Abstract
Background
Rehospitalization rates are higher in African-American than Caucasian patients with heart failure (HF). The reasons for the disparity in outcomes between African-Americans and Caucasians may relate to differences in medication adherence.
Objective
To determine whether medication adherence is a mediator of the relationship between ethnicity and event-free survival in patients with HF.
Methods
Medication adherence was monitored longitudinally in 135 HF patients using the Medication Event Monitoring System (MEMS). Events (ED visits for HF exacerbation, HF and cardiac rehospitalization, and all cause mortality) were obtained by interview and hospital data base review. A series of regression models and survival analyses were conducted to determine whether medication adherence mediated the relationship between ethnicity and event-free survival.
Results
Event-free survival was significantly worse in African-Americans than Caucasians. Ethnicity was a predictor of medication adherence (p = .011). African-Americans were 2.57 times more likely to experience an event than Caucasians (p = .026). Ethnicity was not a predictor of event-free survival after entering medication adherence in the model (p = .06).
Conclusion
Medication adherence was a mediator of the relationship between ethnicity and event-free survival in this sample. Interventions designed to reduce barriers to medication adherence may decrease the disparity in outcomes.
Keywords: medication adherence, heart failure, outcomes, ethnicity, mediator
Introduction
Heart failure (HF) has been described as a “new epidemic”. The number of people diagnosed with HF is expected to double within the next 25 years owing to the aging of the population and improvements in treatment for cardiac conditions resulting in increased survival of cardiovascular diseases.1, 2 Acute exacerbation of HF signs and symptoms commonly requires hospitalizations that may contribute to increased mortality.3
The natural history of HF can now be modified by appropriate pharmacological therapy.4–6 However, pharmacological therapy only benefits patients who take their medications as prescribed. Therefore, medication adherence is essential to achieve better health outcomes.7–14 Poor medication adherence increases the risk of mortality and morbidity15 and leads to high health care costs8, 12, 15 in patients with HF.
There is a higher prevalence of HF among African-Americans compared with the general population (3% vs 2%, respectively).16, 17 African-Americans tend to be diagnosed with HF at an earlier age, have lower left ventricular ejection fractions (LVEF), and worse New York Heart Association (NYHA) functional class at the time of diagnosis than Caucasians.16, 18–20 Moreover, African-American HF patients have higher rehospitalization rates,21–26 longer lengths of hospital stay25 and higher hospital charges25 than Caucasian patients. The reasons for the disparity in outcomes between African-Americans and Caucasians are unclear. Prior researchers have suggested that African-American patients with HF have lower adherence rates than Caucasian patients with HF27, 28 but whether differences in medication adherence may play a role in disparity in outcomes is unknown. Accordingly, the purpose of this study was to determine whether medication adherence is a mediator of the relationship between ethnicity and the composite endpoint of event-free survival of ED visits for HF exacerbation or cardiac or HF hospitalization or mortality in patients with HF.
Methods
Study Design
This was a prospective, longitudinal study in which patients with HF were followed for up to 3.5 years. At baseline, patients' demographic and clinical data were collected by patient interview or medical record review. Outcome data on hospitalizations or survival were assessed monthly by telephone interview and by examining the hospital administrative database.
Samples and Setting
Detailed eligibility criteria and recruitment methods have been published previously.29 Patients of all ethnicities and either gender were recruited from outpatient cardiology clinics and inpatient cardiology wards in Central Kentucky. Patients were enrolled who had a confirmed diagnosis of chronic HF and were on stable doses of HF medications. Patients were excluded if they had obvious cognitive impairment (i.e., not able to give informed consent or participate in an interview), were referred for heart transplantation, or had a co-existing terminal illness such as cancer, or end-stage renal disease. No patient was attending a heart failure disease management program.
Measurement of Variables
Independent variable
Ethnicity was the independent variable in this study. Patient's self-reported ethnicity was collected by patient interview.
Mediator variable
Medication adherence was tested as a potential mediator variable in the study. Medication adherence was measured continuously for 3 months using a microelectronic medication monitoring device (Medication Event Monitoring System [MEMS], AARDEX®-USA, Union City, CA) that is housed in the caps of a medication vial. Real-time data were collected when the cap was removed. Patients kept a diary of cap openings not related to taking medication such as checking their medication supply or filling the bottle. All cap openings unrelated to taking medications were deleted from the analysis. Medication adherence from the MEMS was defined as the dose-count which is the percentage of prescribed doses taken during the 3-month monitoring period.29 Patients who took at least 88% of their prescribed doses were categorized as adherent, while patients who took less than 88% of doses were categorized as non-adherent. This cutpoint was chosen based on prior research demonstrating that adherence at or above this level predicted better event-free survival.30, 31
The MEMS was chosen as the measure of medication adherence because the MEMS is an objective measure considered the new reference standard for the measurement of medication adherence.32–37 Evidence from validation studies of the MEMS confirms that patients rarely remove a pill without taking it.38, 39 For example, in Kimmel et al.'s study,39 anticoagulation control, measured by International Normalized Ratio (INR) was correlated with medication adherence using the MEMS. In addition, using electronic monitoring caps to measure medication adherence can better identify patients who omitted doses than other measures.40 Furthermore, using electronic monitoring caps to measure medication adherence does not alter adherence.41 In one study,41 HIV patients were randomly assigned to one of the following three groups in order to determine the impact of surveillance methods on adherence: MEMS, medication diary, and a no surveillance control group, with adherence measured by a structured interview at baseline and study endpoint. After four weeks, there were no differences in adherence between the three groups, demonstrating that there is no Hawthorne effect associated with using the MEMS.
MEMS data were collected from one HF medication for each patient. Prior research has demonstrated that monitoring one medication provides a valid indicator of all medication-taking behavior.32, 33 The medication chosen to be monitored was based on the following criteria. If the patient was taking a medication twice a day, this medication was chosen for monitoring using the MEMS. If all medications were taken twice or only once per day, then the beta-adrenergic blocking agent was chosen unless the patient was not prescribed one. In those cases, the angiotension-converting-enzyme (ACE) inhibitor or angiotensin receptor blocker was used. If no beta-blocker or ACE inhibitor was prescribed, digoxin or a diuretic was used in the MEMS device.
The MEMS was used for three months to measure patient's medication adherence because three months has been shown to accurately reflect long-term adherence,42, 43 it avoids overburdening the patient with a longer data collection period, and is longer than many studies in which the MEMS was used for less than a month.32, 40, 44
Outcome variable
The outcome variable was the composite end-point of occurrences and time to the following events: ED visits for symptoms of decompensated HF, HF or cardiac hospitalizations and all cause mortality (i.e., event-free survival). Data on event-free survival were obtained by patient/family interview, hospital data base review and review of death certificates and records. During data collection, the date and reasons for ED visits, hospitalization and death were noted. If there was a difference between patient/family report and the hospital records, we carefully reviewed the medical record to confirm the visit date and reason, and discussed the discrepancy with the patient or family. If the ED visit or rehospitalization was outside the system, a patient release was obtained and the medical record was reviewed. In all cases, conflicting data between patient/family report and hospital records were resolved with review of the medical record and interview of the patient and family.
Covariates
New York Heart Association (NYHA) functional class, age, gender, education level, living status, body mass index (BMI), left ventricular ejection fraction (LVEF), medical regimen, patient's attitude toward prescribed medication, knowledge of medication, and barrier to medication adherence were collected as covariates. NYHA was determined by standardized patient interview.45 Patients' age, gender, education level, living status, LVEF and medication regimen (i.e., ACE inhibitors [yes/no], β blockers [yes/no], diuretics [yes/no], digoxin [yes/no], aldosterone antagonist [yes/no]) were collected from the medical record, and patient interview.
Patient's attitudes toward medication adherence, knowledge of medication and barriers to medication adherence were measured using the Attitude, Knowledge, and Barriers subscales of the Medication Adherence Scale (MAS).46 The 3-item Attitude subscale ranges from 0 to 30; higher scores indicate a more positive attitude toward medication adherence. The Knowledge subscale consists of three items. The Knowledge subscale ranges from 0 to 30; higher scores indicate more knowledge of prescribed medication. The Barriers subscale consists of 11 items. Patients were asked to rate how important they think each of these 11 causes of not taking pills. Patients rated on a 10-point scale that is scored from 10 (very important cause) to 0 (not important cause). The total score ranges from 0 to 110 with a higher score reflecting more barriers to adhering to prescribed medication. One example of an item is “cost of medication”. Based on our prior study,46 the MAS is a reliable and valid indicator of attitudes, knowledge and barriers to medication adherence in patients with HF.
Procedure
Permission to conduct the study was obtained from the University of Kentucky (UK) Institutional Review Board (IRB). A trained research nurse confirmed patients' eligibility, explained study requirements to eligible patients, and obtained informed, written consent.
At baseline, patients' sociodemographic and clinical characteristics were collected by interview and medical record review. Detailed written and verbal instructions on use of the MEMS bottle were then given to patients. Patients were instructed to take the specified medicine from the MEMS bottle for three months and to close the cap after each use. A medication diary was given to patients to record unscheduled cap openings. Patients who used a pill box were asked to keep the MEMS bottle beside their pill box and take that medicine from the MEMS bottle.
Patients returned the bottle by mail or in person after three months of continuous use of the MEMS. The data from the MEMS cap were downloaded to a personal computer, printed, and entered into a data base for analysis. Patients were followed for up to 3.5 years to collect data regarding ED visits, hospitalizations and death.
Data Management and Analysis
All data analyses were performed using SPSS, version 16.0; a significance level of .05 was used throughout. Data analysis began with a descriptive examination of all variables, including frequency distributions, means, standard deviations, medians, and interquartile ranges, as appropriate to the level of measurement of the variables.
Patients were divided into adherent and nonadherent groups based on their medication adherence rate measured by the MEMS using a cutpoint of 88%30 and into the African-American or Caucasian group. To compare time to the composite end-point, the log-rank test was used to compare the time to the endpoint between African-Americans and Caucasians. Kaplan-Meier plots were used to graphically depict group differences in event-free survival. Cox proportional hazards regression modeling was used to assess the time to the composite end-point between the two ethnic groups, while controlling for the following potential covariates: age, gender, education level, living status, ejection fraction, baseline NYHA, LVEF, ACE inhibitor use, and beta-blocker use.
To test whether medication adherence was a mediator of the relationship between ethnicity and event-free survival, a series of regression models and Cox-survival analyses were conducted. The test for mediation followed the steps outlined by Baron et al.47–50 Four regression models were run to test for the mediator effect. The first model tested whether ethnicity (the independent variable) was a predictor of the medication adherence (mediator). The second model tested whether medication adherence was a predictor of event-free survival (outcome variable). The third model tested whether ethnicity was a predictor of event-free survival. In the fourth model, both the ethnicity and medication adherence (independent and mediator variables) were entered simultaneously as predictors of the event-free survival (outcome variable). The following conditions must be met if a mediator effect is present: 1) the results of the first, second, and the third models should be significant, and 2) the significance level of the coefficient associated with the independent variable in the fourth model is less significant (partial mediator) or non-significant (full mediator) than in the third model.47, 48, 51
Results
Patient Characteristics
A total of 147 patients with HF were recruited for the study but complete MEMS data were obtained from 135 patients (Table 1). The reasons for incomplete MEMS data from the 12 patients were of technical failure of the cap or loss of the MEMS cap by the patient. The mean age of patients in the sample was 61 years. The average LVEF reflected enrollment of patients with and without systolic dysfunction. Thirty percent of the patients were female. One quarter of the patients did not complete high school education (26%). About two thirds of patients were classified as NYHA class III or IV. Full sample characteristics and comparison of the two ethnic groups were presented in Table 1.
Table 1.
Sample Characteristics
| Characteristics | Total Sample (N = 135) | African-Americans (n = 14) | Caucasians (n = 121) | P |
|---|---|---|---|---|
| Age, years | 61 ± 11 | 57 ± 11 | 61 ± 11 | .227 |
| Female | 41 (30.4) | 8 (57.1) | 33 (27.3) | .026 |
| Education, years | 12.7 ± 3.3 | 13.4 ± 2.0 | 12.5 ± 3.4 | .362 |
| Living alone | 40 (29.6) | 5 (35.7) | 35 (28.9) | .402 |
| Financial status | .633 | |||
| Comfortable | 32 (24.1) | 2 (14.3) | 30 (25.2) | |
| Enough to make ends meet | 71 (53.4) | 8 (57.1) | 63 (52.9) | |
| Not enough to make ends meet | 30 (22.6) | 4 (28.6) | 26 (21.8) | |
| With government or commercial insurance | 125 (92.6) | 11 (78.6) | 114 (94.2) | .316 |
| With government insurance | 106 (78.5) | 10 (71.4) | 96 (79.3) | .499 |
| With commercial insurance | 24 (17.9) | 2 (14.3) | 22 (18.3) | 1.0 |
| Attitudes toward medication adherence | 28.5±3.2 | 27.9±3.2 | 28.6±3.3 | .430 |
| Knowledge of medication | 21.1±8.8 | 20.3±9.8 | 21.2±8.7 | .712 |
| Barriers to medication adherence | 18.8±29.9 | 31.6±32.9 | 17.4±29.3 | .103 |
| Cost of medication | 1.9±3.7 | 3.8±4.4 | 1.6±3.6 | .115 |
| LVEF, % | 34.6 ± 14.2 | 35.2 ± 17.4 | 34.6 ± 13.9 | .905 |
| NYHA functional class | .721 | |||
| I/II | 51 (38.9) | 6 (46.2) | 45 (38.1) | |
| III | 61 (46.6) | 6 (46.2) | 55 (46.6) | |
| IV | 19 (14.5) | 1 (7.7) | 18 (15.3) | |
| Charlson comorbidity index | 3.3 ± 1.7 | 3.2 ± 1.6 | 3.3 ± 1.7 | .800 |
| Hypertension | 103 (79.2) | 10 (76.9) | 93 (79.5) | .732 |
| Diabetes | 63 (47.4) | 6 (42.9) | 57 (47.9) | .783 |
| Stroke | 25 (18.8) | 4 (28.6) | 21 (17.6) | .299 |
| Previous MI | 79 (60.8) | 5 (35.7) | 74 (63.8) | .079 |
| BMI | 31.9 ±7.1 | 34.2 ± 9.7 | 31.6 ± 6.7 | .297 |
| Number of pill taken daily | 13 ± 7.0 | 14.2 ± 9.1 | 12.5 ± 6.5 | .402 |
| Taking ACEI | 97 (71.9) | 10 (71.4) | 87 (71.9) | .595 |
| Taking BB | 120 (88.9) | 12 (85.7) | 108 (89.3) | .480 |
| Medication adherence | 97 (71.9) | 6 (42.9) | 91 (75.2) | .023 |
Data are presented as means ± SD, or N (%), interval level data compared by independent t-test, nominal and categorical by Chi-square; ACEI = angiotensin-converting-enzyme inhibitor; BB = beta blocker; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NYHA = New York Heart Association
Ninety percent of the patients were Caucasians. Of the total sample, 70% were classified as adherent. Caucasian patients were more likely to be adherent compared to the African-American patients. Gender was the only sociodemographic variable that differentiated patients in the two ethnic groups. There were a greater percentage of female patients in the African-American group (57.1%) than in the Caucasian group (27.3%). There were no group differences based on patients' government insurance (i.e., Medicare or Medicaid) status, financial status, BMI, co-morbidities (i.e., diabetes, hypertension, previous myocardial infarction and stroke), or number of pills taken per day. There was no group difference in the type of medication (generic or non-generic) monitored using the MEMS (p = .159).
Ethnicity, medication adherence and event-free survival
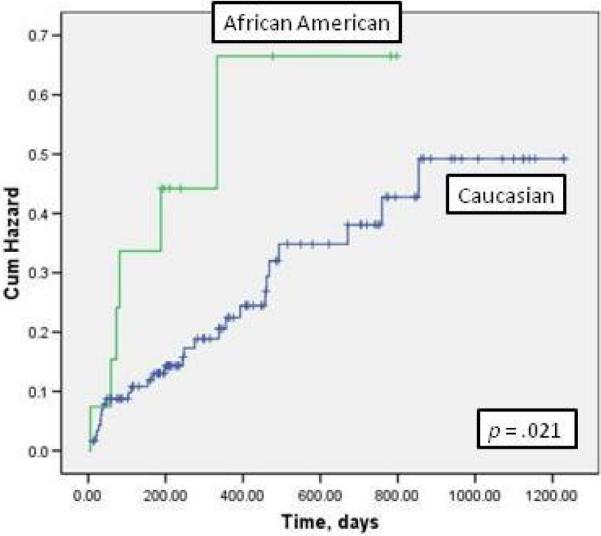
There was no difference in mortality rates between African-American and Caucasian patients. The prevalence of cardiac rehospitalizations was higher in African-American patients than Caucasian patients (47% vs. 19%, p = .004). In Kaplan-Meier analysis, the composite endpoint of event-free survival was significantly worse in African-Americans than in Caucasians (Figure 1).
Figure 1. Hazard Plot of ethnicity and event-free survival.
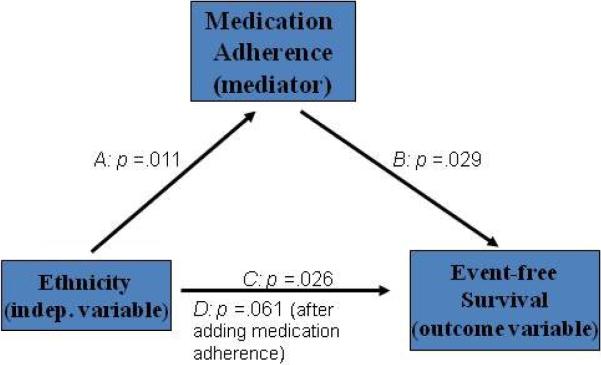
In a series of regression models and Cox-survival analyses medication adherence mediated the relationship between ethnicity and event-free survival based on the following sequence of regression analyses. First, in Path A (Figure 2), ethnicity independently predicted medication adherence (p = .011). Second, in Path B, patients who were nonadherent were 2.11 times more likely to experience an event than adherent patients (p = .029). Third, in Path C, African-Americans were more likely to experience an event than Caucasians (p = .026). In the final Path D, ethnicity was no longer a significant predictor of event-free survival when medication adherence was entered into the model (p = .06). It is important to note that African-Americans were 2.57 times more likely to experience an event than Caucasians in a simple Cox regression model (p = .026). After adjusting for age, gender, education level, living status, NYHA, LVEF, ACEI use and β blocker use, African-Americans were 3.19 times more likely to experience an event than Caucasians (p = .022; Table 2).
Figure 2. Medication adherence is a mediator.
Path A: Test of whether ethnicity is a predictor of medication adherence.
Path B: Test of whether medication adherence is a predictor of event-free survival.
Path C: Test of whether ethnicity is a predictor of event-free survival.
Path D: Test of whether ethnicity and medication adherence together are predictors of event-free survival.
Table 2.
Cox Regression Modeling: Ethnicity on Event-free Survival (N = 135)
| Variables | Hazard Ratio | Wald | Significance |
|---|---|---|---|
| *Simple Cox Regression | |||
| Ethnicity | 2.57 | 4.94 | 0.026 |
| **Multiple Cox Regression | |||
| Ethnicity | 3.189 | 5.241 | 0.022 |
| Age | 1.006 | 0.127 | 0.722 |
| Gender | 0.851 | 0.117 | 0.732 |
| Education | 1.002 | 0.001 | 0.976 |
| Living status | 0.564 | 1.911 | 0.167 |
| LVEF | 0.979 | 1.812 | 0.178 |
| NYHA | 1.007 | 0.001 | 0.982 |
| Taking ACEI | 0.913 | 0.035 | 0.851 |
| Taking BB | 0.343 | 4.364 | 0.037 |
χ2 = 5312, p = 0.021
χ2 = 18.684, p = 0.028
ACEI = angiotensin-converting-enzyme inhibitor; BB = beta blocker; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association
Discussion
A goal of Healthy People 2010 is the elimination of racial/ethnic health disparities.52 African-American patients with HF have a higher rate of hospital readmission than Caucasian patients.22–24, 26 This finding suggests that additional strategies that address factors underlying this disparity in African-American HF patients may be needed. It is important to explore factors associated with racial/ethnic health disparities in patients with HF so that effective interventions to health disparities can be developed. To our knowledge this is the first study to examine mediators of the link between ethnicity and outcomes in HF patients. Our results demonstrated that medication adherence was a mediator of the relationship between ethnicity and event-free survival in patients with HF.
Consistent with other studies, we found that the rates of HF or cardiac rehospitalization were higher in African-American patients than Caucasian patients.21–26, 53, 54 However, reasons for the increased risk for hospitalization in African-Americans compared to Caucasians are unclear. Biological differences between the two ethnic groups and/or differences in psychosocial and behavioral factors may play a role on racial/ethnic disparity in outcomes.24, 26, 55, 56
Currently, there is no direct evidence that biological differences among ethnicities contribute to disparity in outcomes. In some studies, investigators found genetic differences that contribute to the development of HF and identified differences in the etiology of HF between African-Americans and Caucasians. These findings provide some evidence to explain racial/ethnic disparity in outcomes. Advocates of a biological explanation for disparities point to advances in genomic techniques, that allowed investigation of genetic factors that might contribute to racial/ethnic disparity. In one case-comparison study,55 African-American participants with variant alpha2c receptor genotypes were 5 times more likely to have HF than African-Americans with other receptor genotypes. No HF risk was associated with the variant beta1 receptor alone; but, participants who had both variant receptor genotypes had a tenfold increased risk of developing HF. However, to date, there is no direct genetic explanation for the disparity in hospitalization rates.
Another biological explanation is the etiology of HF. Hypertension is the most common cause of HF in African American HF patients and coronary heart disease (CHD) is the most common cause of HF in Caucasian HF patients.16, 17, 19, 57–59 In the large Studies of Left Ventricular Dysfunction (SOLVD) registry, hypertension was found as the etiology of HF in 32% of African Americans and only 4% of Caucasians, while CHD was the etiology in 36% of African-Americans and 73% of Caucasians.56 In our study, hypertension was the cause of HF in 18% of African-Americans and only 5.5% of Caucasians, while CHD was the cause of HF in 35% of African-Americans and 63% of Caucasians. In a large study,60 investigators examined the natural history of HF with preserved left ventricular systolic function in African-American and Caucasian patients over five years. African American patients had a significantly higher mortality risk than white patients (hazard ratio [HR] = 1.34). The racial difference in survival rate was most prominent in patients with a non-ischemic etiology (HR = 1.6) compared with patients with ischemic heart failure. The finding that HF etiology is different between these two ethnic groups and non-ischemic etiology of HF leads to worse outcomes compared to ischemic etiology of HF provides other support for a biological explanation. However, without direct evidence, it is hard to conclude that racial/ethnic disparity in outcomes is due to biological differences. Therefore, more studies are needed to explore direct biological evidence of racial/ethnic difference in outcomes.
Other researchers have reported that differences in rehospitalizations may be related to difference in the prevalence of comorbid conditions and the effectiveness of their treatment,23, 24 differences in socioeconomic factors (e.g., age, access to care),23, 61 differences in psychological factors (e.g., depression),24 and differences in adherence to diet and prescribed medication.26 In our study, medication adherence emerged as a predominant mechanism linking ethnicity and poor outcomes. When we compared sociodemographic and clinical variables between African-American and Caucasian participants, only gender and medication adherence were different between these two ethnic groups. Although the African-American group had a greater percentage of female participants than Caucasian group, gender was not related to event-free survival. This result is consistent with prior studies showing no gender difference in rehospitalization or mortality in HF.62, 63
A major finding in our study was that patients in the Caucasian group were more likely to be adherent than those in the African-American group. Studies of the relationship between ethnicity and medication adherence have produced inconsistent results.27, 32, 64–66 A number of investigators reported no differences in medication adherence based on race/ethnicity.32, 64, 67, 68 However, in three studies,27, 65, 66 African-American participants were less adherent than Caucasian participants. Each of these studies had one or more of the following limitations that may have influenced their results: self-report measures of medication adherence,64, 67, 68 small sample sizes,32, 64 or lack of consistent definitions of medication adherence.27, 32, 64–68
It is unclear why African-Americans might have lower adherence. Investigators have postulated that it is not race alone, but the interaction of race and income that is related to adherence.69 Other investigators found that African-Americans were more likely to experience side effects from taking medication compared to Caucasians.70 Differences in adherence also may be related to differences in depression71 or out-of-pocket prescription costs.72 In a national study,73 African-Americans were 1.38 times more likely to report cost-related nonadherence compared to Caucasians.
In prior studies, investigators reported attitudes toward medication,74, 75 knowledge,75–77 and perceived barriers to medication taking78, 79 were correlated to medication adherence. African-Americans were more likely to have negative attitudes toward medication,80 lower levels of knowledge about medications,81, 82 and more barriers to medication taking.83 Low levels of health literacy81 may account for lower levels of health-related knowledge among African-Americans. However, in another study,84 African-American patients reported that their health care providers were more active in advising and counseling about hypertension care and medication adherence than did Caucasians. African-Americans were found to have more knowledge of the risks/benefits of available therapies, to be more aware of the importance of controlling their blood pressure, and to be equally adherent. These data suggest that disparities in health-related knowledge and attitudes between African-Americans and Caucasians can be eliminated when both patients and health care providers are actively engaged in the health care process.
The association between medication nonadherence and ethnicity could be explained by income, side effects of medication, depression, out-of-pocket prescription costs, cost of medication, attitudes toward medication taking, knowledge of medication and barriers to medication adherence. Our results suggest the need to collect more data of potential factors related to racial/ethnic disparity on medication adherence to provide further insight into the relationships among race/ethnicity, medication adherence and outcomes and to improve medication adherence and reduce racial/ethnic disparity in outcomes.
Limitations
Although we demonstrated significant differences between African-Americans and Caucasians, a larger sample size of African-American HF patients is needed to generalize these results. Our sample only included 10% African-Americans and future studies of this phenomenon should include a larger proportion of African-Americans so that the complex dynamics surrounding adherence and outcomes can be better illuminated. The small sample of African-Americans also may produce unstable results that need to be verified in a larger sample. Thus our findings should be considered exploratory and the need for replication emphasized.
Conclusion
The major finding of this study was that medication adherence was a mediator of the relationship between ethnicity and event-free survival in this sample. Although these data suggest that interventions designed to improve medication adherence in African-Americans may decrease the disparity in outcomes between African-American and Caucasian patients with HF, our findings must be replicated before definitive recommendations can be made.
Acknowledgements
This study was supported by funding from the Philips Medical-American Association of Critical Care Nurses Outcomes Grant, University of Kentucky General Clinical Research Center (M01RR02602), American Heart Association Great River Affiliate Post-doctoral Fellowship to Jia-Rong Wu, grant # R01 NR008567 from the National Institute of Nursing Research and a Center grant to the University of Kentucky, College of Nursing from NIH, NINR, 1P20NR010679. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Because this study was funded from the NIH/NINR, I also would like to inform the publishers of Journal of Cardiac Failure about a new NIH Public access policy as below. “Journal acknowledges that Author retains the right to provide a copy of the final manuscript to the NIH upon acceptance for Journal publication, for public archiving in PubMed Central as soon as possible but no later than 12 months after publication by Journal.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer Statement The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Air Force or the Department of Defense.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Opasich C, Febo O, Riccardi PG, et al. Concomitant factors of decompensation in chronic heart failure. Am. J. Cardiol. 1996;78:354–357. doi: 10.1016/s0002-9149(96)00294-9. [DOI] [PubMed] [Google Scholar]
- 4.Norgard NB, Stark JE. Pharmacotherapy for heart failure with left ventricular dysfunction: beyond angiotensin-converting enzyme inhibitors and beta-blockers. Pharmacotherapy. 2008;28:920–931. doi: 10.1592/phco.28.7.920. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Abraham WT, Albert NM, et al. Prospective evaluation of beta-blocker use at the time of hospital discharge as a heart failure performance measure: results from OPTIMIZE-HF. J. Card. Fail. 2007;13:722–731. doi: 10.1016/j.cardfail.2007.06.727. [DOI] [PubMed] [Google Scholar]
- 6.Rosen D, Decaro MV, Graham MG. Evidence-based treatment of chronic heart failure. Compr. Ther. 2007;33:2–17. doi: 10.1007/s12019-007-0006-0. [DOI] [PubMed] [Google Scholar]
- 7.Chin MH, Goldman L. Factors contributing to the hospitalization of patients with congestive heart failure. Am. J. Public Health. 1997;87:643–648. doi: 10.2105/ajph.87.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chui MA, Deer M, Bennett SJ, et al. Association between adherence to diuretic therapy and health care utilization in patients with heart failure. Pharmacotherapy. 2003;23:326–332. doi: 10.1592/phco.23.3.326.32112. [DOI] [PubMed] [Google Scholar]
- 9.Happ MB, Naylor MD, Roe-Prior P. Factors contributing to rehospitalization of elderly patients with heart failure. J. Cardiovasc. Nurs. 1997;11:75–84. doi: 10.1097/00005082-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Hays RD, Kravitz RL, Mazel RM, et al. The impact of patient adherence on health outcomes for patients with chronic disease in the Medical Outcomes Study. J. Behav. Med. 1994;17:347–360. doi: 10.1007/BF01858007. [DOI] [PubMed] [Google Scholar]
- 11.Joshi PP, Mohanan CJ, Sengupta SP, et al. Factors precipitating congestive heart failure--role of patient non-compliance. J. Assoc. Physicians India. 1999;47:294–295. [PubMed] [Google Scholar]
- 12.Li H, Morrow-Howell N, Proctor EK. Post-acute home care and hospital readmission of elderly patients with congestive heart failure. Health Soc. Work. 2004;29:275–285. doi: 10.1093/hsw/29.4.275. [DOI] [PubMed] [Google Scholar]
- 13.Miura T, Kojima R, Mizutani M, et al. Effect of digoxin noncompliance on hospitalization and mortality in patients with heart failure in long-term therapy: A prospective cohort study. Eur. J. Clin. Pharmacol. 2001;57:77–83. doi: 10.1007/s002280100272. [DOI] [PubMed] [Google Scholar]
- 14.Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med. Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 15.Hope CJ, Wu J, Tu W, et al. Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am. J. Health. Syst. Pharm. 2004;61:2043–2049. doi: 10.1093/ajhp/61.19.2043. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW. Heart failure in African Americans: a cardiovascular engima. J. Card. Fail. 2000;6:183–186. doi: 10.1054/jcaf.2000.17610. [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW. Heart failure in African Americans. Am. J. Cardiol. 2005;96:3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JE, Hellkamp AS, Mark DB, et al. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Am. Heart J. 2008;155:501–506. doi: 10.1016/j.ahj.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancy CW, Abraham WT, Albert NM, et al. Quality of care of and outcomes for African Americans hospitalized with heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) registry. J. Am. Coll. Cardiol. 2008;51:1675–1684. doi: 10.1016/j.jacc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Evangelista LS, Dracup K, Doering LV. Racial differences in treatment-seeking delays among heart failure patients. J. Card. Fail. 2002;8:381–386. doi: 10.1054/jcaf.2002.129234. [DOI] [PubMed] [Google Scholar]
- 21.Alexander M, Grumbach K, Remy L, et al. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. Am. Heart J. 1999;137:919–927. doi: 10.1016/s0002-8703(99)70417-5. [DOI] [PubMed] [Google Scholar]
- 22.Brown DW, Haldeman GA, Croft JB, et al. Racial or ethnic differences in hospitalization for heart failure among elderly adults: Medicare, 1990 to 2000. Am. Heart J. 2005;150:448–454. doi: 10.1016/j.ahj.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Deswal A, Petersen NJ, Urbauer DL, et al. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am. Heart J. 2006;152:348–354. doi: 10.1016/j.ahj.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Mathew J, Wittes J, McSherry F, et al. Racial differences in outcome and treatment effect in congestive heart failure. Am. Heart J. 2005;150:968–976. doi: 10.1016/j.ahj.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 25.Philbin EF, DiSalvo TG. Influence of race and gender on care process, resource use, and hospital-based outcomes in congestive heart failure. Am. J. Cardiol. 1998;82:76–81. doi: 10.1016/s0002-9149(98)00233-1. [DOI] [PubMed] [Google Scholar]
- 26.Rathore SS, Foody JM, Wang Y, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289:2517–2524. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 27.Bagchi AD, Esposito D, Kim M, et al. Utilization of, and adherence to, drug therapy among medicaid beneficiaries with congestive heart failure. Clin. Ther. 2007;29:1771–1783. doi: 10.1016/j.clinthera.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Wu JR, Moser DK, Chung ML, et al. Predictors of medication adherence using a multidimensional adherence model in patients with heart failure. J. Card. Fail. 2008;14:603–614. doi: 10.1016/j.cardfail.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu JR, Moser DK, Chung ML, et al. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. J. Card. Fail. 2008;14:203–210. doi: 10.1016/j.cardfail.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu JR, Lennie TA, De Jong M, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Circulation. 2008;118:S1039. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu JR, Moser DK, De Jong MJ, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Am. Heart J. 2009;157:285–291. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunbar-Jacob J, Bohachick P, Mortimer MK, et al. Medication adherence in persons with cardiovascular disease. J. Cardiovasc. Nurs. 2003;18:209–218. doi: 10.1097/00005082-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Bouvy ML, Heerdink ER, Urquhart J, et al. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: A randomized controlled study. J. Card. Fail. 2003;9:404–411. doi: 10.1054/s1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 34.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin. Ther. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. discussion 1073. [DOI] [PubMed] [Google Scholar]
- 35.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J. Clin. Epidemiol. 2001;54(Suppl 1):S57–60. doi: 10.1016/s0895-4356(01)00457-7. [DOI] [PubMed] [Google Scholar]
- 36.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 37.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med. Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Cheng CW, Woo KS, Chan JC, et al. Association between adherence to statin therapy and lipid control in Hong Kong Chinese patients at high risk of coronary heart disease. Br. J. Clin. Pharmacol. 2004;58:528–535. doi: 10.1111/j.1365-2125.2004.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Arch. Intern. Med. 2007;167:229–235. doi: 10.1001/archinte.167.3.229. [DOI] [PubMed] [Google Scholar]
- 40.Svarstad BL, Chewning BA, Sleath BL, et al. The Brief Medication Questionnaire: A tool for screening patient adherence and barriers to adherence. Patient Educ. Couns. 1999;37:113–124. doi: 10.1016/s0738-3991(98)00107-4. [DOI] [PubMed] [Google Scholar]
- 41.Wagner GJ, Ghosh-Dastidar B. Electronic monitoring: adherence assessment or intervention? HIV clinical trials. 2002;3:45–51. doi: 10.1310/XGXU-FUDK-A9QT-MPTF. [DOI] [PubMed] [Google Scholar]
- 42.Bohachick P, Burke LE, Sereika S, et al. Adherence to angiotensin-converting enzyme inhibitor therapy for heart failure. Prog. Cardiovasc. Nurs. 2002;17:160–166. doi: 10.1111/j.0889-7204.2002.01643.x. [DOI] [PubMed] [Google Scholar]
- 43.Melbourne KM, Geletko SM, Brown SL, et al. Medication adherence in patients with HIV infection: a comparison of two measurement methods. AIDS Read. 1999;9:329–338. [PubMed] [Google Scholar]
- 44.Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12:86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 45.Mills RM, Jr., Haught WH. Evaluation of heart failure patients: Objective parameters to assess functional capacity. Clin. Cardiol. 1996;19:455–460. doi: 10.1002/clc.4960190603. [DOI] [PubMed] [Google Scholar]
- 46.Wu JR, Chung M, Lennie TA, et al. Testing the psychometric properties of the Medication Adherence Scale in patients with heart failure. Heart Lung. 2008;37:334–343. doi: 10.1016/j.hrtlng.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 48.Bennett JA. Mediator and moderator variables in nursing research: Conceptual and statistical differences. Res. Nurs. Health. 2000;23:415–420. doi: 10.1002/1098-240x(200010)23:5<415::aid-nur8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 49.MacKinnon DP, Lockwood CM, Hoffman JM, et al. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacKinnon DP, MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval. Rev. 1993;17:144–158. [Google Scholar]
- 51.Sonnentag S, Zijlstra FR. Job characteristics and off-job activities as predictors of need for recovery, well-being, and fatigue. J. Appl. Psychol. 2006;91:330–350. doi: 10.1037/0021-9010.91.2.330. [DOI] [PubMed] [Google Scholar]
- 52.US Department of Health and Human Services . Healthy People 2010: With Understanding and Improving Health and Objectives for Improving Health. 2nd ed. Vol 1. U.S. Government Printing Office; Washington, DC: 2000. [Google Scholar]
- 53.Lafata JE, Pladevall M, Divine G, et al. Are there race/ethnicity differences in outpatient congestive heart failure management, hospital use, and mortality among an insured population? Med. Care. 2004;42:680–689. doi: 10.1097/01.mlr.0000129903.12843.fc. [DOI] [PubMed] [Google Scholar]
- 54.Prisant LM, Thomas KL, Lewis EF, et al. Racial analysis of patients with myocardial infarction complicated by heart failure and/or left ventricular dysfunction treated with valsartan, captopril, or both. J. Am. Coll. Cardiol. 2008;51:1865–1871. doi: 10.1016/j.jacc.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 55.Small KM, Wagoner LE, Levin AM, et al. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N. Engl. J. Med. 2002;347:1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 56.Bourassa MG, Gurne O, Bangdiwala SI, et al. Natural history and patterns of current practice in heart failure. The Studies of Left Ventricular Dysfunction (SOLVD) Investigators. J. Am. Coll. Cardiol. 1993;22:14A–19A. doi: 10.1016/0735-1097(93)90456-b. [DOI] [PubMed] [Google Scholar]
- 57.Yancy CW. Treatment of heart failure in African Americans: clinical update. Ethn. Dis. 2002;12:S1–19–26. [PubMed] [Google Scholar]
- 58.Yancy CW. Heart failure in African Americans: pathophysiology and treatment. J. Card. Fail. 2003;9:S210–215. doi: 10.1054/s1071-9164(03)00590-6. [DOI] [PubMed] [Google Scholar]
- 59.Yancy CW. The prevention of heart failure in minority communities and discrepancies in health care delivery systems. Med. Clin. North Am. 2004;88:1347–1368. xii–xiii. doi: 10.1016/j.mcna.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 60.East MA, Peterson ED, Shaw LK, et al. Racial differences in the outcomes of patients with diastolic heart failure. Am. Heart J. 2004;148:151–156. doi: 10.1016/j.ahj.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Vaccarino V, Gahbauer E, Kasl SV, et al. Differences between African Americans and whites in the outcome of heart failure: Evidence for a greater functional decline in African Americans. Am. Heart J. 2002;143:1058–1067. doi: 10.1067/mhj.2002.122123. [DOI] [PubMed] [Google Scholar]
- 62.Diercks DB, Fonarow GC, Kirk JD, et al. Risk stratification in women enrolled in the Acute Decompensated Heart Failure National Registry Emergency Module (ADHERE-EM) Acad. Emerg. Med. 2008;15:151–158. doi: 10.1111/j.1553-2712.2008.00030.x. [DOI] [PubMed] [Google Scholar]
- 63.Mullens W, Abrahams Z, Sokos G, et al. Gender differences in patients admitted with advanced decompensated heart failure. Am. J. Cardiol. 2008;102:454–458. doi: 10.1016/j.amjcard.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Evangelista LS, Berg J, Dracup K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart Lung. 2001;30:294–301. doi: 10.1067/mhl.2001.116011. [DOI] [PubMed] [Google Scholar]
- 65.Graveley EA, Oseasohn CS. Multiple drug regimens: Medication compliance among veterans 65 years and older. Res. Nurs. Health. 1991;14:51–58. doi: 10.1002/nur.4770140108. [DOI] [PubMed] [Google Scholar]
- 66.Rich MW, Gray DB, Beckham V, et al. Effect of a multidisciplinary intervention on medication compliance in elderly patients with congestive heart failure. Am. J. Med. 1996;101:270–276. doi: 10.1016/s0002-9343(96)00172-6. [DOI] [PubMed] [Google Scholar]
- 67.Conn V, Taylor S, Miller R. Cognitive impairment and medication adherence. J. Gerontol. Nurs. 1994;20:41–47. doi: 10.3928/0098-9134-19940701-09. [DOI] [PubMed] [Google Scholar]
- 68.Morgan AL, Masoudi FA, Havranek EP, et al. Difficulty taking medications, depression, and health status in heart failure patients. J. Card. Fail. 2006;12:54–60. doi: 10.1016/j.cardfail.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Akincigil A, Bowblis JR, Levin C, et al. Long-Term Adherence to Evidence Based Secondary Prevention Therapies after Acute Myocardial Infarction. J. Gen. Intern. Med. 2007 doi: 10.1007/s11606-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am. J. Med. 2006;119:70, e79–15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Margolick JB, Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J. Acquir. Immune Defic. Syndr. 2005;38:320–328. [PubMed] [Google Scholar]
- 72.Klein D, Turvey C, Wallace R. Elders who delay medication because of cost: health insurance, demographic, health, and financial correlates. Gerontologist. 2004;44:779–787. doi: 10.1093/geront/44.6.779. [DOI] [PubMed] [Google Scholar]
- 73.Gellad WF, Haas JS, Safran DG. Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study. J. Gen. Intern. Med. 2007;22:1572–1578. doi: 10.1007/s11606-007-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 1999;47:555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 75.van der Wal MH, Jaarsma T, Moser DK, et al. Compliance in heart failure patients: The importance of knowledge and beliefs. Eur. Heart J. 2006;27:434–440. doi: 10.1093/eurheartj/ehi603. [DOI] [PubMed] [Google Scholar]
- 76.Welsh JD, Heiser RM, Schooler MP, et al. Characteristics and treatment of patients with heart failure in the emergency department. J. Emerg. Nurs. 2002;28:126–131. doi: 10.1067/men.2002.123074. [DOI] [PubMed] [Google Scholar]
- 77.Kim EY, Han HR, Jeong S, et al. Does knowledge matter?: intentional medication nonadherence among middle-aged Korean Americans with high blood pressure. J. Cardiovasc. Nurs. 2007;22:397–404. doi: 10.1097/01.JCN.0000287038.23186.bd. [DOI] [PubMed] [Google Scholar]
- 78.Bennett SJ, Cordes DK, Westmoreland G, et al. Self-care strategies for symptom management in patients with chronic heart failure. Nurs. Res. 2000;49:139–145. doi: 10.1097/00006199-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 79.Bennett SJ, Milgrom LB, Champion V, et al. Beliefs about medication and dietary compliance in people with heart failure: an instrument development study. Heart Lung. 1997;26:273–279. doi: 10.1016/s0147-9563(97)90084-4. [DOI] [PubMed] [Google Scholar]
- 80.Siegel K, Karus D, Schrimshaw EW. Racial differences in attitudes toward protease inhibitors among older HIV-infected men. AIDS Care. 2000;12:423–434. doi: 10.1080/09540120050123828. [DOI] [PubMed] [Google Scholar]
- 81.Kaplan RC, Bhalodkar NC, Brown DL, et al. Differences by age and race/ethnicity in knowledge about hypercholesterolemia. Cardiol. Rev. 2006;14:1–6. doi: 10.1097/01.crd.0000160308.62033.29. [DOI] [PubMed] [Google Scholar]
- 82.Mochari H, Ferris A, Adigopula S, et al. Cardiovascular disease knowledge, medication adherence, and barriers to preventive action in a minority population. Prev Cardiol. 2007;10:190–195. doi: 10.1111/j.1520-037x.2007.06619.x. [DOI] [PubMed] [Google Scholar]
- 83.Ferguson TF, Stewart KE, Funkhouser E, et al. Patient-perceived barriers to antiretroviral adherence: associations with race. AIDS Care. 2002;14:607–617. doi: 10.1080/0954012021000005434. [DOI] [PubMed] [Google Scholar]
- 84.Kressin NR, Wang F, Long J, et al. Hypertensive patients' race, health beliefs, process of care, and medication adherence. J. Gen. Intern. Med. 2007;22:768–774. doi: 10.1007/s11606-007-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]