes in carotid IMT in youth with type 2 diabetes mellitus and to identify any predictors of increased carotid IMT.
Subjects and Methods
Study population
A total of 129 adolescents and young adults (age range, 10–23 yr) with type 2 diabetes mellitus participated in this study. The diagnosis of type 2 diabetes was based on the American Diabetes Association criteria (6). Specifically, the participants had elevated fasting plasma glucose levels of at least 126 mg/dl, or symptoms of hyperglycemia and random plasma glucose of at least 200 mg/dl, or 2-h plasma glucose of at least 200 mg/dl during an oral glucose tolerance test. All individuals also had no evidence of another specific type of diabetes and were non-insulin requiring in the basal state to prevent diabetic ketoacidosis. A total of 124 subjects were islet cell antibody-negative (glutamic acid decarboxylase, islet cell antigen 512, insulin autoantibodies). Five individuals did not have islet cell antibody data available. Pregnant females were excluded from the study.
A majority of the study population was recruited from individuals with type 2 diabetes followed at the Diabetes Center at Cincinnati Children’s Hospital (n = 124). Of 329 eligible subjects in the Diabetes Center, we approached 140 subjects, beginning with the oldest eligible subjects. A total of 124 consented to participate. Those who participated were similar to the eligible population in mean age (19 vs. 20 yr), gender (62% female vs. 64% female), and mean body mass index (BMI) (36.7 vs. 36.4 kg/m2). The eligible population had a slightly higher percentage of nonwhites than the participants (63 vs. 50%, respectively). Thus, for most demographic categories, the eligible and participant populations were similar. The five remaining subjects were recruited from local medical practices. These individuals did not have islet cell antibody data available.
Before enrollment in the study, written informed consent was obtained from subjects who were at least 18 yr old or the parent or guardian for subjects younger than 18 yr old, and written assent was obtained for subjects less than 18 yr old according to the guidelines established by the Institutional Review Board at Cincinnati Children’s Hospital and in accordance with the Declaration of Helsinki.
Data collection
After a minimum 10-h overnight fast, participants came to the Clinical Research Center at Cincinnati Children’s Hospital Medical Center for an in-person study visit, during which demographic and anthropometric data were collected, fasting venipuncture and blood pressure (BP) were performed, and carotid IMT was measured. Two measures of height were obtained with a calibrated stadiometer (Veeder-Rood, Elizabethtown, NC) by trained personnel. Weight was also measured twice and averaged with a Health-O-Meter electronic scale (model 770; SECA, Hanover, MD). BMI was calculated as kilograms per meter squared. BP was obtained manually with a mercury Sphygmomanometer (W. A. Baum Co., Inc., Copiague, NY) according to the standards of the Fourth Report on Blood Pressure Control in Children (7). Duration of disease was measured from the date of diagnosis to the date of study.
Laboratory
Plasma glucose was measured using a Hitachi model 704 glucose analyzer with intraassay and interassay coefficients of variation of 1.2 and 1.6%, respectively (8). Plasma insulin was measured by RIA using an antiinsulin serum raised in guinea pegs, 125I labeled insulin (Linco, St. Louis, MO) and a double antibody method to separate bound from free tracer. This assay has a sensitivity of 2 pmol and has intra- and interassay coefficients of variation of 5 and 8%, respectively (8). Assays of fasting plasma lipid profiles were carried out in a laboratory that is National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention standardized with the low-density lipoprotein (LDL) cholesterol concentration calculated using the Friedewald equation. IL-6, TNF-α, and C-reactive protein (CRP) were measured using a high sensitivity ELISA. Glycosylated hemoglobin (HbA1c) was measured in red blood cells using HPLC methods.
Carotid IMT
Carotid ultrasound studies were performed by a single registered vascular technologist. The carotid arteries were evaluated with high-resolution B-mode ultrasonography using a GE Vivid 7 ultrasound imaging system (GE Medical Systems, Wauwatosa, WI) with a high-resolution linear array vascular ultrasound centered at 7.5 MHz. For each subject, each carotid wall and segment was examined independently from continuous angles to identify the thickest carotid IMT. Three segments were imaged with left and right sides averaged for the common artery, bulb (bifurcation), and the internal carotid artery. Multiple digital image loops were digitally transmitted using the Camtronics Medical System (Hartland, WI) for offline reading and analyses. A trace technique was employed to measure the maximum carotid thickness from the leading edge. All images were read by a single research-trained vascular technician, who was blinded to subjects and has greater than 3 yr experience reading carotid ultrasound studies. Carotid IMT was measured from the leading edge (lumen-intima) to the leading edge (medial-adventia). This technique was found to be more reproducible than point-to-point measurements (coefficient of variation for repeat readings, 5.3 to 8% for trace vs. 8.4 to 11.6% for point to point for the three carotid segments; Urbina, E. M., unpublished data, 2008).
Statistical analysis
All analyses were performed with Statistical Analysis Software (version 9.1.3; SAS Institute Inc., Cary, NC) (9). Average values for demographic, anthropometric, laboratory values and medication information were obtained for the entire group and by sex. χ2 tests were used to detect differences in race and medication use between sex groups, whereas t tests were used to detect sex differences in age, weight, height, BMI, systolic and diastolic BP. The Wilcoxon rank sum test was used to compare total cholesterol, LDL and high-density lipoprotein (HDL) cholesterol, triglycerides, fasting glucose, HbA1c, TNF-α, IL-6, CRP, and duration of the disease between males and females. Associations of carotid IMT outcomes with demographic, anthropometric, laboratory measures, and medications were analyzed using linear regression modeling with the aid of stepwise and forward procedures. Sex was forced in the models because of apparent sex differences in both outcomes and many independent variables. Normality of outcome variables was evaluated using Shapiro-Wilk tests (P > 0.05 as normal) and visually checked by normal probability plots and QQ-plots. All three outcome variables were not normally distributed. After log transformation, normal distributions were assumed for all three variables. The Shapiro-Wilk test for bulb (log) was of marginal significance (P < 0.0326), but normality was assumed after reviewing the normal quantile plot. Linear relationships of carotid IMT outcomes and individual independent variables were assessed by scatter plot. Residuals of each model were inspected to assure a good fit. Logistic regression analysis was performed to elucidate independent determinants of elevated carotid IMT. An elevated carotid IMT was defined as greater than the 95th percentile for the measure taken in 215 lean healthy control subjects participating in the parent study from which the study data were obtained. For both linear and logistic regression, one TNF-α outlier was excluded from analysis. Fasting insulin was not considered because about half of the patients were on insulin medication. Statin medication was not included in analysis because there were only six patients using it. Secondary interactions between age, race, sex, HbA1c, and duration of disease were also examined.
Results
Table 1 lists the average demographic, anthropometric data, and laboratory and carotid measurements for all subjects, stratified by sex. There were more non-Caucasian and female participants in the study, but there was no significant race difference by sex. Males were taller and heavier with higher systolic BP and lower HDL cholesterol and CRP levels. Males had a significantly thicker common and internal carotid artery, with a trend toward a thicker artery in the bulb (P = 0.0798). Of the subjects on medications, 57% were taking metformin, 45% insulin, 18% an antihypertensive medication, and 5% a lipid-lowering agent.
Table 1.
Characteristics of the study population
| Variables | Females (n = 79) | Males (n = 50) | P values |
|---|---|---|---|
| Caucasians (n) | 29 | 18 | |
| Non-Caucasians (n) | 50 | 32 | |
| Age (yr) | 18.9 ± 3.2 | 18.9 ± 3.2 | |
| Weight (kg) | 101.7 ± 27.3 | 112.4 ± 31.8 | 0.0453a |
| Height (cm) | 165.1.0 ± 7.8 | 176.2 ± 9.6 | <0.0001a |
| BMI (kg/m2) | 37.2 ± 9.4 | 36.0 ± 9.2 | |
| Systolic BP (mm Hg) | 120 ± 12 | 127 ± 11 | 0.0008a |
| Diastolic BP (mm Hg) | 68 ± 13 | 68 ± 16 | |
| Total cholesterol (mg/dl) | 188 ± 37 | 188 ± 48 | |
| LDL cholesterol (mg/dl) | 112 ± 34 | 120 ± 48 | |
| HDL cholesterol (mg/dl) | 48 ± 12 | 41 ± 10 | 0.0010b |
| Triglycerides (mg/dl) | 142 ± 98 | 158 ± 106 | |
| Fasting glucose (mg/dl) | 154 ± 85 | 178 ± 91 | |
| HbA1c (%) | 8.6 ± 3.3 | 8.6 ± 3.3 | |
| Insulin (mU/ml) | 24.4 ± 13.3 | 26.9 ± 20.6 | |
| IL-6 (pg/ml) | 2.9 ± 2.1 | 2.0 ± 1.1 | 0.0299b |
| TNF-α (pg/ml) | 1.9 ± 1.1 | 1.8 ± 1.0 | |
| CRP (mg/liter) | 6.8 ± 6.9 | 3.8 ± 3.6 | 0.0232b |
| Duration of diabetes (yr) | 4.4 ± 2.6 | 4.3 ± 2.9 | |
| Common carotid (mm) | 0.52 ± 0.09 | 0.58 ± 0.11 | 0.0035b |
| Bulb (mm) | 0.51 ± 0.14 | 0.55 ± 0.12 | 0.0798b |
| Internal carotid (mm) | 0.42 ± 0.09 | 0.48 ± 0.11 | 0.0015b |
Data are expressed as mean ± sd.
t Test.
Wilcoxon rank sum test, P value < 0.05.
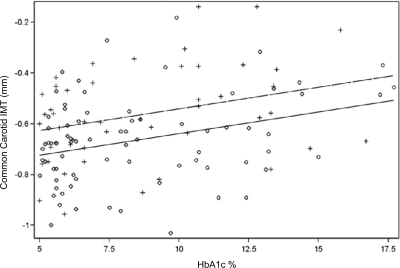
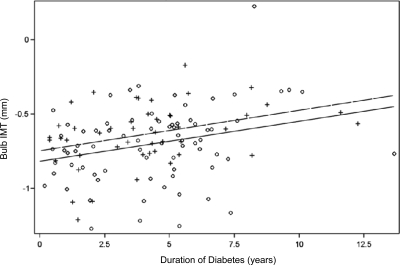
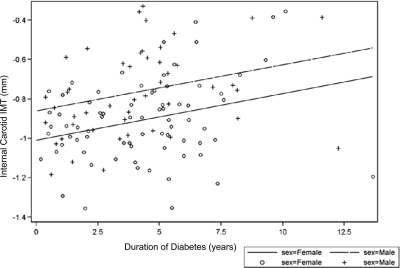
Multiple regression modeling revealed age, HbA1c, and male sex as the only significant determinants of common carotid IMT explaining 26% of the variance. The modeling was then repeated for each of the carotid segments. Table 2 demonstrates that whereas HbA1c was found to be an important determinant in the common carotid artery, duration of diabetes was important in the bulb and internal carotid segments. These linear relationships are illustrated in a scatter plot by sex in Figs. 1–3. Regression modeling demonstrated that male sex was significant in all three carotid segments, whereas elevated BP (systolic or diastolic) was important in the bulb and internal segments. LDL cholesterol was significant in the internal carotid segment. Medications, including metformin, insulin, antihypertensives, and lipid-lowering agents, were included in the regression models and were not significant.
Table 2.
Significant determinates of carotid IMTa
| Regionb | Common carotid | Bulb | Internal carotid |
|---|---|---|---|
| Age | 0.0146 | ||
| Sex (male) | 0.0982 | 0.0999 | 0.1532 |
| Height | 0.0050 | ||
| Systolic BP z-score | 0.0679 | ||
| Diastolic BP z-score | 0.0393 | ||
| LDL cholesterol | 0.0014 | ||
| HbA1c (%) | 0.0131 | ||
| Duration | 0.0244 | 0.0159 | |
| R2 (adjusted) | 0.23 | 0.22 | 0.32 |
All models have P < 0.0001.
All parameter estimates listed have P < 0.05.
Figure 1.
Linear relationship of common carotid IMT and HbA1c.
Figure 2.
Linear relationship of bulb IMT and duration of diabetes.
Figure 3.
Linear relationship of internal carotid IMT and duration of diabetes.
Logistic regression was performed to elucidate independent determinants of elevated carotid IMT. In our youth with type 2 diabetes mellitus, 13.4% had an elevated common carotid IMT, 16.5% had an elevated bulb IMT, and 18.9% had an elevated internal carotid IMT. For the common carotid, sex, HbA1c, insulin administration, and systolic BP z-score (z) were significantly associated with thicker common carotid artery. After controlling for sex, systolic BP z-score, and insulin administration, the odds for a thicker common IMT increased 35% with each 1% increase in HbA1c [95% confidence interval (CI), 1.12–1.63; P = 0.0016]. In the carotid bulb, systolic BP z-score and duration of diabetes were significantly associated with thicker IMT, whereas higher HDL cholesterol was associated with a better outcome. After controlling for systolic BP z and HDL cholesterol, the odds of having a thicker bulb increased by 33% for each year increase in duration of diabetes (95% CI, 1.08–1.65; P = 0.0076). For the internal carotid artery, the odds for a thicker vessel increased with male sex, higher LDL cholesterol or TNF-α levels, lower HDL cholesterol levels, and longer duration of diabetes. After controlling for sex, cholesterol levels, and TNF-α, the odds for thicker internal carotid IMT increased by 29% with each year increase in duration of diabetes (95% CI, 1.01–1.65; P = 0.0399).
Discussion
This study demonstrates that increased carotid IMT is associated with higher HbA1c concentrations and longer duration of type 2 diabetes mellitus in youth. Specifically, the logistic regression analysis established that each 1% increase in HbA1c or each year of duration of diabetes is associated with approximately 30% increased odds of a thicker carotid IMT. These data suggest that poor glycemic control is associated with structural changes in the carotid artery that are consistent with early atherosclerosis. In addition, regression models demonstrated that traditional cardiovascular risk factors, including BP, LDL cholesterol, and male sex, are also important determinants of carotid IMT in this population. To examine whether age, race, or sex intensifies the association between HbA1c or duration or disease and carotid IMT, we included these interaction terms in regression models. We found no significant interactions. These data establish that HbA1c and duration of diabetes are independent factors in the progression of thickening of carotid IMT. Thus, this cross-sectional study demonstrates that HbA1c, duration of diabetes, and traditional cardiovascular risk factors provide individual contributions to the development of atherosclerosis in adolescents and young adults with type 2 diabetes mellitus.
Previous studies in adults have demonstrated an association between glucose control and increased carotid IMT. Doruk et al. (10) reported that healthy adults with normal HbA1c levels (<6%) show no evidence of increased carotid IMT. Other investigators showed impaired glucose tolerance to be associated with an increase in carotid IMT but one third less than that seen in adults with type 2 diabetes mellitus (5). Our data provide evidence that the degree of hyperglycemia is associated with increased carotid IMT in youth. These findings suggest that improvement in glucose control at an early age may reduce the progression of atherosclerosis.
The relationship between sex and carotid IMT is complex. Studies in adults with type 2 diabetes mellitus have demonstrated similar rates of coronary artery disease and mortality in men and premenopausal women. Investigators suggest that the presence of diabetes eliminates the protection from cardiovascular disease in these females (11). However, there are currently no studies documenting similarities or differences in carotid IMT by sex in adults. Carotid IMT studies in youth with type 1 diabetes mellitus have demonstrated conflicting results. Peppa-Patrikiou et al. (12) demonstrated significantly higher carotid IMT values in male vs. female subjects, whereas Yavuz et al. (13) demonstrated no sex differences. To date, we are unaware of any studies that have examined sex differences in adolescents with type 2 diabetes mellitus. Our findings present new data that demonstrate that sex is a significant independent determinant of carotid IMT in adolescents with type 2 diabetes mellitus, and male sex is associated with worse outcomes in carotid IMT. Furthermore, these findings suggest that the protective effect of female sex may still be present in youth with type 2 diabetes mellitus who on average have had disease less than 5 yr.
In adults, Liu et al. (14) reported recently that carotid IMT was significantly associated with the duration of type 2 diabetes mellitus for longer than 2 yr. A few studies in adolescents with type 1 diabetes mellitus have investigated the relationship between duration of diabetes and carotid IMT. Disease duration appeared to be associated with increased carotid IMT in two studies (13,15), but others have not confirmed this association (12,16,17). To our knowledge, duration of diabetes and carotid IMT have not been studied in adolescents with type 2 diabetes mellitus. In the present study, we demonstrate that duration of diabetes is associated with increased carotid IMT in the internal carotid and bulb segments.
In conclusion, HbA1c and disease duration have individual adverse effects on carotid IMT in adolescents with type 2 diabetes mellitus in the presence of traditional cardiovascular risk factors. These adverse effects appear to be more prominent in males. Our findings suggest that the arterial tree is vulnerable to hyperglycemia beginning in youth. Adult data show that carotid IMT is linked to the atherosclerotic process and is associated with an increased risk of myocardial infarction and stroke (5,18). Therefore, developing effective strategies to improve blood glucose control in youth with type 2 diabetes mellitus is essential to prevent or limit the development and progression of cardiovascular disease.
Acknowledgments
The authors greatly acknowledge the excellent sonography work of Connie McCoy, RVT, and the participants of the Type 2 Diabetes Mellitus and Cardiovascular Disease Study and their families.
Footnotes
This work was supported by National Institutes of Health (National Heart, Lung, and Blood Institute) Grant R01 HL076269.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 1, 2009
Abbreviations: BMI, Body mass index; BP, blood pressure; CI, confidence interval; CRP, C-reactive protein; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; IMT, intima-media thickness; LDL, low-density lipoprotein.
References
- Panzram G 1987 Mortality and survival in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 30:123–131 [DOI] [PubMed] [Google Scholar]
- 1990 Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA 264:3018–3024 [DOI] [PubMed] [Google Scholar]
- Schulte-Altedorneburg G, Droste DW, Felszeghy S, Kellermann M, Popa V, Hegedüs K, Hegedüs C, Schmid M, Módis L, Ringelstein EB, Csiba L 2001 Accuracy of in vivo carotid B-mode ultrasound compared with pathological analysis: intima-media thickening, lumen diameter, and cross-sectional area. Stroke 32:1520–1524 [DOI] [PubMed] [Google Scholar]
- Davis PH, Dawson JD, Riley WA, Lauer RM 2001 Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation 104:2815–2819 [DOI] [PubMed] [Google Scholar]
- Brohall G, Odén A, Fagerberg B 2006 Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 23:609–616 [DOI] [PubMed] [Google Scholar]
- 2008 Standards of medical care in diabetes–2008. Diabetes Care 31(Suppl 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 2004 The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576 [PubMed] [Google Scholar]
- Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD 2000 Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes 49:485–491 [DOI] [PubMed] [Google Scholar]
- 2002 SAS OnlineDoc, version 9.1.3. Cary, NC: SAS Institute http://www.sas.com/ [Google Scholar]
- Doruk H, Mas MR, Ateþkan U, Isik AT, Sađlam M, Kutlu M 2005 The relationship between age and carotid artery intima-media thickness, hemoglobin A1c in nondiabetic, healthy geriatric population. Arch Gerontol Geriatr 41:113–119 [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Paradisi G, Cronin J, Crowde K, Hempfling A, Hook G, Baron AD 2000 Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation 101:2040–2046 [DOI] [PubMed] [Google Scholar]
- Peppa-Patrikiou M, Scordili M, Antoniou A, Giannaki M, Dracopoulou M, Dacou-Voutetakis C 1998 Carotid atherosclerosis in adolescents and young adults with IDDM. Relation to urinary endothelin, albumin, free cortisol, and other factors. Diabetes Care 21:1004–1007 [DOI] [PubMed] [Google Scholar]
- Yavuz T, Akçay A, Omerođlu RE, Bundak R, Sükür M 2002 Ultrasonic evaluation of early atherosclerosis in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 15:1131–1136 [DOI] [PubMed] [Google Scholar]
- Liu YP, Zhan WW, Zhang YF, Chen YH, Lin YY, Zhu Y, Ren XP, Li XY, Ning G 2007 Carotid intima-media thickness and stiffness in relation to type 2 diabetes in Chinese. Endocrine 31:289–293 [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Kawamori R, Matsushima H, Nishizawa H, Kodama M, Kajimoto Y, Morishima T, Kamada T 1994 Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes 43:634–639 [DOI] [PubMed] [Google Scholar]
- Frost D, Beischer W 1998 Determinants of carotid artery wall thickening in young patients with type 1 diabetes mellitus. Diabet Med 15:851–857 [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Yoshitake E, Otani T, Uchigata Y, Kawagoe M, Kasahara T, Omori Y 1993 Carotid atherosclerosis in young-aged IDDM associated with diabetic retinopathy and diastolic blood pressure. Diabetes Res Clin Pract 21:155–159 [DOI] [PubMed] [Google Scholar]
- McGill Jr HC, McMahan CA, Herderick EE, Tracy RE, Malcom GT, Zieskeh AW, Strong JP 2000 Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol 20:836–845 [DOI] [PubMed] [Google Scholar]