Abstract
A truncating allele of the cell cycle checkpoint kinase CHK2 is present in 1% of the population, conferring a moderate increase in breast cancer risk, and inactivation of chk2 enhances mammary tumorigenesis in mice with _targeted inactivation of brca1. We used the mouse mammary tumor virus (MMTV) promoter to _target expression of a kinase-dead CHK2 allele (D347A). Mammary tumors, of predominantly micropapillary histology, developed in 40% of MMTV-CHK2-D347A transgenic mice with an average latency of 20 months. Tumors metastasized to lung and spleen; tumor-derived cell lines were frequently aneuploid and showed suppression of irradiation-induced p53 function. Primary hematopoietic malignancies were also observed in the spleen, another site of MMTV expression. The increased rate of tumor formation in MMTV-CHK2-D347A mice, compared with the relatively low incidence in chk2-null mice, provides a model to study modifiers of CHK2-dependent transformation.
Introduction
CHK2 (also known as CHEK2) encodes a serine/threonine kinase that activates cell cycle checkpoints in response to DNA damage, thereby contributing to the maintenance of genomic integrity (1–3). In yeast (S. pombe), the Chk2 orthologue Cds1 mediates the G2-M checkpoint, phosphorylating Cdc25C in response to activation by the ATM/ATR orthologue Rad-3. In mammalian cells, CHK2 is activated by ATM primarily in response to double-stranded DNA damage although the downstream events triggered by CHK2 activation seem to be more complex.
Genetic evidence of CHK2 inactivation in human cancer syndromes supports its relevance in DNA damage response pathways. We initially discovered germ line CHK2 mutations as part of a screen of checkpoint regulators in families with variants of the multi-cancer Li-Fraumeni syndrome that lack characteristic mutations in p53 (4). Subsequent studies by an international CHEK2 consortium showed that a specific truncating mutation, 1100delC, is present in 1% of the population and in 5% of cases with familial breast cancer, suggesting that it confers a low to moderate increase in cancer risk (5). Follow-up studies involving >10,000 breast cancer cases have provided further support for the 1100delC CHK2 mutation as a “modifier” of cancer risk, even in women unselected for family history (6). In aggregate, epidemiologic studies have thus identified CHK2 as a low-penetrance gene, conferring predisposition to breast cancer and potentially to other tumors, including prostate cancer (7–9).
The cellular mechanisms underlying CHK2 function have been defined in cultured cell lines in which activation of CHK2 by double-stranded DNA damage results in the phosphorylation of a variety of _targets, including CDC25A (serine-123), CDC25C (serine-216), p53 (serine-20), and BRCA1 (serine-988), among others. For example, in U2OS osteosarcoma cells, ionizing radiation–induced phosphorylation of p53 on serine-20 by CHK2 seems to mediate p53 stabilization and G1 cell cycle arrest (10). In U2OS and other cancer cell lines, CDC25A degradation is triggered by its phosphorylation by CHK2 in response to DNA damage, thereby preventing activation of cyclin E–associated CDK2 and activating the S-phase checkpoint (11). In embryonic stem cells, maintenance of G2 cell cycle arrest after irradiation is mediated by phosphorylation and inactivation of cdc25c by chk2 (12). A link to BRCA1 is also supported by radiation-induced phosphorylation of serine-988 by CHK2, leading to the dispersal of BRCA1 nuclear dots (13). Nonetheless, the physiologic _targets of CHK2-mediated phosphorylation that are responsible for its suppression of tumorigenesis in vivo remain to be defined.
Two mouse models of chk2 inactivation have been reported: the first _targeting the COOH-terminal kinase domain (14) and the second resulting in an NH2-terminal truncation (exons 2–5; ref. 15). Chk2-null thymocytes derived from both have abnormalities in ionizing radiation–induced p53-mediated apoptosis. A modest increase in tumor susceptibility was reported in one strain following dimethylbenzanthracene-induced skin carcinogenesis (15) whereas the other developed rare lymphomas and a single case of mammary carcinoma (14). More recently, inactivation of chk2 was found to enhance mammary tumorigenesis induced by mammary-specific _targeting of brca1 (16). This observation contrasts with human epidemiologic studies in which increased prevalence of CHK2 1100delC in familial breast cancer cohorts, but not in BRCA1-linked pedigrees, was proposed as evidence of functional redundancy between the BRCA1 and CHK2 pathways (5, 6). Interestingly, Kim et al. (17) have recently created mice lacking the murine equivalent of brca1 serine-988, the phosphorylation _target of activated chk2. These mice were predisposed to the development of late-onset tumors, thus supporting a direct role of chk2 on brca1 function.
Here, we tested the role of chk2 in mammary tumorigenesis by _targeted overexpression of a kinase-dead D347A mutant, which functions as a dominant-negative protein (10, 11, 13). Following serial pregnancies to drive expression of the mouse mammary tumor virus (MMTV) promoter, mice harboring the transgene reproducibly developed multiple mammary carcinomas, a subset of which showed metastases to lung and spleen. Immortalized cell lines generated from CHK2-D347A–derived mammary tumors showed attenuation of the p53-dependent response to DNA damage. These results support a role for CHK2 inactivation in breast tumorigenesis.
Materials and Methods
DNA constructs and transgenic mice
The transgenic expression construct was derived from human CHK2 cDNA encoding the D347A mutation, driven by the MMTV-LTR, with 650 bp of β-globin intron sequence and 580 bp of hGH poly(A) sequence. The linearized MMTV-CHK2-D347A construct was injected into fertilized FVB/N pronuclei (Beth Israel Deaconess Hospital Transgenic Facility) resulting in seven transgenic mice that contained the transgene as assessed by Southern blot of tail DNA. Immunoblot analyses of mammary and splenic tissue taken from progeny postpartum females confirmed the expression of the CHK2 transgene (human-specific CHK2 antibody from Santa Cruz Biotechnology, Santa Cruz, CA).
All mice were provided routine care in accordance with institutional guidelines and genotyped using standard procedures. Colonies of FVB/N-Tg(MMTV-CHK2-D347A) mice were repeatedly bred to wild-type FVB mice (Taconic Farms, Germantown, NY) and monitored for the development of mammary tumors. Mice were sacrificed on tumor development or at 2 years of age. FVB/N-Tg(MMTVneu) 202 Mu1 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and mated to Tg(MMTV-CHK2-D347A) mice to yield doubly heterozygous Tg(MMTV-CHK2-D347A/c-neu) or heterozygous c-neu transgenic lines. These mice were not mated during the period of tumor development.
Cell culture and cytogenetic studies
After dissection, tissues from mammary tumors, mammary glands, spleens, lungs, liver, and brain were immediately placed in freshly prepared 4% paraformaldehyde for subsequent histologic analyses or were frozen on dry ice and placed at −80°C for biochemical studies. To establish mammary tumor cell lines, tumor fragments were minced in MAM media (DMEM/F12, Life Technologies, Inc., Gaithersburg, MD; epidermal growth factor, 5 ng/mL, Sigma; insulin 1 μg/mL, Sigma; fetal bovine serum, 2.1%; penicillin/streptomycin, 1%) and treated with collagenase or trypsin-EDTA (Life Technologies). Attached cells were maintained in MAM media. For karyotype analysis, proliferating cultures of CHK2-D347A, c-myc, and c-neu tumor cells were submitted to the Dana-Farber/Harvard Cancer Center Cytogenetics Core facility for Giemsa banding. For BRCA1 staining, cultured tumor cells were irradiated and stained with antibody to BRCA1 phosphoserine-988 (Santa Cruz Biotechnology) followed by immunofluorescence microscopy.
CHK2 expression and p53 analysis
To show the dominant-negative properties of CHK2-D347A in vitro, primary human foreskin fibroblasts were retrovirally infected with the MSCV-SVG vector or MSCV-SVG containing CHK2-D347A, and immunoblotting of lysates was done using antibody recognizing human p53 (Santa Cruz Biotechnology). Following tumor development, frozen mouse tissue was pulverized with a chilled mortar and pestle, lysed in SDS sample buffer, followed by SDS-PAGE and probing of immunoblots with human-specific anti-CHK2 antibody (Santa Cruz Biotechnology). Cultured c-myc, c-neu, or CHK2-D347A derived mammary tumor cells were lysed in SDS sample buffer and immunoblotted with antibodies to CHK2, CDC25A (Santa Cruz Biotechnology), p53 (Novocastra), or tubulin (Upstate, Charlottesville, VA). For Northern blots, total RNA was isolated from cultured tumor cells (RNeasy kit, Qiagen, Valencia, CA) and analyzed by standard methods. All experiments involving γ irradiation were done using a cesium irradiator. Big Dye (ABI) sequencing reactions were done on genomic DNA using primers spanning each exon of p53.
Results and Discussion
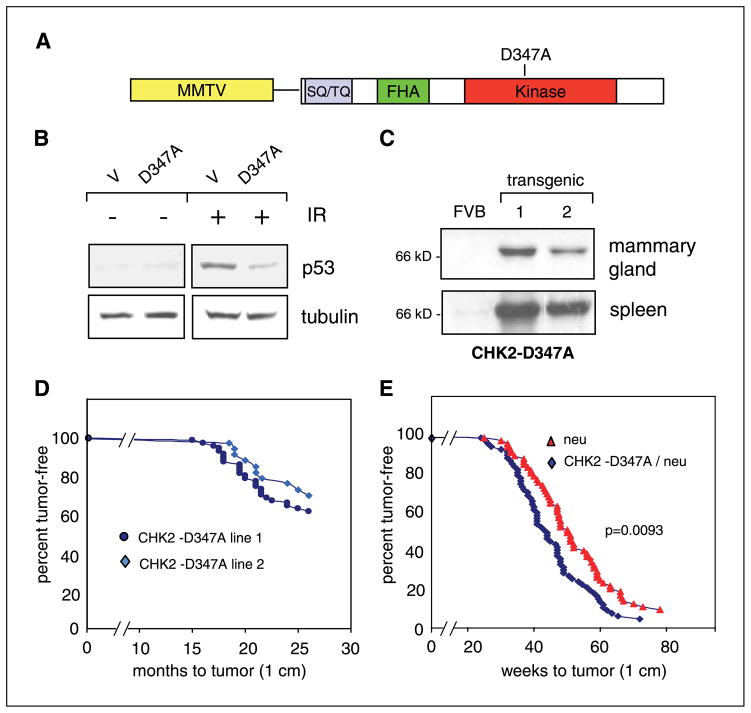
Substitution of alanine for aspartic acid at codon 347 within the kinase domain of CHK2 inactivates its enzymatic activity and has been shown to function as a dominant negative in cultured cell lines (10, 11, 13). Accordingly, we generated a CHK2-D347A construct (Fig. 1A) and confirmed its effect following retroviral infection into primary human foreskin fibroblasts. Ionizing radiation–induced stabilization of p53 (Fig. 1B) and induction of the _target genes MDM2 and p21 (not shown) were suppressed in cells expressing CHK2-D347A compared with vector-infected cells. An MMTV-driven CHK2-D347A construct was used to generate seven founder transgenic mice, of which two with the highest expression of the dominant-negative construct within MMTV-LTR _target tissues (including mammary gland and spleen in both lines) were selected for further study (Fig. 1C).
Figure 1.
Induction of mammary tumors by MMTV-CHK2-D347A. A, schematic diagram of the CHK2-D347A transgene containing the MMTV promoter driving expression of full-length cDNA encoding human CHK2, including the serine/threonine/glutamine-rich motif (SQ/TQ), the forkhead-associated homology domain (FHA), and the kinase domain in which aspartic acid is replaced by alanine at amino acid position 347. B, Western blot of protein lysates from primary human foreskin fibroblasts infected with either MSCV-SVG retroviral vector (V) or MSCV-SVG-CHK2-D347A (D347A). Lysates from infected cells were harvested 90 minutes after 10 Gy of ionizing radiation (IR). Equal microgram quantities of lysates were analyzed using antibody to human p53 or tubulin (control). C, mammary gland and spleen from wild-type FVB and two MMTV-CHK2-D347A lines. Equal quantities of lysates were immunoblotted and probed with antibody to human CHK2. D, Kaplan-Meier curve of tumor-free survival in two lines of MMTV-CHK2-D347A transgenic mice. In line 1 (dark blue), 27 of 70 mice (39%) developed palpable mammary tumors (1 cm) with an average latency of 20 months. In line 2 (light blue), 10 of 33 mice (30%) developed tumors with an average latency of 21.5 months. E, Kaplan-Meier curve of tumor-free survival in MMTV-c-neu mice (red) and MMTV-CHK2-D347A/c-neu double heterozygotes (blue). Fifty-nine c-neu mice developed mammary tumors with an average latency of 49.5 weeks and 64 CHK2-D347A mice developed mammary tumors with an average latency of 44.2 weeks (P = 0.0093).
Female transgenic mice expressing MMTV-CHK2-D347A were monitored for the development of mammary tumors (Fig. 1D and E). Of 70 females bred repeatedly to maximize expression of CHK2-D347A, 27 (39%) developed at least one histologically confirmed mammary carcinoma. The majority of these mice developed multiple tumors with an average latency of 20 months (range 15–26 months; Fig. 1D, transgenic line 1). Of six mice that were sacrificed at 24 months without gross evidence of mammary tumors, three (50%) had mammary hyperplasia on histologic analyses. Thirty-three females from CHK2-D347A line 2 were also studied, of which 10 (30%) developed mammary carcinomas with an average latency of 21.5 months (range, 18.5–26 months; Fig. 1D). Of the 37 mice from both lines that developed mammary tumors, metastatic lesions to the lung were detected in five cases and a metastatic lesion to the spleen was detected in one case (Table 1).
Table 1.
Histologies of tumors from MMTV-CHK2-D347A mice
| Mammary tumor histology | n = 47 |
| Micropapillary/papillary | 12 (26%) |
| Adenocarcinoma | 27 (57%) |
| Adenosquamous/squamous | 8 (17%) |
| Spleen histology | n = 31 |
| Myeloid hyperplasia | 3 (10%) |
| Myeloid dysplasia | 2 (6%) |
| Increased myeloid immaturity | 2 (6%) |
| Myeloid leukemia | 1 (3%) |
| Lymphoid leukemia | 1 (3%) |
| Site of metastatic disease | n = 32 |
| Lung | 5 (15%) |
| Spleen | 1 (3%) |
NOTE: N refers to number of cases examined from mice representing both transgenic lines with gross evidence of mammary abnormality. Mice without gross evidence of tumor were not routinely submitted for histologic examination. Three mice developed clinical mammary tumors but were not available for histologic analyses. The majority of mice developed multiple tumors, sometimes with several histologies occurring in the same mouse.
All tumors tested expressed the CHK2-D347A transgene, which was readily distinguished from endogenous mouse chk2 using a human-specific antibody. The predominating histology of mammary carcinomas was micropapillary in at least 9 of 47 cases analyzed from both transgenic lines and papillary in an additional 3 cases (Table 1; Fig. 2). Micropapillary tumors are characterized by epithelial cells with short papillae lacking a distinct fibrovascular stalk, an unusual histology that has been previously described in STAT 5 and CK2α-driven mammary tumors (18, 19) and is clearly distinguishable from that of the well-studied erbB2-driven or c-myc-driven mammary tumor models (20, 21). A subset of CHK2-D347A–driven tumors had other histologies, including epithelioid, spindle cell, intermediate cell, glandular, and adenosquamous. Mammary hyperplasia was common in mice without tumors as well as in the unaffected glands of those that developed carcinomas. Interestingly, mammary tumors from conditional brca1-null mice with and without p53 inactivation also showed a variety of histologic phenotypes, ranging from hyperplasia to high-grade invasive adenocarcinomas with various morphologic features (22).
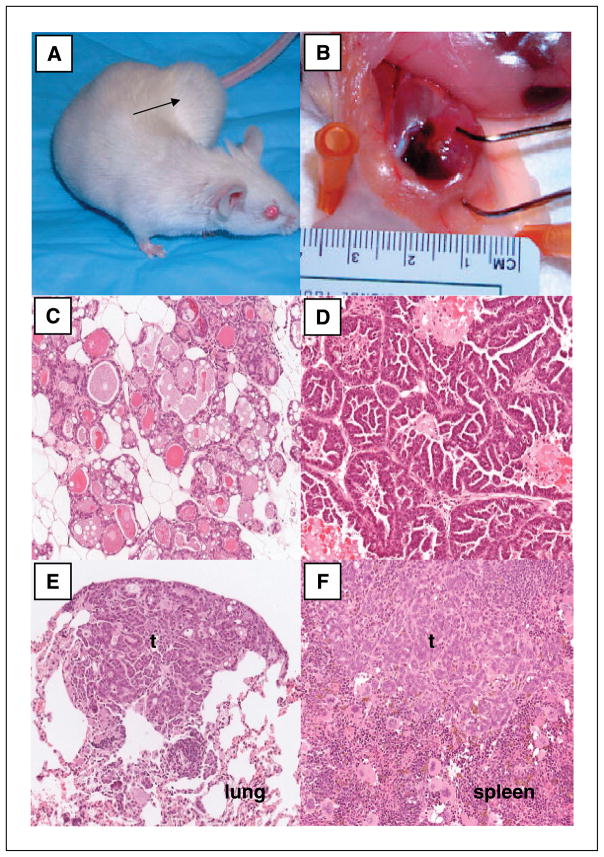
Figure 2.
Histopathology of MMTV-CHK2-D347A–driven tumors. A, MMTV-CHK2-D347A mouse with mammary tumor (black arrow). B, dissected mammary gland from a CHK2-D347A mouse. Upper arm of tweezer marks the tumor; lower tweezer arm marks unaffected mammary tissue. C to F, representative tissue sections stained with H&E showing mammary hyperplasia (C), mammary tumor with micropapillary histology (D), metastatic tumor (t) to the lung (E), and metastatic tumor to the spleen (F). Magnification, ×20.
The FVB strain is commonly used in the creation of transgenic mice because of its reproductive fitness and large zygotic pronuclei (23). Serial mating and advancing age in FVB mice have been linked recently to the development of pituitary adenomas, which secrete prolactin and contribute to mammary hyperplasia and, rarely, formation of noninvasive tumors (24). As expected, pituitary abnormalities were observed in a subset of CHK2-D347A mice; of 45 pituitaries analyzed, 28 had a pituitary adenoma and 6 showed hyperplasia. However, these pituitary abnormalities were not required for mammary tumor formation: of 11 CHK2-D347A mice with histologically normal pituitary glands, 5 developed mammary tumors and 2 had mammary hyperplasia. Furthermore, the characteristics of spontaneous mammary tumors in FVB mice differ from those observed in MMTV-CHK2-D347A strains. Whereas mammary hyperplasia is common under the influence of prolactin, mammary tumors are infrequent, occur singly, have a predominantly adenosquamous histology, and do not metastasize (24, 25). The frequent occurrence of multiple tumors in MMTV-CHK2-D347A mice, their metastatic potential, and distinct histology all point to a CHK2-driven effect. Nonetheless, it is possible that the combination of mammary gland proliferation associated with serial breeding, together with expression of CHK2-D347A, may underlie the striking mammary carcinogenesis observed here, which was not evident in chk2-null mice (see below).
Whereas this study was designed to investigate the contribution of chk2 function in mammary tumorigenesis, mice from the two transgenic lines also developed primary abnormalities of the spleen, an organ that is also _targeted by MMTV and which showed high levels of CHK2-D347A expression in both lines. Spleens from 9 of 31 mice analyzed had hematopoietic abnormalities ranging from myeloid hyperplasia to frank leukemia (Table 1). Such MMTV-driven abnormalities in the spleen have also been reported with c-myc transgenic mice (21). Of note, chk-2 null mice were reported to develop spontaneous lymphomas (15). Together with the hematopoietic abnormalities in CHK2-D347A mice, these observations support a role for CHK2 in tumorigenesis beyond that observed in mammary tissue.
The role of CHK2 as a guardian of genomic stability and the epidemiologic data indicating that CHK2 inactivation may function as a genetic modifier suggested that its loss may facilitate tumor formation driven by other stimuli. Therefore, we tested for potential interactions between MMTV-CHK2-D347A and a well-characterized mouse model of mammary tumorigenesis, MMTV-c-neu overexpression (26). Sixty-four virgin CHK2-D347A/c-neu double heterozygous mice and 59 c-neu heterozygous mice were monitored for tumor development in the absence of serial mating. The average tumor-free survival was 44.2 weeks in the CHK2-D347A/c-neu mice as compared with 49.5 weeks in the c-neu mice (P = 0.0093; Fig. 1E), indicating a potential collaborative effect between chk2 inactivation and c-neu overexpression in determining mammary tumor predisposition. The spectrum of histology observed in tumors from double heterozygous mice reflected that of c-neu rather than CHK2-D347A.
To study the characteristics of CHK2-D347A–driven tumor cells, we established cell lines from 20 of 24 (83%) primary mammary tumors. Remarkably, such cell lines were readily established from CHK2-D347A tumors, which is unusual for most mouse mammary tumor models. In contrast, the single cell line that could be generated from a mammary tumor arising spontaneously in a wild-type FVB mouse with a pituitary adenoma underwent senescence within three passages. As previously noted, immortalized tumor cell lines were infrequently generated from MMTV-c-neu tumors (10%) although establishment of MMTV-c-myc cell lines was generally successful (90%). CHK2-D347A–derived cell lines showed persistent expression of the transgene by both immunoblotting and reverse transcription-PCR analyses (data not shown).
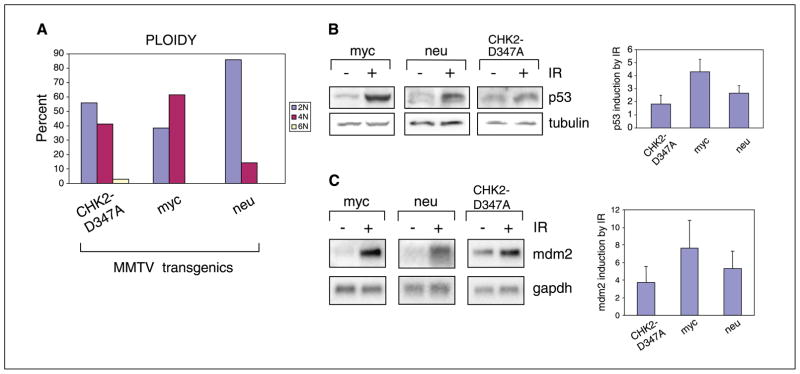
Given the role of CHK2 in the maintenance of genomic stability, we first evaluated the chromosomal integrity of cells from CHK2-D347A tumors. Karyotypes from early-passage cells derived from these tumors showed varying degrees of abnormality, ranging from gain or loss of single chromosomes to gross aneuploidy (Fig. 3A). By comparison, metaphase chromosomes from c-neu-driven tumor cells were predominantly euploid whereas chromosomes from c-myc-driven tumor cells were more frequently aneuploid, consistent with previously published karyotypic analyses (27, 28). No chromosomal abnormalities characteristic of BRCA1 defects, such as quadriradial chromosomes (29), were detected in CHK2-D347A tumor cell lines.
Figure 3.
Functional abnormalities in MMTV-CHK2-D347A–derived tumor cells. A, ploidy of mitoses from seven independent CHK2-D347A tumor–derived cell lines (56% of mitoses were 2N, 41% were 4N, 3% were 6N), two c-myc cell lines (38% of mitoses were 2N, 62% were 4N), and two c-neu cell lines (86% of mitoses were 2N, 14% were 4N). Cells were karyotyped at passages 2 to 5. Virtually all CHK2-D347A cells with 2N ploidy exhibited gains or losses of individual chromosomes. B, quantitation of p53 protein levels following ionizing radiation. Immunoblot analysis of lysates from c-myc, c-neu, and CHK2-D347A mammary tumor-derived cells following 10-Gy ionizing radiation. Equal quantities of protein lysates (tubulin control) were analyzed 1 hour after ionizing radiation and representative samples are shown. Densitometric analysis of multiple immunoblots showing p53 expression following ionizing radiation for three cell lines derived from CHK2-D347A, c-myc, and c-neu tumors. Columns, mean fold induction (standardized to baseline); bars, SD. C, Northern blot analysis of mdm2 expression in c-myc, c-neu, and CHK2-D347A mammary tumor cells 2 hours after ionizing radiation (10 Gy). Representative samples are shown with glyceraldehyde-3-phosphate dehydrogenase (gapdh) control. Densitometric quantitation of multiple experiments showing mdm2 induction by ionizing radiation for CHK2-D347A tumors (nine cell lines), c-myc tumors (five cell lines), and c-neu tumors (three cell lines). Columns, mean fold induction (standardized to baseline); bars, SD.
The development of a mouse model for mammary tumorigenesis also made it possible to test the integrity of specific cellular pathways in epithelial tumor cells driven at least in part by chk2 inactivation. Previous analyses of downstream pathways in cells with inactivated chk2 have primarily been carried out in normal fibroblasts (14, 15). We first tested the integrity of the p53 pathway using nine cell lines derived from CHK2-D347A mouse tumors. None of these lines had acquired a mutation in p53 either in vivo or during culture in vitro as determined by nucleotide sequencing (data not shown). Gamma irradiation of these cells resulted in reduced stabilization of p53 protein levels as compared with cell lines derived from either MMTV-c-myc or MMTV-c-neu tumors (Fig. 3B). Irradiation of CHK2-D347A tumor cells also resulted in reduced induction of the p53-_target gene mdm2 (Fig. 3C), consistent with the partial compromise of p53-dependent ionizing radiation–induced DNA damage signals reported in chk2-null thymocytes (14, 15). Interestingly, levels of p53 protein and mdm2 mRNA were generally elevated in the absence of ionizing radiation in CHK2-D347A tumor–derived cells, possibly reflecting baseline genomic instability in these cells. Whereas the function of p53 as a regulator of mdm2 was compromised in CHK2-D347A tumor cells, we could not detect a reproducible functional alteration of p53-dependent cell cycle checkpoints in CHK2-D347A tumor cells as compared with cells from c-neu and c-myc tumors (data not shown).
In contrast to the p53 pathway, we did not detect consistent abnormalities in either cdc25A or brca1 pathways in CHK2-D347A tumor cells. In U2OS cells, phosphorylation of CDC25A by CHK2 contributes to its degradation following irradiation (11) but CHK2-D347A mammary tumor cells generally showed preservation of irradiation-induced cdc25A degradation, as did c-myc and c-neu tumor-derived cells (data not shown). In addition, formation of brca1 nuclear dots following ionizing radiation was not altered in CHK2-D347A tumors cells as shown by immunofluorescence staining using antibody to phosphoserine-988 (not shown). Thus, despite their similar delayed tumorigenesis phenotype, the molecular events driving tumorigenesis in our dominant negative CHK2 model may differ from the _targeted disruption of BRCA1 serine-988 recently reported by Kim et al. (17). Thus, the quantitative defect in p53-mediated DNA-damage response evident in CHK2-D347A mammary tumor-derived cells may contribute to the tumorigenic effect of CHK2 inactivation in vivo.
Concluding Remarks
Modifier or low-penetrance genes are now thought to account for most cases of breast cancer predisposition within the general population (30). Given their prevalence in the population, CHK2 mutations seem to constitute the first such low-penetrance allele identified in breast cancer, with an associated cancer risk confirmed by multiple epidemiologic analyses. Because few high-prevalence/low-penetrance cancer genes have yet been identified, the functional properties of CHK2 may be particularly informative in searching for other genes that also contribute to cancer predisposition in the general population. CHK2 is noteworthy in that it functions at a critical crossroad in multiple pathways that mediate the response to DNA damage, including p53 itself, but functional redundancy with CHK1 is likely to explain the mild phenotype of CHK2 inactivation in cultured cells, in mouse models, and potentially in human tumorigenesis. Population-based analyses of other genetic variants in checkpoint regulators will be required to determine whether they may also function as modifiers in human breast cancer.
The mouse model described here differs from the phenotype of chk2-null mice, which showed only modest tumor susceptibility. This might result from differences in genetic background associated with the FVB/N strain (CHK2-D347A) and C57BL/6 mice (chk2-null). Alternatively, tumor development in CHK2-D347A mice may reflect additional properties attributable to overexpression of a dominant-negative protein, such as sequestration of components of the DNA-damage response. Finally, it is also possible that the repeated breeding protocol used to induce MMTV-LTR expression provides a hormonally driven proliferative stimulus that cooperates with inactivation of chk2 in mediating tumorigenesis. Such potential interactions between genetic and hormonal factors are particularly relevant, given the known contribution of estrogen exposure to human breast cancer.
Whereas the precise mechanism of tumorigenesis initiated by the dominant-negative CHK2 transgene awaits further experiments, the fact that these mice develop tumors provides the first opportunity to study chk2-mediated signaling in primary malignant epithelial cells driven by chk2 inactivation. This mouse model may prove particularly valuable in studying the interaction between genetic and hormonal risk factors in mammary carcinogenesis.
Acknowledgments
Grant support: NIH grant CA84066 (D.A. Haber); the NIH Specialized Program of Research Excellence on Breast Cancer at Massachusetts General Hospital (D.A. Haber); the Aid for Cancer Research Foundation (E.L. Kwak); and National Cancer Institute grant R01 CA089140 and National Center for Research Resources consortium grant U42 RR014905 for the histopathology in this article (R.D. Cardiff).
We thank Dr. Yixue Cao for MMTV-c-myc mice, Dr. Craig Bassing for assistance with chromosome analyses and helpful discussion, Dr. Leif Ellisen for primary human fibroblasts, Charlotte Manning for guidance in establishing mammary tumor cell lines, and Drs. Sandra Orsulic and Lee Zou for comments on the manuscript.
References
- 1.Zhou B-B, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–9. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 2.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–54. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 3.Bartek J, Falck J, Lukas J. Chk2 kinase—a busy messenger. Nat Rev Mol Cell Biol. 2001;2:877–86. doi: 10.1038/35103059. [DOI] [PubMed] [Google Scholar]
- 4.Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999;86:2528–31. doi: 10.1126/science.286.5449.2528. [DOI] [PubMed] [Google Scholar]
- 5.Meijers-Heijboer H the CHEK2-Breast Cancer Consortium. Low-penetrance susceptibility to breast cancer due to CHEK2*1100delC in noncarriers of BRCA1 or BRCA1 mutations. Nat Genet. 2002;31:55–9. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 6.Easton D the CHEK2 Breast Cancer Case-Control Consortium. CHEK2*1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. 2004;74:1175–82. doi: 10.1086/421251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong X, Wang L, Taniguchi K, et al. Mutations in CHEK2 associated with prostate cancer risk. Am J Hum Genet. 2003;72:270–80. doi: 10.1086/346094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seppala EH, Ikonen T, Monomen N, et al. CHEK2 variants associate with hereditary prostate cancer. Br J Cancer. 2003;89:1966–70. doi: 10.1038/sj.bjc.6601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cybulski C, Huzarski T, Gorski B, et al. A novel founder CHEK2 mutation is associated with increased prostate cancer risk. Cancer Res. 2004;64:2677–9. doi: 10.1158/0008-5472.can-04-0341. [DOI] [PubMed] [Google Scholar]
- 10.Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev. 2002;14:278–88. [PMC free article] [PubMed] [Google Scholar]
- 11.Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410:842–7. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- 12.Hirao A, Kong Y-Y, Matsuoka S, et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–7. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 13.Lee J-S, Collins KM, Brown AL, Lee C-H, Chung JH. HCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–4. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 14.Hirao A, Cheung A, Duncan G, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–32. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takai H, Naka K, Okada Y, et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McPherson JP, Lemmers B, Hirao A, et al. Collaboration of Brca1 and Chk2 in tumorigenesis. Genes Dev. 2004;18:1–10. doi: 10.1101/gad.1192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SS, Cao L, Cuiling L, et al. Uterus hyperplasia and increased carcinogen-induced tumorigenesis in mice carrying a _targeted mutation of the chk2 phosphorylation site in brca1. Mol Cell Biol. 2004;24:9498–507. doi: 10.1128/MCB.24.21.9498-9507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iavnilovitch E, Cardiff RD, Groner B, Barash I. Deregulation of STAT5 expression and activation causes mammary tumors in transgenic mice. Int J Cancer. 2004;112:607–19. doi: 10.1002/ijc.20484. [DOI] [PubMed] [Google Scholar]
- 19.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–57. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- 20.Rosner A, Miyoshi K, Landesman-Bollag E, et al. Histological differences between erbB/ras and wnt pathway transgenic mammary tumors. Am J Pathol. 2002;161:1087–97. doi: 10.1016/S0002-9440(10)64269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leder A, Pattengale PK, Kuo A, Stewart TA, Leder P. Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell. 1986;45:485–95. doi: 10.1016/0092-8674(86)90280-1. [DOI] [PubMed] [Google Scholar]
- 22.Brodie SG, Xu X, Qiao W, et al. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene. 2001;20:7514–23. doi: 10.1038/sj.onc.1204929. [DOI] [PubMed] [Google Scholar]
- 23.Taketo M, Schroeder AC, Mobraaten LE, et al. FVB/N: An inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88:2065–9. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakefield LM, Thordarson G, Nieto A, et al. Spontaneous pituitary a bnormalities and mammary hyperplasia in FVB/NCr mice: implications for mouse modeling. Comp Med. 2003;53:424–32. [PubMed] [Google Scholar]
- 25.Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler L. Prolactin induces ERα-positive and ERα-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–74. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu proto-oncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montagna C, Andrechek ER, Padilla-Nash H, Muller WJ, Reid T. Centrosome abnormalities, recurring deletions of chromosome 4, and genomic amplification of HER/neu define mouse mammary gland adenocarcinomas induced by mutant HER/neu. Oncogene. 2002;21:890–8. doi: 10.1038/sj.onc.1205146. [DOI] [PubMed] [Google Scholar]
- 28.Weaver ZA, McCormack SJ, Liyanage M, et al. A recurring pattern of chromosomal aberrations in mammary gland tumors of MMTV-cmyc transgenic mice. Genes Chromosomes Cancer. 1999;25:251–60. [PubMed] [Google Scholar]
- 29.Xu X, Weaver Z, Linke SP. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–95. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 30.Balmain A, Gray J, Ponder B. The genetics and genomics of cancer. Nat Genet Supp. 2003;33:238–44. doi: 10.1038/ng1107. [DOI] [PubMed] [Google Scholar]