Abstract
Many hemoglobin-derived peptides are present in mouse brain, and several of these have bioactive properties including the hemopressins, a related series of peptides that bind to cannabinoid CB1 receptors. Although hemoglobin is a major component of red blood cells, it is also present in neurons and glia. To examine whether the hemoglobin-derived peptides in brain are similar to those present in blood and heart, we used a peptidomics approach involving mass spectrometry. Many hemoglobin-derived peptides are found only in brain and not in blood, whereas all hemoglobin-derived peptides found in heart were also seen in blood. Thus, it is likely that the majority of the hemoglobin-derived peptides detected in brain are produced from brain hemoglobin and not erythrocytes. We also examined if the hemopressins and other major hemoglobin-derived peptides were regulated in the Cpefat/fat mouse; previously these mice were reported to have elevated levels of several hemoglobin-derived peptides. Many, but not all of the hemoglobin-derived peptides were elevated in several brain regions of the Cpefat/fat mouse. Taken together, these findings suggest that the post-translational processing of alpha and beta hemoglobin into the hemopressins, as well as other peptides, is upregulated in some but not all Cpefat/fat mouse brain regions.
Keywords: peptide processing, neuropeptide, cannabinoid, hemoglobin, carboxypeptidase E
INTRODUCTION
Neuropeptides play a large role in a variety of physiological processes including sleep, feeding, pain, and many more (Strand 2003). Most neuropeptides are produced within the secretory pathway by the selective cleavage of precursors at specific sites (Eipper et al. 1986). After synthesis, the bioactive molecules are stored in vesicles and secreted upon stimulation. In addition to peptides produced within the secretory pathway, a number of peptides have been described that are derived from cytosolic proteins. In some cases, these cytosolic protein-derived peptides have been found to have neuropeptide-like signaling properties. Some examples of this class of peptides include diazepam binding inhibitor (DBI), hippocampal cholinergic neurostimulating peptide (HCNP), hemorphins, neokyotorphin, and the hemopressins. DBI is a fragment of acyl-Coenzyme A-binding protein that displaces diazepam binding to GABAA receptors(Costa & Guidotti 1991). HCNP is a fragment of phosphatidylethanolamine-binding protein that enhances the differentiation of hippocampal neurons (Ojika et al. 2000). Alpha and beta chains of hemoglobin are precursors of numerous bioactive peptides, some of which are the hemorphins, neokyotorphin, and the hemopressins. The hemorphins, derived from the beta chain, bind to opiate receptors and neokyotorphin, derived from the alpha chain, binds angiotensin receptors (Fukui et al. 1983, Nyberg et al. 1997, Zhao et al. 1997). The hemopressins are a family of peptides derived from either alpha hemoglobin (RVD-hemopressin-α and VD-hemopressin-α) or beta hemoglobin (VD-hemopressin-β) which bind to the CB1 cannabinoid receptor (Gomes et al. 2009). This family also includes the 9 amino acid hemopressin peptide which is antinociceptive, reduces blood pressure, and acts as an inverse agonist at the CB1 receptors (Dale et al. 2005b, Heimann et al. 2007, Rioli et al. 2003, Dale et al. 2005a).
Carboxypeptidase E (CPE) is an enzyme involved in the biosynthesis of numerous neuropeptides and other secretory pathway-derived peptides (Fricker 1988). Cpefat/fat mice lack functional CPE due to a point mutation in the gene that causes the substitution of a Pro for a Ser and results in mis-folded enzyme (Naggert et al. 1995). In Cpefat/fat mice, the mature forms of many neuropeptides are substantially reduced and the processing intermediates containing C-terminal basic residues are greatly elevated (Zhang et al. 2008, Fricker et al. 1996, Rovere et al. 1996, Che & Fricker 2005, Che et al. 2005a, Lim et al. 2006). Additionally, previous reports identified several hemoglobin-derived peptides in Cpefat/fat mice that contained C-terminal basic residues, some of which were present at higher levels in the Cpefat/fat mice than in the wild-type littermates (Bures et al. 2001, Lim et al. 2006, Che et al. 2005a).
Hemoglobin is responsible for oxygen delivery to the respiring tissues of the body and is a well-known constituent of red blood cells. In addition, hemoglobin has recently been found to be expressed in many diverse cell types. In 1999, Liu and colleagues found beta hemoglobin mRNA and protein in activated macrophages (Liu et al. 1999). In the past few years, hemoglobin mRNA and/or protein has been detected in lens cells, alveolar epithelial type II and Clara cells, kidney messangeal cells, and epithelial and stromal cells of the endometrium (Wride et al. 2003, Bhaskaran et al. 2005, Newton et al. 2006, Nishi et al. 2008, Haase 2008, Dassen et al. 2008, Ullal et al. 2008). Most recently, alpha and beta hemoglobin mRNA and protein expression were detected in nigral and mesencephalic dopaminergic, striatal GABAergic, and cortical pyramidal neurons and glial cells (Richter et al. 2009, Medvedeva et al. 2009, Pinto et al. 2009, Biagioli et al. 2009). Hemoglobin mRNA expression was also detected in cultured neurons and in differentiated but not in undifferentiated human neuroblastoma cells (Richter et al. 2009, Medvedeva et al. 2009, Pinto et al. 2009, Biagioli et al. 2009). Moreover, hemoglobin expression in mouse brain was suggested to be upregulated in response to ischemia, and the processing of hemoglobin into peptides in mouse brain was rapidly elevated by global ischemia (Gomes et al. 2009, He et al. 2009). Together, these data suggest that hemoglobin-derived peptides could be endogenous signaling molecules within the central nervous system.
To gain a better understanding of the regulation of hemoglobin-derived peptides within the central nervous system, we used mass spectrometry and quantitative peptidomics techniques to compare peptides from wild-type (WT) and Cpefat/fat mice brains. Because it is reasonable to expect that peptides detected upon peptidomics analysis of mouse brain could be derived from blood cells, we also compared the peptidome of brain and blood and analyzed if the major hemoglobin-derived peptides detected in brain were common to blood; these analyses revealed substantial differences between hemoglobin-derived peptides, as well as many other peptides, between blood and brain. We also investigated the hemoglobin-derived peptidome of the heart, a highly vascularized tissue that presumably has hemoglobin only from blood. We found that unlike the brain, hemoglobin derived peptides in heart were not different from those seen in blood. Accordingly, we observed that many hemoglobin peptides were increased in the Cpefat/fat mice brain whereas most peptides derived from other cytosolic proteins were not substantially affected in these mice. Western blot analysis and quantitative real-time PCR indicate that the increase in hemoglobin-derived peptides was not simply due to elevated hemoglobin protein and mRNA production. These data indicate that the effect of the fat mutation was not a non-specific increase in protein turnover, and suggest a more general mechanism for the post-translational processing of hemoglobin not directly involving CPE. Taken together, these findings suggest that hemoglobin-derived peptides are regulated and specifically produced in mouse brain.
MATERIALS AND METHODS
Materials
Hydrochloric acid (6 N, sequanal grade, constant boiling) and trifluoroacetic acid were purchased from Pierce (Rockford, IL, USA). Acetonitrile of HPLC grade was obtained from Fisher Scientific (Fair Lawn, NJ, USA). Dimethylsulfoxide (DMSO), sodium hydroxide, sodium phosphate, glycine, and hydroxylamine hydrochloride were obtained from Sigma-Aldrich, Inc. (St Louis, MO, USA. Water was purified with a Milli-Q system (Millipore, Bedford, MA, USA). The 4-trimethylammoniumbutyryl (TMAB) stable isotopic labeling reagents, D0 and D9 forms of 3-(2,5-dioxopyrrolidin-1-yloxycarbonyl)propyl trimethylammonium butyrate (abbreviated as D0-TMAB and D9-TMAB), were synthesized as described previously (Che & Fricker 2005, Zhang et al. 2002).
Quantitative peptidomics
For peptidomics of the blood, C57BKS/J WT animals were decapitated and 300μl of peripheral blood was collected into low-retention tubes. The samples were immediately placed in a boiling water bath for 1 minute and frozen in dry ice. Samples were then prepared for mass spec analysis as previously described for brain tissues (Che et al. 2005b, Che et al. 2007, Zhang et al. 2008, Che & Fricker 2002). Briefly, for peptide extraction, each sample of blood was sonicated 40 times at 1 pulse/second in 600 μl ice-cold water using an ultrasonic processor (W-380; Ultrasonic Inc., Farmingdale, NY, USA). The homogenates were incubated in a 70°C water bath for 20 min, cooled on ice and then acidified with 0.1 M HCl to a final concentration of 10 mM HCl. The homogenates were centrifuged at 13,000g for 30 min at 4°C and the supernatant was transferred to a low-retention tube and 1/3 final volume of 0.2 M phosphate buffer (pH 9.5) was added.
Because the data on the blood peptidome was to be compared to previous studies on the brain peptidome that involved isotopically-labeled samples to provide quantitative information, the same isotopic labels were used for the analysis of the blood peptidome. For isotopic labeling, 15 mg of D0-TMAB or D9- was dissolved in 37.5 μl DMSO. Labeling was performed as described previously (Che & Fricker 2005). Briefly, 5.7 μl of TMAB solutions were added separately to each blood extract and incubated for 10 min at 23–25°C. Then 1.0 M NaOH was added to adjust the pH to 9.5 and the sample extract solution was incubated another 10 min at 23–25°C. These two steps were repeated six more times to ensure that all peptides were completely labeled. The mixture was then incubated at 23–25°C for two hours and then 60 μl of 2.5 M glycine was added to quench the remaining TMAB reagents. After labeling, D0 and D9 TMAB labeled samples were pooled and filtered through an Amicon Ultracel 10 kDa centrifugal filter device (Millipore) to remove proteins larger than 10 kDa in size. To remove any labels from Tyr residues in the peptides, the filtrate was adjusted to pH 9.0 with 1.0 M NaOH, six microliters of 2.0 M hydroxylamine in DMSO was added, and the sample was incubated for 10 min. This was repeated two more times, for a total added volume of 18 μl hydroxylamine. The peptides were desalted with a PepClean™ C-18 spin column (Pierce) and eluted out of the column with 160 μl of 70% acetonitrile and 0.5% trifluoroacetic acid in water. The samples were frozen, evaporated in a vacuum centrifuge, and stored at −70°C until analysis on a quadrupole time-of-flight mass spectrometer (Ultima Q-TOF, Waters/Micromass, Manchester, UK). The peptide mixture was desalted online for 15 min using a Symmetry C18 trapping column (5 μm particles, 180 μm i.d.× 20 mm, Waters, USA) and the trapped peptides were then separated by elution with a water/acetonitrile 0.1% formic acid gradient through a BEH 130 -C18 column (1.7 μm particles, 100 μm i.d. × 100 mm, Waters, USA), at a flow rate of 600 nl/min, as previously described (Berti et al. 2009). Data were acquired in data-dependent mode and selected peptides dissociated by collisions with argon.
For peptidomics of heart, BALB/C animals were decapitated and heart was extracted, placed in a low retention tube, and microwave irradiated to raise the temperature of the tissue to 80°C. Heart was then immediately frozen and samples were processed for mass spec analysis as previously described for brain (Che et al. 2005b, Che et al. 2007, Zhang et al. 2008, Che & Fricker 2002)
Experiments using Cpefat/fat and WT mice were previously published (Zhang et al. 2008, Lim et al. 2006). However, in these previous publications these data were analyzed specifically for neuropeptides and a small number of other protein fragments. In the present study, we reanalyzed the data for hemoglobin-derived peptides as well as a large number of abundant peptides that arise from cytosolic proteins. The quantitative analysis and identification of peptides was performed as previously described (Zhang et al. 2008, Lim et al. 2006).
Western blot
C57BKS/J Cpefat/fat and WT mice were transcardially perfused with 40 ml ice cold sterile saline to reduce peripheral blood. Brains were then removed and olfactory bulb and striatum were excised. Peripheral blood was used as a positive control. Protein was extracted after sonication in lysis buffer (pH – 8.0) containing 50 mM Tris, 120 mM NaCl, 0.5% NP-40, 100 mM Sodium Fluoride, 200 μM sodium vanadate with protease inhibitor (complete mini, EDTA-free, Roche Diagnostics). The protein concentration was determined using the Bradford method (BioRad). A 10–20% Tris-HCl gel was loaded with 70 μg protein (olfactory bulb), 100 μg protein (striatum), and 0.25 μg protein (blood) per lane and electrotransferred to a nitrocellulose membrane. The membrane was then incubated overnight at 4°C with 1:1000 dilution of either alpha or beta hemoglobin antisera (mouse monoclonal Anti-HBA1and HBB, Sigma). Following repeated washes, the membrane was then incubated with 1:3000 dilution of IRDye800 conjugated affinity purified anti-mouse IgG antibody (Rockland Immunochemicals, Inc.) for 1 hour. Following several washes, the membrane was scanned and bands were quantified on Li-Cor using Odyssey V3.0 software.
Quantitative real-time PCR
C57BKS/J Cpefat/fat and WT Mice were transcardially perfused with ice cold sterile saline. Brains were removed and olfactory bulb and striatum were extracted. Total RNA was isolated from each brain region using the RNeasy Lipid Tissue Mini Kit (Qiagen). cDNA was generated from 1 μg total RNA and random hexamers using the superscript III first strand kit (Invitrogen). A 1:10 dilution of the cDNA template was used for the real-time PCR. Primers for hemoglobin α and β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed and purchased from Invitrogen. Primer sequences are alpha hemoglobin forward 5′TGT GGA TCC CGT CAA CTT CAA; alpha hemoglobin reverse 5′TTT GTC CAG AGA GGC ATG CAC; beta hemoglobin forward 5′ACC ACT AAG CCC CTT TTC TGC T; beta hemoglobin reverse 5′GCT AGA TGC CCA AAG GTC TTC A; GAPDH forward 5′GCA AAG TGG AGA TTG TTG CCA T; GAPDH reverse 5′CCT TGA CTG TGC CGT TGA ATT T. The amplicon size for hemoglobin alpha is 106 bp, for hemoglobin beta is 106 bp, and for GAPDH is 107 bp. For these PCRs, SYBR green (Power SYBR Green PCR Master Mix, Applied Biosystems) was the fluorescent tag. All PCRs were performed on a 7900HT Real Time Thermal cycler (Applied Biosystems). The thermal cycling conditions comprised of an initial uracil-DNA glycosylase decontamination step at 50°C for 2 min, a denaturing step at 95°C for 10 min, and 40 cycles of 10 sec at 95°C, 20 sec at 60°C, and 30 sec at 72°C, followed by a dissociation curve stage at 95°C, 60°C, and 95°C, each for 15 sec. All samples were run in triplicate. Quantitative values were obtained from the threshold cycle number (Ct) at which the increase in the signal associated with exponential growth of PCR products was first detected with SDS 2.1 software (Applied Biosystems). The fold-change in expression was calculated using the ΔΔCt method, with GAPDH as an internal control.
RESULTS
To assess whether hemoglobin-derived peptides in blood could contribute to the peptides detected in the brain, we performed peptidomic analysis of blood and brain using the same methods. Overall, of the 38 alpha hemoglobin-derived peptides detected in brain, only 19 were also detected in the analysis of blood (Table 1, Figure 1A, and Table S1). Similarly, of the 10 beta hemoglobin-derived peptides detected in brain, only 4 were also detected in blood (Table 1, Figure 1A and Table S1). Only one of the three hemopressin peptides (RVD-hemopressin-α) found in brain tissue (Gomes et al., 2009) was detected in blood (Table 1 and Table S1). Conversely, 12 peptides derived from alpha and beta hemoglobin were found only in blood and not detected in brain (Table 1, Figure 1A). Additionally, 39 non-hemoglobin-derived peptides were detected in the blood analyses; these peptides were derived from 18 blood proteins including albumin, apolipoprotein, fibrinogen, kininogen, kallikreins, and several others (Table 1 and Table S1). Of these 39 peptides, 37 were detected in blood but not in brain, while only 2 were detected in both blood and brain. Thus, the comparative profile of peptides in brain versus blood is much different and it is likely that the hemoglobin-derived peptides detected in the brain tissue samples arise from hemoglobin-expressing brain cells and not from blood contamination.
Table 1.
Summary of peptidomic analysis of blood and brain.
| Protein | Number of different peptides found in: | ||
|---|---|---|---|
| Blood Onlya | Brain Onlyb | Blood and Brainc | |
| Albumin | 3 | 1 | 0 |
| Alpha-2-HS-glycoprotein | 1 | 0 | 0 |
| Apolipoprotein A-I | 2 | 0 | 0 |
| Apolipoprotein A-II | 3 | 0 | 0 |
| Apolipoprotein C-I | 2 | 0 | 0 |
| Apolipoprotein C-III | 1 | 0 | 0 |
| Apolipoprotein E | 1 | 0 | 0 |
| Apolipoprotein J | 1 | 0 | 0 |
| Complement component 3 | 1 | 0 | 0 |
| Enhancer of polycomb homolog 1 | 1 | 0 | 0 |
| Fibrinogen alpha | 8 | 0 | 1 |
| Fibrinogen beta | 2 | 1 | 0 |
| Kallikrein 1-related peptidase b1 | 1 | 0 | 0 |
| Kallikrein 1-related peptidase b22 | 2 | 0 | 0 |
| Kallikrein 1-related peptidase b27 | 2 | 0 | 0 |
| Kallikrein 1-related peptidase b3 | 1 | 0 | 0 |
| Kallikrein 1-related peptidase b9 | 1 | 0 | 0 |
| Kininogen-1 | 2 | 0 | 1 |
| Pregnancy zone protein | 2 | 0 | 0 |
| Hemoglobin alpha | 5 | 19 | 19 |
| Hemoglobin beta | 7 | 6 | 4 |
Data from Table S1
Hemoglobin data from (Gomes et al. 2009). Albumin peptide sequence is GEYGFQNAILVR and Fibrinogen beta peptide is ADDDYDEPTDSLDAR
Data from Table S1 and Gomes et al. 2009
Figure 1.
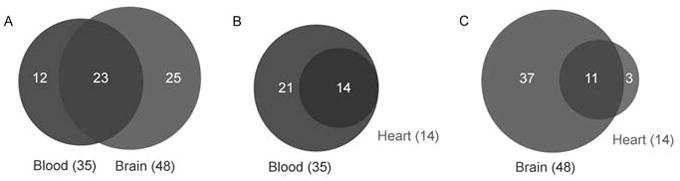
Comparison of hemoglobin derived peptidome between tissues. A. Hemoglobin-derived peptides found in blood versus those found in brain, overlap of circles distinguishes those peptides that were seen in both blood and brain. Comparison of blood and heart is in panel B and brain versus heart is in panel C.
As a control for the brain peptidomic analysis, we also analyzed the hemoglobin-derived peptidome of the heart. The heart is a tissue that is highly vascularized and there is no evidence to suggest that the heart expresses hemoglobin mRNA or protein. Therefore, we compared the peptidome of the heart and blood to see if there is a distinction between the peptide pools, as observed between brain and blood. Altogether, 14 hemoglobin-derived peptides were detected in the heart extracts, and all 14 of these were also seen in blood; no peptides were specific to only heart (Figure 1B). We also compared the hemoglobin-derived heart peptidome to that of brain, and found that of the 48 hemoglobin peptides found in brain, only 11 were also seen in heart (Figure 1C). These data further suggest that many of the hemoglobin-derived peptides in brain are produced from brain hemoglobin.
Next, we investigated the peptide levels in distinct brain regions of wild-type and Cpefat/fat mice, using a quantitative peptidomics approach. This method uses stable isotopic tags to label peptides present in different samples. Then, the samples are pooled, purified, and analyzed by liquid chromatography and mass spectrometry. Previous analysis of these data detected >150 secretory pathway peptides and a large number of peptides derived from cytosolic proteins, although this latter group were not included in the previous analysis (Che & Fricker 2002, Lim et al. 2006, Zhang et al. 2008). The present study re-analyzed the previous mass spectrometry data to focus on hemoglobin-derived peptides and other commonly detected peptides derived from cytosolic proteins. Representative data are shown in Figure 2. A number of peptides derived from alpha and beta hemoglobin were detected, including RVD-hemopressin-α and VD-hemopressin-α (Table 2). Both RVD-hemopressin-α and VD-hemopressin-α were elevated in Cpefat/fat mice as compared to WT controls in olfactory bulb, with levels 3-4-fold higher (Table 2). RVD-hemopressin-α was also found to be elevated in striatum (>6 fold) but not in the prefrontal cortex, which showed a Cpefat/fat mice to WT mice ratio of 1.2 (Table 2). Other alpha hemoglobin derived peptides showed an increase in Cpefat/fat mice as compared to WT mice, and these increases were also limited to the olfactory bulb and striatum, with no increases observed in the prefrontal cortex. A beta hemoglobin-derived peptide, VV-hemorphin-7, was found to be elevated >10 fold in the hypothalamus (Table 2).
Figure 2.
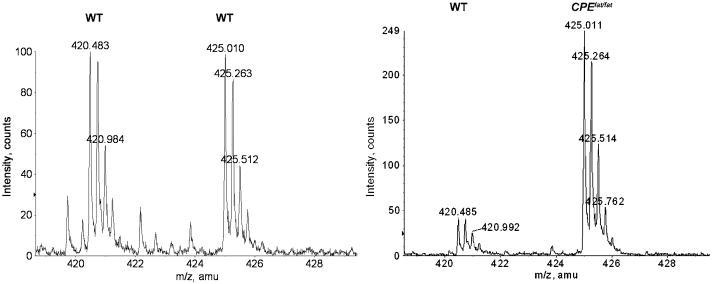
Representative spectra showing levels of peptides in wild-type and Cpefat/fat mice. Left panel: ions at m/z 420.483 and 425.010 (monoisotopic peaks) correspond to mono-D0- and D9- TMAB labeled RVD-hemopressin-α in olfactory bulb in an experiment evaluating WT vs WT mice. Ratio of D0 to D9 is 1.0, illustrating no change in peptide level in WT mice. Right panel: ions at 420.485 and 425.011 (monoisotopic peaks) correspond to mono-D0- and D9-TMAB labeled RVD-hemopressin-α in olfactory bulb in an experiment evaluating Cpefat/fat mice vs WT mice. Quantification of this spectra illustrates RVD-hemopressin-α is 5.5 fold higher in the Cpefat/fat mouse sample as compared to the WT mouse sample.
Table 2.
Relative levels of hemoglobin-derived peptides in Cpefat/fat mice.
| Protein | Sequence | Brain Region | Cpefat/fat:WT ± SEM (n) |
|---|---|---|---|
| Alpha Hemoglobin | |||
| LSDLHAHKL | Olf Bulb | 3.65 ± 1.06 (4) | |
| VDPVNFKLLSH | Olf Bulb | 4.81 ± 3.31 (2) | |
| AGHLDDLPGALSAL | Olf Bulb | 6.91 ± 5.39 (2) | |
| RVDPVNFKLLSH | Olf Bulb | 3.43 ± 0.68 (8) | |
| RVDPVNFKLLSH | PFCx | 1.20 ± 0.19 (3) | |
| RVDPVNFKLLSH | Striatum | >6 (4) | |
| ASHHPADFTPAVHA | Olf Bulb | 3.37 ± 1.72 (2) | |
| ANAAGHLDDLPGALSAL | Olf Bulb | 7.18 ± 5.42 (2) | |
| KFLASVSTVLTSKYR | PFCx | 1.20 ± 0.21 (2) | |
| (FTPAVHASLDKFLASVSTVLTSKYR) | PFCx | 1.31 ± 0.31 (2) | |
| Beta Hemoglobin | |||
| VVYPWTQRY | Hypo | >10 (2) | |
The relative levels of peptides were calculated by the ratio of peak intensity of the D0-TMAB-and the D9-TMAB-labeled peptide pairs. Cpefat/fat:WT indicates the ratio of peptide in Cpefat/fat mice relative to WT mice with standard error of the mean (SEM) for n replicates. Abbreviations: Olf Bulb, olfactory bulb; PFCx, prefrontal cortex; Hypo, hypothalamus; ND, not detected.
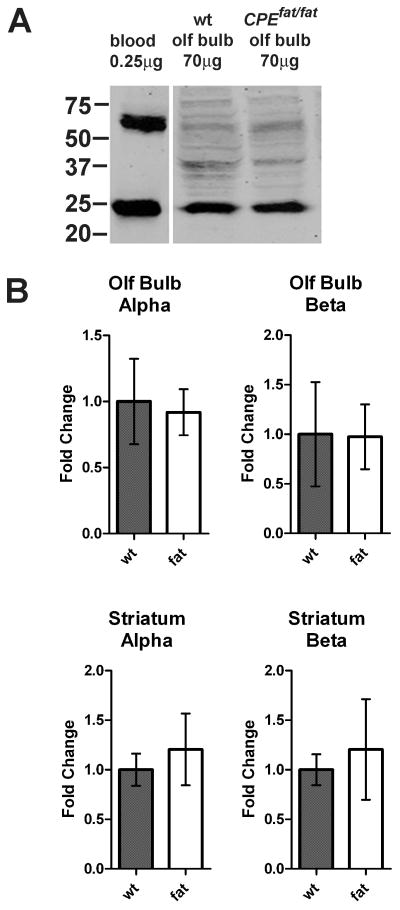
To test if the increase in hemoglobin-derived peptides was a result of elevated levels of hemoglobin mRNA levels in brain, we examined levels of alpha and beta hemoglobin mRNA using quantitative real-time PCR and compared WT and Cpefat/fat mice that were perfused with saline to reduce the contamination from blood. For this, oligonucleotides were created that gave productsof ~100 nucleotides and had comparable melting temperatures to each other and to the control oligonucleotides (GAPDH). Analysis of the PCR product showed bands of the correct size (data not shown). Both male and female mice were included in this analysis because the peptidomics analysis also used both genders in roughly equal numbers. No major differences were observed in comparing the male and female data, so these groups were pooled to increase the overall n value for the experiment. Nosignificantdifference in the levels of alpha and beta hemoglobin mRNAwere found in any of the Cpefat/fat mouse brain regions, relative to wild-type mice (Figure 3). In addition, hemoglobin proteinlevels in the various brain regions of saline-perfused WT and Cpefat/fat mice were determined using Western blot analysis. Representative data are shown in Figure 4A. Quantitation of the results from striatum and olfactory bulb for alpha and beta hemoglobin showed generally comparable levels between wild-type and Cpefat/fat mice, with no statistical significance between groups (Figure 4B).
Figure 3.
Quantitative real-time PCR of hemoglobin mRNA levels in wild-type and Cpefat/fat mice. Results are shown as the fold-change in expression. Fold change was calculated using the ΔΔCt method, with GAPDH as an internal control. Both male and female mice used. n= 5 Cpefat/fat mice and 6 WT mice. Error bars represent standard error of the mean.
Figure 4.
Western blot analysis of hemoglobin protein levels in wild-type and Cpefat/fat mice. A: Representative data for alpha hemoglobin in olfactory bulb and blood. Hemoglobin monomer is seen at approximately 24 kD. B: Quantitation of results from multiple analyses. n= 5 Cpefat/fat mice and 6 WT mice. Error bars represent standard error of the mean.
Because the levels of hemoglobin mRNA and protein are not altered in Cpefat/fat mouse brain, relative to WT mouse brain, it is likely that the increase in some of the hemopressins and other hemoglobin-derived peptides in the mutant mice (Table 2) is due to elevated processing of hemoglobin. To test if other peptides derived from cytosolic proteins are elevated in the Cpefat/fat mice, we analyzed the data comparing Cpefat/fat and WT mice for a number of different cytosolic peptides that were previously identified in brain peptidomics analyses (Table S3). For most of the cytosolic peptides Cpefat/fat/WT ratios were approximately 1.0, indicating that the vast majority of peptides from cytosolic proteins are not altered in the Cpefat/fat mice(Table S3). A small number of peptides show ratios around 2.0 or higher, but in most cases the error range for these is large, indicating that the peptide is variable among mice and not necessarily elevated in the Cpefat/fat mice. For example, one peptide that appears to be much higher in the Cpefat/fat mice than in WT mice is KTEWLDGKHVVF from peptidylprolyl isomerase A (Table S3). This peptide appeared to be elevated approximately 6-fold in Cpefat/fat mouse striatum, but had a large error range (±3.7). Furthermore, this peptide was not comparably altered in several other brain regions in which it was detected, and many other fragments of the same protein were only slightly elevated in the Cpefat/fat mice. Interestingly, one other cytosolic proteinthat showed consistent increases in processing in the Cpefat/fat was the precursor of DBI, acyl-CoA-binding protein (Costa & Guidotti 1991); all three different fragments of this protein detected in our analysis were found to be present in the Cpefat/fat mice at levels ~2 fold higher than the levels in WT mice (Table S3).
DISCUSSION
The discovery that alpha- and beta-hemoglobin derived peptides are present in mouse brain and bind to CB1 cannabinoid receptors raised the possibility that these molecules represent novel endogenous signaling molecules. A central question is whether these peptides are actually produced in brain, or are merely a result of the blood that is inevitably present in brain. Although perfusion can be employed to reduce or eliminate blood cells from tissues, to avoid postmortem production of hemoglobin-derived peptides due to ischemia for peptidomics analysesit is necessary to rapidly heat the brain using microwave irradiation(Che et al. 2005b)or other methods(Skold et al. 2007). Thus, the brain, blood, and heart peptidome were compared in the present study in order to evaluate whether the hemoglobin-derived peptides were common to all samples or if brain peptides were distinct from those present in blood and heart. The finding that hemoglobin-derived peptides detected in brain are largely distinct from those in blood and heart implies that the processing pathways are tissue-selective and that the brain peptides are not simply the result of blood contamination. This was further supported by a comparison of the heart hemoglobin-derived peptidome with those of blood; the large overlap between heart and blood suggests that blood is the source of the hemoglobin peptides found in heart, while the small overlap between brain and either blood or heart suggests distinct origin for the brain peptides. Furthermore, of the 39 non-hemoglobin-derived peptides detected in blood, only 2 were also found in the analyses of brain peptides. Collectively, these results strongly suggest that the brain hemoglobin peptides are locally produced and that the contribution of the blood peptidome to the brain peptidome is minimal.
Another important question from the present studies concerns the mechanism of production of the hemopressins and the physiological regulation of these molecules. The previous finding that Lys- and Arg-extended forms of hemoglobin were elevated in the Cpefat/fat mice (Bures et al. 2001, Lim et al. 2006, Che et al. 2005a) raised the possibility that the processing involved CPE; most of the peptides found to be elevated in these mutant mice are substrates of this enzyme (Che et al. 2005a, Che & Fricker 2005, Fricker et al. 1996, Lim et al. 2006, Rovere et al. 1996, Zhang et al. 2008). Although CPE is in the secretory pathway and hemopressinsare expected to be produced in the cytosol, it is conceivable that transporters like TAP1 and TAP2 could pump these peptides into the endoplasmic reticulum and then they would encounter CPE along the secretory pathway (Koopmann et al. 1997). However, our finding that the increase in many hemoglobin peptides in the Cpefat/fat mice is not limited to those peptides with C-terminal Lys- or Arg-residues (Table 2) indicates that this cytosolic peptide increment is indirect and unrelated to the catalytic action of CPE. The finding that the hemopressinsand several other hemoglobin-derived peptides increase in some, but not all brain regions of Cpefat/fat mice without accompanying changes in hemoglobin protein or mRNA levels, suggests regulation at the post-translational processing level.
Overall, the level of upregulation of hemoglobin-derived peptides in Cpefat/fat is generally much lower than the degree of upregulation of neuropeptide processing intermediates. For example, peptides derived from pro-opiomelanocortin that have C-terminal lysine or arginine are >20 fold more abundant in Cpefat/fat mice brains as compared to WT mice (Che et al. 2005a). Furthermore, a fragment of procholecystokinin containing a C-terminal -Gly-Arg-Arg extension was found at levels estimated to be 13–51-fold higher in Cpefat/fat mice as compared to WT controls (Cain et al. 1997, Wang et al. 1998). Although some neuropeptides are not greatly affected by the absence of CPE activity in the Cpefat/fat mice, this is presumably due to the presence of a related enzyme, carboxypeptidase D, in the trans Golgi apparatus and immature secretory vesicles (Varlamov & Fricker 1998). In contrast to the large increases in levels of most Lys/Arg-extended neuropeptide intermediates and large decreases in levels of the mature neuropeptides previously found in Cpefat/fat mice, the hemopressins and other related hemoglobin-derived peptides detected in this study either showed relatively modest 2–10 fold increases or no change, depending on brain region. The magnitude of the changes in hemoglobin peptides detected in the present study is in general agreement with a previous peptidomics studies that reported changes in hemoglobin-derived peptides with C-terminal Lys and/or Arg in Cpefat/fat mice (Bures et al. 2001). The Bures et al study used affinity columns to isolate the Lys-and/or Arg-extended peptides, a technique developed to identify neuropeptide processing intermediates in Cpefat/fat mice (Che et al. 2001, Fricker et al. 2000). Because the hemopressins, and most other hemoglobin-derived peptides, do not contain C-terminal Lys/Arg residues, they would not have been detected in the previous studies that used this technique.
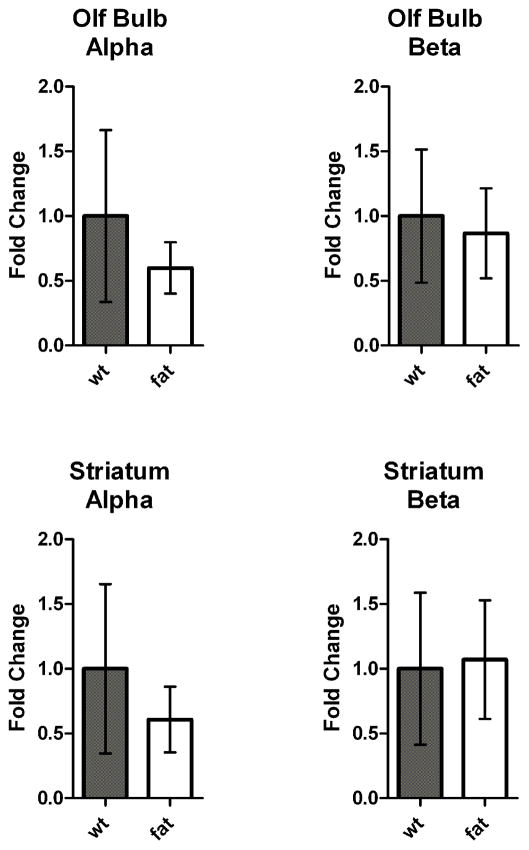
Because CPE resides in the secretory pathway and removes basic residues from the C-terminus of peptides (Fricker & Snyder 1982, Fricker & Snyder 1983), it makes sense that many neuropeptide processing intermediates that contain C-terminal Lys/Arg residues are increased in the Cpefat/fat mice which lack functional CPE (Fricker et al. 1996). However, it is not clear by what mechanism a CPE defect in Cpefat/fat mice leads to altered levels of the hemopressins and other hemoglobin-derived peptides. This upregulation is not likely due to increased levels of hemoglobin production as levels of its mRNA and protein were not significantly different between Cpefat/fat mice and WT mice (Figures 2 and 3). Therefore, it is likely that the post-translational processing of alpha and beta hemoglobin is upregulated in some brain regions of Cpefat/fat mice. The upregulation of the hemoglobin derived peptides is not due to a general large-scale breakdown of all cytosolic proteins because most peptides derived from cytosolic proteins are present in Cpefat/fat and WT mice at generally comparable levels (Figure 5). This suggests that there is specificity for hemoglobin breakdown in the Cpefat/fat mice and that by some unknown mechanism the post translational processing of both alpha and beta hemoglobin is affected, leading to increased hemoglobin derived peptides.
Figure 5.
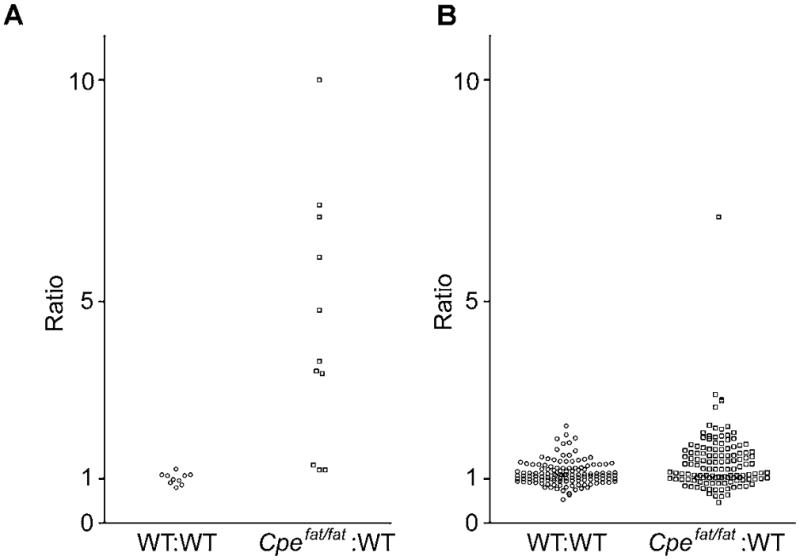
Ratio of hemoglobin-derived and other cytosolic protein-derived peptides in WT:WT and Cpefat/fat:WT mice. A. Relative amounts of hemoglobin-derived peptides in groups of WT mice as compared to other WT mice (left) and Cpefat/fat mice as compared to WT mice (right). Data points with a ratio of “10” refer to a ≥10 fold increase in Cpefat/fat mice as compared to WT mice. For identity of peptides, see Table 2. B. Ratios of peptides derived from cytosolic proteins other than hemoglobin. For identify of peptides, see Table S3.
In addition to alpha and beta hemoglobin, the only other protein detected in the present study that appeared to consistently show elevated processing into peptides in the Cpefat/fat mice was DBI, a putative peptide antagonist of the binding of benzodiazepine to the GABAA receptor (Costa & Guidotti 1991). It is interesting that peptides derived from all three of these proteins have been found to be bioactive, and more specifically, to interact with neurotransmitter receptors (Gomes et al. 2009, Nyberg et al. 1997, Ivanov et al. 1997, Costa & Guidotti 1991, Heimann et al. 2007). The hemopressins, hemorphins, and DBI are distinct from classical neuropeptides that are produced in the secretory pathway and released from cells upon stimulation. It is possible that the cytosol-derived bioactive peptides are analogous to signaling molecules such as nitric oxide or anandamide which are not stored in vesicles. Instead, these “non-classical neurotransmitters” are made on demand by enzymes that are stimulated by various pathways, and then constitutively secreted. It is therefore plausible that hemopressins, hemorphins, and other peptides derived from cytosolic proteins represent “non-classical neuropeptides” which are synthesized upon stimulation and then constitutively secreted. Regulation, which is central to a proposed role as a signaling molecule, would occur at the level of synthesis rather than secretion. The major finding of the present study suggesting that the hemopressins and other hemoglobin derived peptides are upregulated in the Cpefat/fat mice supports this proposed role as non-classical neuropeptides.
Increased levels of hemoglobin-derived peptides have also been reported in another mutant animal model, Purkinje cell degeneration (pcd) mice (Berezniuk et al. 2010). Pcd mice have a spontaneous mutation in the cytosolic carboxypeptidase CCP1/Nna1 (Fernandez-Gonzalez et al. 2002, Mullen et al. 1976). In a recent peptidomics study comparing peptides in pcd and WT mouse brain extracts, all hemoglobin-derived peptides were found to be elevated greater than 2-fold in the pcd mutant brains; these peptides included four alpha hemoglobin-derived and two beta hemoglobin-derived peptides (Berezniuk et al. 2010). Although the hemopressins were not reported in the Berezniuk et al study, RVD-hemopressin-α was seen in pcd brains in an additional study and was found to be elevated >5 fold as compared to control brains (I. Berezniuk and L. Fricker, unpublished). Furthermore, this increase in RVD-hemopressin-α and other hemoglobin-derived peptides was not seen in the hearts of pcd animals as compared to WT controls (J. Sironi and L. Fricker, unpublished). These studies further support the idea that hemopressins detected in brain extracts are produced from brain hemoglobin and are not contaminants from erythrocytes present within the brain.
There are many remaining questions that need to be answered to complete our understanding on this emerging field. Key questions involve the enzymatic pathway required for the production of the cytosolic peptides, the mechanism(s) by which this pathway is regulated, whether the peptides are secreted, and if so, the mechanism of this secretion. It is likely that the ubiquitin-proteasome pathway is involved in the production of hemopressin and other cytosolic peptides, although this needs to be directly investigated. It is also likely that the peptides are secreted from the cytosol; many intracellular peptides, such as annexins, thymosin, and interleukin-β are known to be secreted from the cytosol (Goya & Bolognani 1999, Gerke & Moss 2002, Simi et al. 2007). Further studies are required to understand the pathophysiological function of the hemopressins and the other cytosolic-derived peptides and to determine the mechanism by which hemopressins are increased in Cpefat/fat mouse brain. In the present study, RVD-hemopressin-α and VD-hemopressin-α were detected in the olfactory bulb; in rodent brain, very high levels of CB1 receptor expression are detected in the anterior olfactory nucleus (Tsou et al. 1998, Lein et al. 2007). Hemopressin peptides were also found in the striatum and prefrontal cortex in the present study, which also have high levels of CB1 receptor expression. An interesting area for further research will be to investigate if hemoglobin is differentially processed in various cells/brain regions, as suggested by the present data.
Supplementary Material
Acknowledgments
This work was primarily supported by National Institutes of Health grant DA-04494 (to L.D.F.). Mass spectrometry was performed in the Laboratory for Macromolecular Analysis and Proteomics of the Albert Einstein College of Medicine, directed by Dr. Ruth Angeletti, and in Brazil through the Rede de Proteoma do Estado de São Paulo in the Laboratório Nacional de Luz Sincrotron, Campinas, SP, Brazil, and supported in part by Fundação de Amparo a Pesquisa do Estado de São Paulo 04/04933-2, 04/14846-0 and Financiadora de Estudos e Projetos A-03/134 (to E.S.F.) and by fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (E.S.F. and L.M.C.). We are thankful to Prof. Fabio Gozzo, Universidade de Campinas, for his immeasurable support with mass spectrometry. Quantitative PCR was performed in the genomics facility of Albert Einstein College of Medicine. Thanks are also due to Larkin Elderon for assistance with the analysis of the blood peptidome.
References
- Berezniuk I, Sironi J, Callaway MB, Castro LM, Hirata IY, Ferro ES, Fricker LD. CCP1/Nna1 functions in protein turnover in mouse brain: Implications for cell death in Purkinje cell degeneration mice. Faseb J. 2010 doi: 10.1096/fj.09-147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti DA, Morano C, Russo LC, et al. Analysis of intracellular substrates and products of thimet oligopeptidase in human embryonic kidney 293 cells. The Journal of biological chemistry. 2009;284:14105–14116. doi: 10.1074/jbc.M807916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran M, Chen H, Chen Z, Liu L. Hemoglobin is expressed in alveolar epithelial type II cells. Biochemical and biophysical research communications. 2005;333:1348–1352. doi: 10.1016/j.bbrc.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioli M, Pinto M, Cesselli D, et al. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15454–15459. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bures EJ, Courchesne PL, Douglass J, et al. Identification of incompletely processed potential carboxypeptidase E substrates from CpEfat/CpEfat mice. Proteomics. 2001;1:79–92. doi: 10.1002/1615-9861(200101)1:1<79::AID-PROT79>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Cain BM, Wang W, Beinfeld MC. Cholecystokinin (CCK) levels are greatly reduced in the brains but not the duodenums of Cpe(fat)/Cpe(fat) mice: a regional difference in the involvement of carboxypeptidase E (Cpe) in pro-CCK processing. Endocrinology. 1997;138:4034–4037. doi: 10.1210/endo.138.9.5490. [DOI] [PubMed] [Google Scholar]
- Che FY, Biswas R, Fricker LD. Relative quantitation of peptides in wild-type and Cpe(fat/fat) mouse pituitary using stable isotopic tags and mass spectrometry. J Mass Spectrom. 2005a;40:227–237. doi: 10.1002/jms.742. [DOI] [PubMed] [Google Scholar]
- Che FY, Fricker LD. Quantitation of neuropeptides in Cpe(fat)/Cpe(fat) mice using differential isotopic tags and mass spectrometry. Analytical chemistry. 2002;74:3190–3198. doi: 10.1021/ac015681a. [DOI] [PubMed] [Google Scholar]
- Che FY, Fricker LD. Quantitative peptidomics of mouse pituitary: comparison of different stable isotopic tags. J Mass Spectrom. 2005;40:238–249. doi: 10.1002/jms.743. [DOI] [PubMed] [Google Scholar]
- Che FY, Lim J, Pan H, Biswas R, Fricker LD. Quantitative neuropeptidomics of microwave-irradiated mouse brain and pituitary. Mol Cell Proteomics. 2005b;4:1391–1405. doi: 10.1074/mcp.T500010-MCP200. [DOI] [PubMed] [Google Scholar]
- Che FY, Yan L, Li H, Mzhavia N, Devi LA, Fricker LD. Identification of peptides from brain and pituitary of Cpe(fat)/Cpe(fat) mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9971–9976. doi: 10.1073/pnas.161542198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che FY, Zhang X, Berezniuk I, Callaway M, Lim J, Fricker LD. Optimization of neuropeptide extraction from the mouse hypothalamus. Journal of proteome research. 2007;6:4667–4676. doi: 10.1021/pr060690r. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life sciences. 1991;49:325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- Dale CS, Pagano Rde L, Rioli V. Hemopressin: a novel bioactive peptide derived from the alpha1-chain of hemoglobin. Memorias do Instituto Oswaldo Cruz. 2005a;100(Suppl 1):105–106. doi: 10.1590/s0074-02762005000900017. [DOI] [PubMed] [Google Scholar]
- Dale CS, Pagano Rde L, Rioli V, Hyslop S, Giorgi R, Ferro ES. Antinociceptive action of hemopressin in experimental hyperalgesia. Peptides. 2005b;26:431–436. doi: 10.1016/j.peptides.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Dassen H, Kamps R, Punyadeera C, Dijcks F, de Goeij A, Ederveen A, Dunselman G, Groothuis P. Haemoglobin expression in human endometrium. Human reproduction (Oxford, England) 2008;23:635–641. doi: 10.1093/humrep/dem430. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Mains RE, Herbert E. Peptides in the nervous system. Trends Neurosci. 1986;9:463–468. [Google Scholar]
- Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, Morgan JI, Zuo J. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science (New York, NY. 2002;295:1904–1906. doi: 10.1126/science.1068912. [DOI] [PubMed] [Google Scholar]
- Fricker LD. Carboxypeptidase E. Annual review of physiology. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- Fricker LD, Berman YL, Leiter EH, Devi LA. Carboxypeptidase E activity is deficient in mice with the fat mutation. Effect on peptide processing. The Journal of biological chemistry. 1996;271:30619–30624. doi: 10.1074/jbc.271.48.30619. [DOI] [PubMed] [Google Scholar]
- Fricker LD, McKinzie AA, Sun J, et al. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci. 2000;20:639–648. doi: 10.1523/JNEUROSCI.20-02-00639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD, Snyder SH. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:3886–3890. doi: 10.1073/pnas.79.12.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD, Snyder SH. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. The Journal of biological chemistry. 1983;258:10950–10955. [PubMed] [Google Scholar]
- Fukui K, Shiomi H, Takagi H, Hayashi K, Kiso Y, Kitagawa K. Isolation from bovine brain of a novel analgesic pentapeptide, neo-kyotorphin, containing the Tyr-Arg (kyotorphin) unit. Neuropharmacology. 1983;22:191–196. doi: 10.1016/0028-3908(83)90008-4. [DOI] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiological reviews. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Gomes I, Grushko JS, Golebiewska U, et al. Novel endogenous peptide agonists of cannabinoid receptors. Faseb J. 2009;23:3020–3029. doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya RG, Bolognani F. Homeostasis, thymic hormones and aging. Gerontology. 1999;45:174–178. doi: 10.1159/000022082. [DOI] [PubMed] [Google Scholar]
- Haase VH. Hemoglobin in the kidney: breaking with traditional dogma. J Am Soc Nephrol. 2008;19:1440–1441. doi: 10.1681/ASN.2008060594. [DOI] [PubMed] [Google Scholar]
- He Y, Hua Y, Keep R, Wang M, Xi G. Brain hemoglobin expression after intracerebral hemorrhage. Sfn Abstract. 2009:148.29. [Google Scholar]
- Heimann AS, Gomes I, Dale CS, et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VT, Karelin AA, Philippova MM, Nazimov IV, Pletnev VZ. Hemoglobin as a source of endogenous bioactive peptides: the concept of tissue-specific peptide pool. Biopolymers. 1997;43:171–188. doi: 10.1002/(SICI)1097-0282(1997)43:2<171::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Koopmann JO, Hammerling GJ, Momburg F. Generation, intracellular transport and loading of peptides associated with MHC class I molecules. Current opinion in immunology. 1997;9:80–88. doi: 10.1016/s0952-7915(97)80163-x. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lim J, Berezniuk I, Che FY, Parikh R, Biswas R, Pan H, Fricker LD. Altered neuropeptide processing in prefrontal cortex of Cpe (fat/fat) mice: implications for neuropeptide discovery. Journal of neurochemistry. 2006;96:1169–1181. doi: 10.1111/j.1471-4159.2005.03614.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Zeng M, Stamler JS. Hemoglobin induction in mouse macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6643–6647. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedeva VP, Richter F, Chesselet MF. Hemoglobin mRNA expression can be detected and up-regulated in differentiated but not in undifferentiated human neuroblastoma SH-SY5Y. Sfn Abstract. 2009:630.9. [Google Scholar]
- Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nature genetics. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Newton DA, Rao KM, Dluhy RA, Baatz JE. Hemoglobin is expressed by alveolar epithelial cells. The Journal of biological chemistry. 2006;281:5668–5676. doi: 10.1074/jbc.M509314200. [DOI] [PubMed] [Google Scholar]
- Nishi H, Inagi R, Kato H, Tanemoto M, Kojima I, Son D, Fujita T, Nangaku M. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J Am Soc Nephrol. 2008;19:1500–1508. doi: 10.1681/ASN.2007101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg F, Sanderson K, Glamsta EL. The hemorphins: a new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers. 1997;43:147–156. doi: 10.1002/(SICI)1097-0282(1997)43:2<147::AID-BIP8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ojika K, Mitake S, Tohdoh N, Appel SH, Otsuka Y, Katada E, Matsukawa N. Hippocampal cholinergic neurostimulating peptides (HCNP) Progress in neurobiology. 2000;60:37–83. doi: 10.1016/s0301-0082(99)00021-0. [DOI] [PubMed] [Google Scholar]
- Pinto M, Biagioli M, Cesselli D, et al. Alpha- and beta- globin expression in mesencephalic dopaminergic neurons and glial cells. Sfn Abstract. 2009:630.27. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter F, Meurers BH, Zhu C, Medvedeva VP, Chesselet MF. Neurons express hemoglobin alpha- and beta-chains in rat and human brains. The Journal of comparative neurology. 2009;515:538–547. doi: 10.1002/cne.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioli V, Gozzo FC, Heimann AS, et al. Novel natural peptide substrates for endopeptidase 24.15, neurolysin, and angiotensin-converting enzyme. The Journal of biological chemistry. 2003;278:8547–8555. doi: 10.1074/jbc.M212030200. [DOI] [PubMed] [Google Scholar]
- Rovere C, Viale A, Nahon J, Kitabgi P. Impaired processing of brain proneurotensin and promelanin-concentrating hormone in obese fat/fat mice. Endocrinology. 1996;137:2954–2958. doi: 10.1210/endo.137.7.8770919. [DOI] [PubMed] [Google Scholar]
- Simi A, Lerouet D, Pinteaux E, Brough D. Mechanisms of regulation for interleukin-1beta in neurodegenerative disease. Neuropharmacology. 2007;52:1563–1569. doi: 10.1016/j.neuropharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Skold K, Svensson M, Norrman M, Sjogren B, Svenningsson P, Andren PE. The significance of biochemical and molecular sample integrity in brain proteomics and peptidomics: stathmin 2–20 and peptides as sample quality indicators. Proteomics. 2007;7:4445–4456. doi: 10.1002/pmic.200700142. [DOI] [PubMed] [Google Scholar]
- Strand FL. Neuropeptides: general characteristics and neuropharmaceutical potential in treating CNS disorders. Progress in drug research. Fortschritte der Arzneimittelforschung. 2003;61:1–37. doi: 10.1007/978-3-0348-8049-7_1. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Ullal AJ, Litaker RW, Noga EJ. Antimicrobial peptides derived from hemoglobin are expressed in epithelium of channel catfish (Ictalurus punctatus, Rafinesque) Developmental and comparative immunology. 2008;32:1301–1312. doi: 10.1016/j.dci.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Varlamov O, Fricker LD. Intracellular trafficking of metallocarboxypeptidase D in AtT-20 cells: localization to the trans-Golgi network and recycling from the cell surface. Journal of cell science. 1998;111 (Pt 7):877–885. doi: 10.1242/jcs.111.7.877. [DOI] [PubMed] [Google Scholar]
- Wang W, Cain BM, Beinfeld MC. Adult carboxypeptidase E-deficient fat/fat mice have a near-total depletion of brain CCK 8 accompanied by a massive accumulation of glycine and arginine extended CCK: identification of CCK 8 Gly as the immediate precursor of CCK 8 in rodent brain. Endocrine. 1998;9:329–332. doi: 10.1385/ENDO:9:3:329. [DOI] [PubMed] [Google Scholar]
- Wride MA, Mansergh FC, Adams S, Everitt R, Minnema SE, Rancourt DE, Evans MJ. Expression profiling and gene discovery in the mouse lens. Molecular vision. 2003;9:360–396. [PubMed] [Google Scholar]
- Zhang R, Sioma CS, Thompson RA, Xiong L, Regnier FE. Controlling deuterium isotope effects in comparative proteomics. Analytical chemistry. 2002;74:3662–3669. doi: 10.1021/ac025614w. [DOI] [PubMed] [Google Scholar]
- Zhang X, Che FY, Berezniuk I, Sonmez K, Toll L, Fricker LD. Peptidomics of Cpe(fat/fat) mouse brain regions: implications for neuropeptide processing. Journal of neurochemistry. 2008;107:1596–1613. doi: 10.1111/j.1471-4159.2008.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Garreau I, Sannier F, Piot JM. Opioid peptides derived from hemoglobin: hemorphins. Biopolymers. 1997;43:75–98. doi: 10.1002/(SICI)1097-0282(1997)43:2<75::AID-BIP2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.