Abstract
The study of experimental hypertension and the development of drugs with selective inhibitory effects on the enzymes and receptors constituting the components of the circulating and tissue renin-angiotensin systems have led to newer concepts of how this system participates in both physiology and pathology. Over the last decade, a renewed emphasis on understanding the role of angiotensin-(1–7) and angiotensin-converting enzyme 2 in the regulation of blood pressure and renal function has shed new light on the complexity of the mechanisms by which these components of the renin angiotensin system act in the heart and in the kidneys to exert a negative regulatory influence on angiotensin converting enzyme and angiotensin II. The vasodepressor axis composed of angiotensin-(1–7)/angiotensin-converting enzyme 2/mas receptor emerges as a site for therapeutic interventions within the renin-angiotensin system. This review summarizes the evolving knowledge of the counterregulatory arm of the renin-angiotensin system in the control of nephron function and renal disease.
Keywords: blood pressure, hypertension, renal function, renin, renal disease
for more than a century the kidney has maintained a position of dominance as a primary suspect in the pathogenesis of arterial hypertension given its critical function in the regulation of body fluid volumes and its role in controlling arterial pressure through the regulation of fluid balance and as the predominant source for the synthesis and the secretion of renin. The demonstration by Goldblatt et al. (54) that the placement of a clamp at the level of the renal artery was associated with a blood pressure elevation that could persist for several months or even years became the definitive underpinning to the exploration of how the kidneys either cause or contribute to the pathogenesis of arterial hypertension. Sixty-eight years later, Cervenka et al. (24) would demonstrate the essentiality of intrarenal expression of receptors to angiotensin II (ANG II) in mediating the hypertensive response due to ischemia through their report that clipping of a renal artery failed to induce the development of two-kidney, one-clip hypertension in ANG II receptor knockout mice.
Of the many regulatory mechanisms affecting nephron function, the influence of a kidney-borne renin-angiotensin system continues to gain acceptance (101, 102). Regulated independently from the circulating renin-angiotensin system, intrarenal formation of ANG II modulates solute and water transport across the renal tubules and the filtration of proteins through the glomerular barrier. In addition, ANG II trophic actions may contribute to renal pathology in part by increasing collagen deposition. On the opposite side of the story, Fasciolo (35), in the Andean city of Mendoza, Argentina, first articulated the concept that the kidneys possessed an antihypertensive action that could buffer the pressor actions mediated by the renin-dependent formation of ANG II in the circulation. While the pursuit of this concept by others met with relative success (97, 98), the recent characterization of the actions of angiotensin-(1–7) [ANG-(1–7)] and its further elaboration as a component of the now named angiotensin-converting enzyme 2 (ACE2)/ANG-(1–7)/mas axis (43), provides an alternate explanation as to how components within the intrarenal renin-angiotensin system function to counteract the hypertensive effects of ANG II in the long-term regulation of body fluids and arterial pressure. This review summarizes the evidence for the actions of the ANG-(1–7)/ACE2/mas axis in the regulation of renal function and its participation in renal disease.
The ANG-(1–7)/ACE2/mas Axis
General considerations.
The heptapeptide ANG-(1–7), generated from either ANG I or ANG II, acts to oppose the vasoconstrictor, proliferative, and profibrotic actions of ANG II in the circulation, cardiac, vascular, and renal tissues (37, 43). ANG-(1–7) is generated from ANG I through the hydrolytic activity of the tissue endopeptidases neprilysin (neutral endopeptidase 24.11), prolyl-endopeptidase 24.26, and thimet oligopeptidase 24.15 (150). ACE2, acting as a monocarboxypeptidase to cleave the peptide bond between proline and a hydrophobic C-terminal residue (145), degrades ANG II into ANG-(1–7) (118, 135). ACE2 is found in vascular endothelial cells, cardiac myocytes, the testes, liver, and the gut. In the kidney, ACE2 is found primarily in the luminal surface of the tubular epithelium (16, 134), a finding that contrasts with the more generalized distribution of ACE (148). The diversity of the enzymes contributing to ANG-(1–7) formation may be a function of tissue-specific localization and access to the corresponding substrates (either ANG I or ANG II) within either the extracellular or intracellular compartments. Therefore, the actions of ANG-(1–7) may be regulated in part through the control of when and where the dual substrates are expressed. Vascular endothelial cells having a rich content of both prolyl endopeptidase 24.26 and neprilysin explains their predominant role for ANG-(1–7) formation from ANG I in the systemic and coronary vascular circulations while the abundant expression of neprilysin in deep proximal renal tubules and brush border membranes (86, 125) catalyzes the formation of ANG-(1–7) from ANG I as well as its degradation into ANG-(1–4) (38). In experiments conducted in spontaneously hypertensive rat (SHR) urine, ANG-(1–7) hydrolysis into the inactive ANG-(1–4) fragment was suppressed in the presence of omapatrilat, a specific inhibitor of both ACE and neprilysin (38).
ACE2 is an exopeptidase that catalyzes the conversion of ANG I to the nonapeptide ANG-(1–9) or the conversion of ANG II to the heptapeptide ANG-(1–7). Studies by Vickers et al. (145) and Rice et al. (118) demonstrated a primary role of ACE2 in converting ANG II into ANG-(1–7) with an efficacy >400-fold compared with the hydrolytic action of ACE2 in forming ANG-(1–9). ACE2 protein levels are significantly downregulated in the kidneys of hypertensive (28), diabetic (133), and pregnant rats (14, 16), suggesting a negative regulatory role of ACE2 in blood pressure control. In addition, ACE2 mutant mice develop late-onset glomerulonephritis resembling diabetic nephropathy (106).
The heptapeptide ANG-(1–7) binds to the mas receptor, a seven-transmembrane protein with domains containing sequences characteristic of G protein-coupled receptors (157). The mas receptor is expressed in renal cortical and proximal tubular cells and also afferent arterioles and the apical surface of the tubular epithelium (1, 130). In mutant mice lacking the mas receptor, the specific ANG-(1–7) binding to renal tissue was eliminated and this was associated with loss of the antidiuretic response to ANG-(1–7) after water loading as well as blunting of ANG-(1–7)-mediated vascular endothelial relaxation in aortic rings (124). We have confirmed that the mas receptor conveys the signaling actions of ANG-(1–7) in cardiac myocytes in culture (131) and further showed that ANG-(1–7) stimulates a MAPK phosphatase (48). The obtunding actions of ANG II as a negative regulator of ACE2 mRNA in neural and cardiac tissues suggests that ANG II has a direct effect on influencing the hydrolytic activity of ACE2 in ANG-(1–7) formation (47, 49). Although in our studies ANG-(1–7) showed no direct effect on the expression of ACE2 mRNA (48), the heptapeptide blocked the negative effect of ANG II in inhibiting cardiac ACE2 transcripts in cardiac myocytes and astrocytes in culture (47, 49).
Effects of the ANG-(1–7)/ACE2/mas axis on renal hemodynamics.
As addressed by us elsewhere (37), the systemic vasodilator effects of ANG-(1–7) are not consistently observed in animals with intact baroreceptor reflexes or in conditions in which there is no activation of the renin- angiotensin system. In contrast, in rats made areflexic by spinal cord destruction (7), the SHR (8, 9), and canine renal hypertension (100), dose-dependent decreases in arterial pressure can be readily demonstrated. Similarly, a robust vasodilator response may be obtained in rat isolated blood vessels (103), piglet pial arteries (92), and rabbit afferent renal arterioles (117). The consistent demonstration of ANG-(1–7) vasodilator actions in isolated vessel preparations underscores the actions of ANG-(1–7) as a paracrine local regulator of vascular tone, as the concentrations of the ligand near its receptor site are always much higher than those found in the circulation. Because the importance of the prevailing level of renin-angiotensin system activity in unmasking the vasodilator actions of ANG-(1–7) has not been consistently recognized, it may account for a failure of others to uncover vasodilator properties of the peptide (141, 152).
Table 1 summarizes the hemodynamic and tubular actions of the ANG-(1–7)/ACE2/mas axis. ANG-(1–7)-mediated increases in renal blood flow are abolished by blockade of the mas receptor or inhibition of prostaglandin release and nitric oxide in SHR and Wistar-Kyoto (WKY) controls (31, 119, 120). As reviewed elsewhere, ANG-(1–7) growth-inhibitory properties (46) can be demonstrated in rat proximal tubular cells where the heptapeptide inhibits ANG II-stimulated phosphorylation of MAPK and transforming growth factor-β1 (130).
Table 1.
Summary of major actions of ANG-(1–7) in the nephron
| Hemodynamic and Water Transport Actions |
|---|
| Intrarenal infusion of ANG-(1–7) in normotensive rats reduces renal plasma flow and increases absolute and fractional sodium excretion without changes in glomerular filtration rate. Blockade of ANG-(1–7) receptors reverses these effects (19). |
| Antidiuretic effects of ANG-(1–7) are associated in male Wistar rats with increases in urinary Na+ concentration, urinary osmolality, and reduction in creatinine clearance. These effects are blocked by administration of A-779 or losartan (5). |
| ANG-(1–7) stimulates substantial diuresis and natriuresis in the isolated rat kidney (30). |
| Intrarenal infusions of ANG-(1–7) in the dog are followed by increases in water, sodium, and urea (but not potassium) excretion rates. This effect is not completely blocked by the AT1 antagonists and not at all by the AT2 receptor antagonist PD123319 (59). |
| The aquaporin-1-mediated antidiuretic effect of ANG-(1–7) in female virgin rats is reversed in the pregnant ones (62, 73). |
| ANG-(1–7)-enhanced water transport in rat inner medullary collecting duct is abolished by both A-779 and a vasopressin V2 receptor antagonist (90). |
| The nonpeptide AVE 0991 is shown to mimic the effects of ANG-(1–7) binding on mas receptors while A-779 completely blocks the antidiuretic effects of AVE 0991. In vitro receptor autoradiography in C57BL/6 mice showed that the specific binding of 125I-ANG-(1–7) to mouse kidney slices was displaced by AVE while the nonpeptide ANG-(1–7) agonist displaced the binding of 125I-ANG-(1–7) in mas-transfected monkey kidney cells and of rhodamine-ANG-(1–7) in mas-transfected Chinese hamster ovary cells (113). |
| Actions on Electrolyte Transport Across Renal Tubules |
|---|
| Regulation of kidney epithelial electrolyte transport by ANG-(1–7) may involve activation of PLA2 (4). |
| ANG-(1–7) selectively modulates the Na+-ATPase activity present in basolateral membranes of kidney proximal tubules through a losartan-sensitive receptor (22). Stimulatory effect of ANG II on the Na+-ATPase activity in proximal tubules is reversed, in a dose-dependent manner, by ANG-(1–7) through the mas receptor (82). Later studies from the same laboratory indicate that ANG-(1–7) stimulates Na+-ATPase activity through the AT1R-Gq protein-PI-PLC β-PKC pathway (83). |
| Bradykinin counteracts the stimulatory effect of ANG-(1–7) on the proximal tubule Na+-ATPase activity (23). |
| Basolateral membrane Na+ -ATPase activity of inner cortex from pig kidney is comparably inhibited by both ANG II and ANG-(1–7) (29). |
| At physiological levels ANG-(1–7) induces stimulation of bicarbonate transport in proximal straight renal tubules through an AT1 receptor mechanism (51). |
| In LLC-PK1 cells, ANG-(1–7) inhibits high glucose stimulated p38 MAPK and increases Src homology 2-containing protein tyrosine phosphatase-1 in a dose-dependent fashion. Effects are blocked by the ANG-(1–7) antagonist A-779 (52). |
| Enhanced phosphatidylcholine biosynthesis by ANG-(1–7) in the rat renal cortex is mediated by a non-AT1/AT2 receptor (53). |
| The generation of ANG IV [ANG-(3–7)] (from the NH2-terminal metabolism of ANG-(1–7) is implicated in eliciting a decrease in energy-dependent solute transport in proximal renal tubules (56). ANG-(1–7)-derived ANG IV binds with high affinity to AT4 receptors in Madin-Darby bovine kidney (MDBK) epithelial cells (57). |
| Infusion of ANG-(1–7) stimulates release of 6-keto-PGF1α in both urine and perfusate of isolated rat kidneys. Concomitant treatment with indomethacin causes a robust decrease in ANG-(1–7)-mediated diuresis and natriuresis (62). |
| ANG II-induced angiotensin-converting enzyme 2 downregulation in human kidney tubular cells is associated with ACE upregulation through activation of ERK1/2 or p38 MAPK (79). Immortalized mouse POD convert ANG I to ANG-(1–7) preferentially, and the conversion is blocked by a neprilysin inhibitor (142). Isolated rat glomeruli also generate ANG-(1–7) from ANG I (143). |
| ANG-(1–7) inhibits ANG II-stimulated phosphorylation of MAPK in proximal tubular cells, while the peptide given alone has no effect (130). |
| In inactin-anesthetized Munich-Wistar-Fromter rats, intratubular application of small doses of ANG-(1–7) has no effect on tubular reabsorption in proximal convoluted or distal tubule. Intratubular ANG-(1–7) at a concentration of 10(−8) M increase reabsorption in Henle's loop by an AT1 receptor-mediated mechanism (139). |
ANG-(1–7) and tubuloglomerular balance.
In 1996 we first reported that “a constant intrarenal infusion of ANG-(1–7) at 0.1 and 1 nmol·min−1·kg−1 had minimal effects on renal blood flow and blood pressure and resulted in an elevated urinary excretion of Na and water compared with the time-control saline-infused group” (58). We further showed that ANG-(1–7) inhibition of the transport-dependent O2 consumption was abolished by pretreatment with the Na+-K+-ATPase inhibitor ouabain in fresh suspensions of rat proximal tubules in a concentration-dependent fashion (58). These studies followed the report that the ANG-(1–7) increase in glomerular filtration rate was associated with a substantial natriuretic and diuretic response in isolated rat kidneys (30). The inhibitory effects of ANG-(1–7) on transcellular sodium transport is associated with activation of phospholipase A2 (4), increased phosphatidylcholine (53), and release of cyclooxygenase products (27) (Table 1). Inhibition of Na+-K+-ATPase activity by ANG-(1–7) may be under dual regulation of both AT1 and AT2 receptors (29), although in the rat low ANG-(1–7) (10−12 M) concentrations increase fluid reabsorption while the opposite is true at higher concentrations (10−8 M) (51). While natriuretic actions of ANG-(1–7) have been confirmed in experiments in which rats have been given ACE inhibitors (155) or ANG-(1–7) infusions (70, 121), the issue remains controversial since other studies in water-loaded animals demonstrated antidiuretic actions of ANG-(1–7) that are blocked in the presence of the mas receptor antagonist [d-Ala7]-ANG-(1–7) (A-779) (123). As noted by Dilauro and Burns (32), differences in experimental designs, anesthesia, and site-specific effects along the nephron structures may account for reported variations of ANG-(1–7) effects on renal hemodynamics and salt and water excretion (Table 1). In our opinion, additional factors need to be considered. Cross talk between the mas and AT1 and AT2 receptors provides alternate explanations since supraphysiological actions of ANG-(1–7) can be blocked by AT1 receptor antagonists in Chinese hamster ovary cells transfected with AT1 receptors (26). Moreover, Kostenis et al. (81) reported the occurrence of heterooligomerization between the mas and AT1 receptors. Evidence that ANG-(1–7) may augment the vasodilator actions of bradykinin through binding to either AT2 or mas receptors (13, 23, 36, 55, 88, 108, 122) is another indication of a complex regulation of the net signaling mechanisms determining the actions of ANG II and ANG-(1–7) at the receptor level. In this connection, the possibility we suggest is that ANG-(1–7) may be acting as an endogenous allosteric modulator of either the AT1 or AT2 receptor. As reviewed by Christopoulos et al. (25, 91), allosteric modulators are defined as ligands that bind to a site on the receptor that is topographically distinct from the orthosteric binding site. Based on this consideration, ANG-(1–7) may act as an endogenous allosteric modulator of AT1 or AT2 receptors by changing their binding and functional responses.
Contribution of ACE2 to nephron function.
ACE2 within the kidneys colocalizes with ACE in proximal and distal tubules and in podocytes within the glomerulus (87, 133, 156). In mice, expression of ACE2 in the tunica media of renal arterioles contrasts with the expression of ACE in the vascular endothelium of these arterioles (129). As first shown by us in the heart (68) and the aorta (65, 66), administration of an ANG II receptor antagonist leads to an increase kidney arteriole ACE2 gene expression (129). In keeping with previous studies (28), renal expression of ACE2 was reduced by kidney disease and subtotal nephrectomy (78). Similar reductions in ACE2 activity were obtained in isolated glomeruli from streptozotocin-induced diabetic rats, and this was accompanied by increased content of glomerular angiotensinogen and ANG II (84). Neoexpression of ACE2 occurs in glomeruli and peritubular capillary endothelium of patients with primary and secondary renal disease, including transplanted kidneys (85). Interestingly, urinary ACE2 expression correlates with proteinuria in patients with diabetic nephropathy (147). Little is known about the expression of mas receptors in renal structures. However, a study by Velkoska et al. (144) reported increased cortical ACE binding and medullary mas receptor expression associated with reduced cortical and medullary ACE2 activity in the remnant kidney following subtotal nephrectomy. Measurements of ACE2 and ACE gene transcripts in renal biopsies obtained from type 2 diabetic patients provides insight into the opposing regulation of the enzymes involved in ANG II and ANG-(1–7) formation (116). In this study, the expression pattern of ACE2 and ACE mRNAs was measured by real-time PCR in laser microdissected renal biopsies from 13 diabetic and 8 control patients (116). A reduced expression of ACE2 mRNA was found in both the glomerular and proximal tubular compartments of diabetic subjects while the opposite was found for ACE mRNA (116). In agreement with these studies, the ANG II-mediated upregulation of ACE and downregulation of ACE2 in hypertensive nephropathy was shown to occur via activation of ERK1/2 and p38 MAPK (79).
The diverse nature of the structures composing the nephron, the functional unit of the kidney, provides a particular challenge in assessing the expression of renin-angiotensin system proteins and their effects. This is exemplified by the diverse well-characterized distribution of renin-angiotensin system components along the nephron by Navar and colleagues (75, 101, 102, 114, 115). Nevertheless, the current evidence suggest that the ACE2/ANG-(1–7)/mas axis is represented in the various components of the nephron, colocalizes with angiotensinogen, renin, ACE, ANG II, and AT1 receptors, and responds to stimuli and injury in a manner that agrees with a renoprotective role to buffer the actions of the ACE/ANG II/AT1 receptor axis. The demonstration of ACE2-dependent formation of ANG-(1–7) in sheep kidney, a species in which the organ closely resembles the human structure, provides further evidence for its role in the regulation of renal function (126). Importantly, our own studies first showed the presence of ANG-(1–7) in urine of both experimental animals (38, 40, 71, 76, 77, 109, 126, 155) and humans (41, 42, 89). In humans, ANG-(1–7) excretion exceeded those for ANG I and ANG II while in untreated essential hypertensive subjects the urinary content of ANG-(1–7) was markedly reduced compared with normal subjects (41, 42).
The ANG-(1–7)/ACE2/mas Axis in Human Disease
In advancing the proposal that ANG-(1–7) counteracts the vasoconstrictor, growth-promoting, and profibrotic actions of ANG II (39), there was an obvious need to investigate the role of this heptapeptide in contributing to diseases of the cardiovascular system. A first glimpse into this possibility was provided when the magnitude of the antihypertensive effects of chronic ACE inhibition in essential hypertensive subjects was found to correlate with increased urinary levels of ANG-(1–7) (89). The further demonstration that untreated essential hypertensive subjects had reduced levels of urinary ANG-(1–7) excretion (41) buttressed the idea that a defect in either the synthesis or actions of renally produced ANG-(1–7) may contribute to the expression of the disease we call hypertension. From experimental studies to observations in humans, evidence continues to accumulate as to a role of the ANG-(1–7)/ACE2/mas axis in the pathogenesis of hypertension, diabetes, and renal disease. In this context, upregulation of ACE2 expression in prehypertensive subjects suggests its participation as a compensatory albeit transient effect in disease progression (74). Our studies in transgenic rats overexpressing the renin gene (71) and the report that administration of either an ANG-(1–7) antagonist or an ACE2 inhibitor worsened the course of two-kidney, one-clip hypertension (20) are certainly in agreement with this possibility. As illustrated in Fig. 1, increased tissue expression of renin in mRen2.Lewis congenic hypertensive rats is associated with large decreases in renal ACE2 activity and ACE2 gene transcripts. In addition, the presence of a significant correlation between renal ACE2 activity and plasma ANG-(1–7) levels further documents the role of ACE2 in regulating the formation of ANG-(1–7) from ANG II. Protective effects of the ANG-(1–7)/ACE2/mas axis in renal disease (6, 21, 80, 85, 127, 128, 140, 144, 146, 147, 159) and experimental and human diabetic nephropathy (10, 11, 33, 95, 104, 116, 132, 133, 151, 153, 156, 158) have been demonstrated in several studies, and a therapeutic potential for recombinant ACE2 (rACE2) is derived by the recent finding that its administration in mice obtunds the pressor actions of ANG II while increasing plasma ANG-(1–7) levels (152). While several studies support a renoprotective role of the ANG-(1–7) axis in kidney disease (3, 6, 11, 17, 18, 20, 31, 45, 69, 80, 85, 95, 96, 112, 128, 133, 144, 156, 159), Esteban et al. (34) found a proinflammatory effect of exogenously administered ANG-(1–7) in mas knockout mice. Their study contrasts with the recent finding by Zhang et al. (159), who showed that ANG-(1–7) administration reversed chemically induced glomerulonephritis in rats.
Fig. 1.
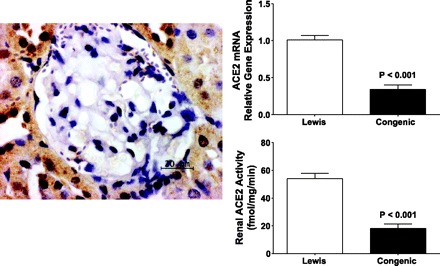
Composite of immunoreactive expression of angiotensin-converting enzyme 2 (ACE2) in renal cortex of Lewis normotensive (Lewis) denoting significant staining in proximal tubules while there is minimal immunoreactive ACE2 activity in the glomerulus. Values are means ± SE of renal ACE2 mRNA and ACE2 activity as reported elsewhere (71). Hypertension in congenic mRen2.Lewis rats is associated with marked decreases in renal ACE2 mRNA and ACE2 activity. Data are from studies reported elsewhere (71).
It should be stressed that additional findings in other disease states such as cirrhosis (2, 60, 61, 63, 64, 105, 107, 149, 154), systemic sclerosis (111), and even cancer (50, 93, 110) further underscore the possible participation of the ANG-(1–7)/ACE2/mas axis as a compensatory mechanism in these disorders. Novel exploratory research on the role of the ANG-(1–7)/ACE2/mas axis in pregnancy (12, 14–16, 138) and the development of eclampsia (94) is another indication of how the intrinsic regulatory mechanisms within the renin-angiotensin system govern homeostasis.
Novel ANG I-Forming Peptides
A brief review of renin-angiotensin system components within the kidney is amplified by the discovery of an alternate precursor for the formation of ANG II and ANG-(1–7) through the characterization of proangiotensin 12 by Nagata and collaborators (99). Proangiotensin 12 [ANG-(1–12)] is an upstream propeptide to ANG I first isolated from the rat small intestine (99). ANG-(1–12) constricted aortic strips and, when infused intravenously, raised blood pressure in rats. The vasoconstrictor response to ANG-(1–12) was abolished by either captopril or the ANG II type I receptor blocker CV-11974 (99). Work from our laboratory has confirmed the existence of ANG-(1–12) in cardiac and renal tissues of WKY and SHR and further demonstrated increased cardiac content of ANG-(1–12) in the heart of SHR (72).
To date it has been generally accepted that angiotensinogen is the only known functional substrate for the generation of ANG peptides by renin. Because the level of angiotensinogen in humans and other species is close to the KM value for renin, changes in angiotensinogen levels have been shown to influence directly the activity of the renin-angiotensin system (101, 102). In the rat, the sequence of the dodecapeptide ANG-(1–12) is Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Leu11-Tyr12. Since renin specifically cleaves the Leu10-Leu11 bond of rat angiotensinogen to form ANG I, the cleavage between the two aromatic residues Tyr12-Tyr13 for the liberation of ANG-(1–12) may not be accounted for by the action of renin. We have confirmed this idea in several models of experimental hypertension in which cardiac metabolism of ANG-(1–12) into ANG II and ANG-(1–7) occurred through a non-renin-dependent pathway (137). In addition, content of ANG-(1–12) was increased in the heart of WKY rats in which renin was absent from the circulation and the heart 48 h postnephrectomy (44). The discovery of ANG-(1–12) and the studies conducted to date (67) suggest an alternate answer of how tissues process the primary precursor that gives rise to the tissue formation of ANG II and ANG-(1–7), independently from the circulation. While additional work is clearly necessary, the data reviewed here clearly suggest that the regulation of renin-angiotensin system influence in blood pressure control and tissue function is tissue selective and embodies multiple mechanisms for expression of the biologically active peptides.
GRANTS
In addition to support provided by National Heart, Lung, and Blood Institute Grant PO1 HL051952, the author gratefully acknowledges grant support provided by Unifi (Greensboro, NC) and the Farley-Hudson Foundation (Jacksonville, NC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Alenina N, Xu P, Rentzsch B, Patkin EL, Bader M. Genetically altered animal models for Mas and angiotensin-(1–7). Exp Physiol 93: 528–537, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Alfany-Fernandez I, Casillas-Ramirez A, Bintanel-Morcillo M, Brosnihan KB, Ferrario CM, Serafin A, Rimola A, Rodes J, Rosello-Catafau J, Peralta C. Therapeutic _targets in liver transplantation: angiotensin II in nonsteatotic grafts and angiotensin-(1–7) in steatotic grafts. Am J Transplant 9: 439–451, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Amao OL. Angiotensin-converting enzyme 2 (ACE2) gene and protein expression in diabetic patients without nephropathy. Kidney Int 75: 1118–1119, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Andreatta-van Leyen S, Romero MF, Khosla MC, Douglas JG. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1–7). Kidney Int 44: 932–936, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Baracho NC, Simoes-e-Silva AC, Khosla MC, Santos RA. Effect of selective angiotensin antagonists on the antidiuresis produced by angiotensin-(1–7) in water-loaded rats. Braz J Med Biol Res 31: 1221–1227, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Batlle D, Soler MJ, Wysocki J. New aspects of the renin-angiotensin system: angiotensin-converting enzyme 2—a potential _target for treatment of hypertension and diabetic nephropathy. Curr Opin Nephrol Hypertens 17: 250–257, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Benter IF, Diz DI, Ferrario CM. Cardiovascular actions of angiotensin(1–7). Peptides 14: 679–684, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Benter IF, Ferrario CM, Morris M, Diz DI. Antihypertensive actions of angiotensin-(1–7) in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 269: H313–H319, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin-(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with l-NAME. Am J Physiol Heart Circ Physiol 290: H684–H691, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Benter IF, Yousif MH, Cojocel C, Al-Maghrebi M, Diz DI. Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol 292: H666–H672, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 28: 25–33, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol 93: 658–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension 27: 523–528, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Brosnihan KB, Neves LA, Anton L, Joyner J, Valdes G, Merrill DC. Enhanced expression of Ang-(1–7) during pregnancy. Braz J Med Biol Res 37: 1255–1262, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Brosnihan KB, Neves LA, Chappell MC. Does the angiotensin-converting enzyme (ACE)/ACE2 balance contribute to the fate of angiotensin peptides in programmed hypertension? Hypertension 46: 1097–1099, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, Penninger J, Ferrario CM. Enhanced renal immunocytochemical expression of ANG-(1–7) and ACE2 during pregnancy. Hypertension 42: 749–753, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Burchill L, Velkoska E, Dean RG, Lew RA, Smith AI, Levidiotis V, Burrell LM. Acute kidney injury in the rat causes cardiac remodelling and increases angiotensin-converting enzyme 2 expression. Exp Physiol 93: 622–630, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Burgelova M, Kramer HJ, Teplan V, Thumova M, Cervenka L. Effects of angiotensin-(1–7) blockade on renal function in rats with enhanced intrarenal Ang II activity. Kidney Int 67: 1453–1461, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Burgelova M, Kramer HJ, Teplan V, Velickova G, Vitko S, Heller J, Maly J, Cervenka L. Intrarenal infusion of angiotensin-(1–7) modulates renal functional responses to exogenous angiotensin II in the rat. Kidney Blood Press Res 25: 202–210, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Burgelova M, Vanourkova Z, Thumova M, Dvorak P, Opocensky M, Kramer HJ, Zelizko M, Maly J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1–7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens 27: 1988–2000, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Burns KD. The emerging role of angiotensin-converting enzyme-2 in the kidney. Curr Opin Nephrol Hypertens 16: 116–121, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Caruso-Neves C, Lara LS, Rangel LB, Grossi AL, Lopes AG. Angiotensin-(1–7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta 1467: 189–197, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Caruso-Neves C, Provenzano K, Luz FF, Santos FM, Fernandes MS, Leao-Ferreira LR, Lopes AG. Bradykinin counteracts the stimulatory effect of angiotensin-(1–7) on the proximal tubule Na+-ATPase activity through B2 receptor. Regul Pept 110: 207–212, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension 40: 735–741, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Christopoulos A. Allosteric binding sites on cell-surface receptors: novel _targets for drug discovery. Nat Rev Drug Discov 1: 198–210, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Clark MA, Tallant EA, Diz DI. Downregulation of the AT1A receptor by pharmacologic concentrations of angiotensin-(1–7). J Cardiovasc Pharmacol 37: 437–448, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Clark MA, Tallant EA, Tommasi E, Bosch S, Diz DI. Angiotensin-(1–7) reduces renal angiotensin II receptors through a cyclooxygenase-dependent mechanism. J Cardiovasc Pharmacol 41: 276–283, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002 [DOI] [PubMed] [Google Scholar]
- 29. De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NC, Cardozo FP, Abi-Abib R, Fernandes MS, Santos DP, Caruso-Neves C. Angiotensin II and angiotensin-(1–7) inhibit the inner cortex Na+-ATPase activity through AT2 receptor. Regul Pept 120: 167–175, 2004 [DOI] [PubMed] [Google Scholar]
- 30. DelliPizzi AM, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin(1–7). Br J Pharmacol 111: 1–3, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dharmani M, Mustafa MR, Achike FI, Sim MK. Effects of angiotensin 1–7 on the actions of angiotensin II in the renal and mesenteric vasculature of hypertensive and streptozotocin-induced diabetic rats. Eur J Pharmacol 561: 144–150, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Dilauro M, Burns KD. Angiotensin-(1–7) and its effects in the kidney. ScientificWorldJournal 9: 522–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ebermann L, Spillmann F, Sidiropoulos M, Escher F, Heringer-Walther S, Schultheiss HP, Tschope C, Walther T. The angiotensin-(1–7) receptor agonist AVE0991 is cardioprotective in diabetic rats. Eur J Pharmacol 590: 276–280, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Esteban V, Heringer-Walther S, Sterner-Kock A, de BR, van den Engel S, Wang Y, Mezzano S, Egido J, Schultheiss HP, Ruiz-Ortega M, Walther T. Angiotensin-(1–7) and the g protein-coupled receptor MAS are key players in renal inflammation. PLoS One 4: e5406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fasciolo JC, Risler N. [A peptide of renal origin depressing vascular reactivity.] C R Seances Soc Biol Fil 168: 1140–1141, 1974 [PubMed] [Google Scholar]
- 36. Fernandes L, Fortes ZB, Nigro D, Tostes RC, Santos RA, Catelli De Carvalho MH. Potentiation of bradykinin by angiotensin-(1–7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension 37: 703–709, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension 55: 445–452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Iskandar SS, Dean RH, Diz DI. Vasopeptidase inhibition and Ang-(1–7) in the spontaneously hypertensive rat. Kidney Int 62: 1349–1357, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7). Hypertension 30: 535–541, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int 68: 2189–2196, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Ferrario CM, Martell N, Yunis C, Flack JM, Chappell MC, Brosnihan KB, Dean RH, Fernandez A, Novikov SV, Pinillas C, Luque M. Characterization of angiotensin-(1–7) in the urine of normal and essential hypertensive subjects. Am J Hypertens 11: 137–146, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Ferrario CM, Smith RD, Brosnihan B, Chappell MC, Campese VM, Vesterqvist O, Liao WC, Ruddy MC, Grim CE. Effects of omapatrilat on the renin-angiotensin system in salt-sensitive hypertension. Am J Hypertens 15: 557–564, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289: H2281–H2290, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol 296: H1184–H1192, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferreira AJ, Pinheiro SV, Castro CH, Silva GA, Silva AC, Almeida AP, Bader M, Rentzsch B, Reudelhuber TL, Santos RA. Renal function in transgenic rats expressing an angiotensin-(1–7)-producing fusion protein. Regul Pept 137: 128–133, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Freeman EJ, Chisolm GM, Ferrario CM, Tallant EA. Angiotensin-(1–7) inhibits vascular smooth muscle cell growth. Hypertension 28: 104–108, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol 290: C420–C426, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol 295: C1169–C1174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 295: H2373–H2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gallagher PE, Tallant EA. Inhibition of human lung cancer cell growth by angiotensin-(1–7). Carcinogenesis 25: 2045–2052, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Garcia NH, Garvin JL. Angiotensin 1–7 has a biphasic effect on fluid absorption in the proximal straight tubule. J Am Soc Nephrol 5: 1133–1138, 1994 [DOI] [PubMed] [Google Scholar]
- 52. Gava E, Samad-Zadeh A, Zimpelmann J, Bahramifarid N, Kitten GT, Santos RA, Touyz RM, Burns KD. Angiotensin-(1–7) activates a tyrosine phosphatase and inhibits glucose-induced signalling in proximal tubular cells. Nephrol Dial Transplant 24: 1766–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gironacci MM, Fernandez-Tome MC, Speziale E, Sterin-Speziale N, Pena C. Enhancement of phosphatidylcholine biosynthesis by angiotensin-(1–7) in the rat renal cortex. Biochem Pharmacol 63: 507–514, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension. I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59: 347–379, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gorelik G, Carbini LA, Scicli AG. Angiotensin 1–7 induces bradykinin-mediated relaxation in porcine coronary artery. J Pharmacol Exp Ther 286: 403–410, 1998 [PubMed] [Google Scholar]
- 56. Handa RK. Angiotensin-(1–7) can interact with the rat proximal tubule AT4 receptor system. Am J Physiol Renal Physiol 277: F75–F83, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Handa RK. Binding and signaling of angiotensin-(1–7) in bovine kidney epithelial cells involves the AT4 receptor. Peptides 21: 729–736, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1–7): in vivo and in vitro studies. Am J Physiol Renal Fluid Electrolyte Physiol 270: F141–F147, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Heller J, Kramer HJ, Maly J, Cervenka L, Horacek V. Effect of intrarenal infusion of angiotensin-(1–7) in the dog. Kidney Blood Press Res 23: 89–94, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Herath CB, Lubel JS, Jia Z, Velkoska E, Casley D, Brown L, Tikellis C, Burrell LM, Angus PW. Portal pressure responses and angiotensin peptide production in rat liver are determined by relative activity of ACE and ACE2. Am J Physiol Gastrointest Liver Physiol 297: G98–G106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, Smith AI, Burrell LM, Angus PW. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1–7) levels in experimental biliary fibrosis. J Hepatol 47: 387–395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hilchey SD, Bell-Quilley CP. Association between the natriuretic action of angiotensin-(1–7) and selective stimulation of renal prostaglandin I2 release. Hypertension 25: 1238–1244, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Huang ML, Li X, Meng Y, Xiao B, Ma Q, Ying SS, Wu PS, Zhang ZS. Upregulation of angiotensin converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol Physiol 37: e1–e6, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Huang Q, Xie Q, Shi CC, Xiang XG, Lin LY, Gong BD, Zhao GD, Wang H, Jia NN. Expression of angiotensin-converting enzyme 2 in CCL4-induced rat liver fibrosis. Int J Mol Med 23: 717–723, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Igase M, Kohara K, Nagai T, Miki T, Ferrario CM. Increased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin II type 1 receptor blockade. Hypertens Res 31: 553–559, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1–7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 289: H1013–H1019, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Ganten D, Chappell MC, Ferrario CM, Diz DI. Chronic immunoneutralization of brain angiotensin-(1–12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am J Physiol Regul Integr Comp Physiol 297: R111–R115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43: 970–976, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1–7)-Mas receptor axis. Hypertens Res 32: 533–536, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Iyer SN, Averill DB, Chappell MC, Yamada K, Allred AJ, Ferrario CM. Contribution of angiotensin-(1–7) to blood pressure regulation in salt-depleted hypertensive rats. Hypertension 36: 417–422, 2000 [DOI] [PubMed] [Google Scholar]
- 71. Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol 291: H2166–H2172, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 294: H2614–H2618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Joyner J, Neves LA, Stovall K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) serves as an aquaretic by increasing water intake and diuresis in association with downregulation of aquaporin-1 during pregnancy in rats. Am J Physiol Regul Integr Comp Physiol 294: R1073–R1080, 2008 [DOI] [PubMed] [Google Scholar]
- 74. Keidar S, Strizevsky A, Raz A, Gamliel-Lazarovich A. ACE2 activity is increased in monocyte-derived macrophages from prehypertensive subjects. Nephrol Dial Transplant 22: 597–601, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension 53: 344–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kohara K, Brosnihan KB, Chappell MC, Khosla MC, Ferrario CM. Angiotensin-(1–7): a member of circulating angiotensin peptides. Hypertension 17: 131–138, 1991 [DOI] [PubMed] [Google Scholar]
- 77. Kohara K, Brosnihan KB, Ferrario CM. Angiotensin(1–7) in the spontaneously hypertensive rat. Peptides 14: 883–891, 1993 [DOI] [PubMed] [Google Scholar]
- 78. Koitka A, Cooper ME, Thomas MC, Tikellis C. Angiotensin converting enzyme 2 in the kidney. Clin Exp Pharmacol Physiol 35: 420–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol 172: 1174–1183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Konoshita T, Wakahara S, Mizuno S, Motomura M, Aoyama C, Makino Y, Kawai Y, Kato N, Koni I, Miyamori I, Mabuchi H. Tissue gene expression of renin-angiotensin system in human type 2 diabetic nephropathy. Diabetes Care 29: 848–852, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111: 1806–1813, 2005 [DOI] [PubMed] [Google Scholar]
- 82. Lara LS, Bica RB, Sena SL, Correa JS, Marques-Fernandes MF, Lopes AG, Caruso-Neves C. Angiotensin-(1–7) reverts the stimulatory effect of angiotensin II on the proximal tubule Na+-ATPase activity via a A779-sensitive receptor. Regul Pept 103: 17–22, 2002 [DOI] [PubMed] [Google Scholar]
- 83. Lara LS, Correa JS, Lavelle AB, Lopes AG, Caruso-Neves C. The angiotensin receptor type 1-Gq protein-phosphatidyl inositol phospholipase Cbeta-protein kinase C pathway is involved in activation of proximal tubule Na+-ATPase activity by angiotensin(1–7) in pig kidneys. Exp Physiol 93: 639–647, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Leehey DJ, Singh AK, Bast JP, Sethupathi P, Singh R. Glomerular renin angiotensin system in streptozotocin diabetic and Zucker diabetic fatty rats. Transl Res 151: 208–216, 2008 [DOI] [PubMed] [Google Scholar]
- 85. Lely AT, Hamming I, van GH, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol 204: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 86. Li C, Booze RM, Hersh LB. Tissue-specific expression of rat neutral endopeptidase mRNAs. Ann NY Acad Sci 780: 145–155, 1996 [DOI] [PubMed] [Google Scholar]
- 87. Li N, Zimpelmann J, Cheng K, Wilkins JA, Burns KD. The role of angiotensin converting enzyme 2 in the generation of angiotensin 1–7 by rat proximal tubules. Am J Physiol Renal Physiol 288: F353–F362, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Li P, Chappell MC, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) augments bradykinin-induced vasodilation by competing with ACE and releasing nitric oxide. Hypertension 29: 394–400, 1997 [DOI] [PubMed] [Google Scholar]
- 89. Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1–7) in essential hypertension. J Hypertens 14: 799–805, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Magaldi AJ, Cesar KR, de Araujo M, Sim̃oes e Silva AC, Santos RA. Angiotensin-(1–7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflügers Arch 447: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 91. May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 47: 1–51, 2007 [DOI] [PubMed] [Google Scholar]
- 92. Meng W, Busija DW. Comparative effects of angiotensin-(1–7) and angiotensin II on piglet pial arterioles. Stroke 24: 2041–2044, 1993 [DOI] [PubMed] [Google Scholar]
- 93. Menon J, Soto-Pantoja DR, Callahan MF, Cline JM, Ferrario CM, Tallant EA, Gallagher PE. Angiotensin-(1–7) inhibits growth of human lung adenocarcinoma xenografts in nude mice through a reduction in cyclooxygenase-2. Cancer Res 67: 2809–2815, 2007 [DOI] [PubMed] [Google Scholar]
- 94. Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine 18: 239–245, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis 51: 613–623, 2008 [DOI] [PubMed] [Google Scholar]
- 96. Modrall JG, Sadjadi J, Brosnihan KB, Gallagher PE, Yu CH, Kramer GL, Bernstein KE, Chappell MC. Depletion of tissue angiotensin-converting enzyme differentially influences the intrarenal and urinary expression of angiotensin peptides. Hypertension 43: 849–853, 2004 [DOI] [PubMed] [Google Scholar]
- 97. Muirhead EE. Renal vasodepressor mechanisms: the medullipin system. J Hypertens Suppl 11: S53–S58, 1993 [PubMed] [Google Scholar]
- 98. Muirhead EE, Brown GB, Germain GS, Leach BE. The renal medulla as an antihypertensive organ. J Lab Clin Med 76: 641–651, 1970 [PubMed] [Google Scholar]
- 99. Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 350: 1026–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 100. Nakamoto H, Ferrario CM, Fuller SB, Robaczewski DL, Winicov E, Dean RH. Angiotensin-(1–7) and nitric oxide interaction in renovascular hypertension. Hypertension 25: 796–802, 1995 [DOI] [PubMed] [Google Scholar]
- 101. Navar LG. The intrarenal renin-angiotensin system in hypertension. Kidney Int 65: 1522–1532, 2004 [DOI] [PubMed] [Google Scholar]
- 102. Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep 5: 135–143, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Neves LA, Averill DB, Ferrario CM, Aschner JL, Brosnihan KB. Vascular responses to angiotensin-(1–7) during the estrous cycle. Endocrine 24: 161–165, 2004 [DOI] [PubMed] [Google Scholar]
- 104. Oliveira MA, Carvalho MH, Nigro D, Passaglia RC, Fortes ZB. Angiotensin-(1–7) and bradykinin interaction in diabetes mellitus: in vivo study. Peptides 23: 1449–1455, 2002 [DOI] [PubMed] [Google Scholar]
- 105. Osterreicher CH, Taura K, De MS, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM, Brenner DA. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology 50: 929–938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol 168: 1808–1820, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut 54: 1790–1796, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Paula RD, Lima CV, Britto RR, Campagnole-Santos MJ, Khosla MC, Santos RA. Potentiation of the hypotensive effect of bradykinin by angiotensin-(1–7)-related peptides. Peptides 20: 493–500, 1999 [DOI] [PubMed] [Google Scholar]
- 109. Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2).Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol 295: H10–H20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Petty WJ, Miller AA, McCoy TP, Gallagher PE, Tallant EA, Torti FM. Phase I and pharmacokinetic study of angiotensin-(1–7), an endogenous antiangiogenic hormone. Clin Cancer Res 15: 7398–7404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pignone A, Rosso AD, Brosnihan KB, Perfetto F, Livi R, Fiori G, Guiducci S, Cinelli M, Rogai V, Tempestini A, Bartoli F, Generini S, Ferrario CM, Cerinic MM. Reduced circulating levels of angiotensin-(1–7) in systemic sclerosis: a new pathway in the dysregulation of endothelial-dependent vascular tone control. Ann Rheum Dis 66: 1305–1310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhaes JA, da Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RA, Simoes e Silva AC. Genetic deletion of the angiotensin-(1–7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int 75: 1184–1193, 2009 [DOI] [PubMed] [Google Scholar]
- 113. Pinheiro SV, Simoes e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, Pesquero JB, Walther T, Alenina N, Bader M, Bleich M, Santos RA. Nonpeptide AVE 0991 is an angiotensin-(1–7) receptor Mas agonist in the mouse kidney. Hypertension 44: 490–496, 2004 [DOI] [PubMed] [Google Scholar]
- 114. Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens 3: 96–104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 74: 1610–1616, 2008 [DOI] [PubMed] [Google Scholar]
- 117. Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802, 2002 [DOI] [PubMed] [Google Scholar]
- 118. Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383: 45–51, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol 284: H1985–H1994, 2003 [DOI] [PubMed] [Google Scholar]
- 120. Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49: 185–192, 2007 [DOI] [PubMed] [Google Scholar]
- 121. Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1–7): an update. Regul Pept 91: 45–62, 2000 [DOI] [PubMed] [Google Scholar]
- 122. Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen CJ, Carvalho WS, Simoes e Silva AC, Khosla MC. Characterization of a new angiotensin antagonist selective for angiotensin-(1–7): evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensin receptors. Brain Res Bull 35: 293–298, 1994. [DOI] [PubMed] [Google Scholar]
- 123. Santos RA, Simoes e Silva AC, Magaldi AJ, Khosla MC, Cesar KR, Passaglio KT, Baracho NC. Evidence for a physiological role of angiotensin-(1–7) in the control of hydroelectrolyte balance. Hypertension 27: 875–884, 1996 [DOI] [PubMed] [Google Scholar]
- 124. Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de BI, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Schulz R, Sakane Y, Berry C, Ghai R. Characterisation of neutral endopeptidase 3.4.24.11 (NEP) in the kidney: comparison between normotensive, genetically hypertensive and experimentally hypertensive rats. J Enzyme Inhib 4: 347–358, 1991 [DOI] [PubMed] [Google Scholar]
- 126. Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol 292: F82–F91, 2007 [DOI] [PubMed] [Google Scholar]
- 127. Simoes e Silva AC, Diniz JS, Pereira RM, Pinheiro SV, Santos RA. Circulating renin angiotensin system in childhood chronic renal failure: marked increase of angiotensin-(1–7) in end-stage renal disease. Pediatr Res 60: 734–739, 2006 [DOI] [PubMed] [Google Scholar]
- 128. Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int 72: 614–623, 2007 [DOI] [PubMed] [Google Scholar]
- 129. Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol 296: F398–F405, 2009 [DOI] [PubMed] [Google Scholar]
- 130. Su Z, Zimpelmann J, Burns KD. Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 69: 2212–2218, 2006 [DOI] [PubMed] [Google Scholar]
- 131. Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289: H1560–H1566, 2005 [DOI] [PubMed] [Google Scholar]
- 132. Tikellis C, Bialkowski K, Pete J, Sheehy K, Su Q, Johnston C, Cooper ME, Thomas MC. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes 57: 1018–1025, 2008 [DOI] [PubMed] [Google Scholar]
- 133. Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension 41: 392–397, 2003 [DOI] [PubMed] [Google Scholar]
- 134. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000 [DOI] [PubMed] [Google Scholar]
- 135. Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1–7) in transgenic Ren-2 hypertensive rats. Am J Physiol Heart Circ Physiol 292: H3019–H3024, 2007 [DOI] [PubMed] [Google Scholar]
- 137. Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol 294: H2242–H2247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Valdes G, Neves LA, Anton L, Corthorn J, Chacon C, Germain AM, Merrill DC, Ferrario CM, Sarao R, Penninger J, Brosnihan KB. Distribution of angiotensin-(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 27: 200–207, 2006 [DOI] [PubMed] [Google Scholar]
- 139. Vallon V, Richter K, Heyne N, Osswald H. Effect of intratubular application of angiotensin 1–7 on nephron function. Kidney Blood Press Res 20: 233–239, 1997 [DOI] [PubMed] [Google Scholar]
- 140. van der Wouden EA, Henning RH, Deelman LE, Roks AJ, Boomsma F, de Zeeuw D. Does angiotensin (1–7) contribute to the anti-proteinuric effect of ACE-inhibitors. J Renin Angiotensin Aldosterone Syst 6: 96–101, 2005 [DOI] [PubMed] [Google Scholar]
- 141. van der Wouden EA, Ochodnicky P, van Dokkum RP, Roks AJ, Deelman LE, de Zeeuw D, Henning RH. The role of angiotensin(1–7) in renal vasculature of the rat. J Hypertens 24: 1971–1978, 2006 [DOI] [PubMed] [Google Scholar]
- 142. Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol 293: F398–F407, 2007 [DOI] [PubMed] [Google Scholar]
- 143. Velez JC, Ryan KJ, Harbeson CE, Bland AM, Budisavljevic MN, Arthur JM, Fitzgibbon WR, Raymond JR, Janech MG. Angiotensin I is largely converted to angiotensin (1–7) and angiotensin (2–10) by isolated rat glomeruli. Hypertension 53: 790–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Velkoska E, Dean RG, Burchill LJ, Levidiotis V, Burrell LM. Reduction in renal ACE2 expression in subtotal nephrectomy is ameliorated with ACE inhibition. Clin Sci (Lond) 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002 [DOI] [PubMed] [Google Scholar]
- 146. Wang G, Lai FM, Lai KB, Chow KM, Kwan CH, Li KT, Szeto CC. Urinary mRNA expression of ACE and ACE2 in human type 2 diabetic nephropathy. Diabetologia 51: 1062–1067, 2008 [DOI] [PubMed] [Google Scholar]
- 147. Wang G, Lai FM, Lai KB, Chow KM, Kwan CH, Li KT, Szeto CC. Discrepancy between intrarenal messenger RNA and protein expression of ACE and ACE2 in human diabetic nephropathy. Am J Nephrol 29: 524–531, 2009 [DOI] [PubMed] [Google Scholar]
- 148. Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem 280: 39353–39362, 2005 [DOI] [PubMed] [Google Scholar]
- 149. Warner FJ, Lubel JS, McCaughan GW, Angus PW. Liver fibrosis: a balance of ACEs? Clin Sci (Lond) 113: 109–118, 2007 [DOI] [PubMed] [Google Scholar]
- 150. Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24. 11 Life Sci 52: 1461–1480, 1993 [DOI] [PubMed] [Google Scholar]
- 151. Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 171: 438–451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wysocki J, Ye M, Rodriguez E, Gonzalez-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D. _targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension 55: 90–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006 [DOI] [PubMed] [Google Scholar]
- 154. Xia CY, Li L, Liu HM, Cong WM. High expression of angiotensin-converting enzyme and angiotensin-converting enzyme 2 in preservation injury after liver transplantation in rats. Hepatol Res 39: 1118–1124, 2009 [DOI] [PubMed] [Google Scholar]
- 155. Yamada K, Iyer SN, Chappell MC, Brosnihan KB, Fukuhara M, Ferrario CM. Differential response of angiotensin peptides in the urine of hypertensive animals. Regul Pept 80: 57–66, 1999 [DOI] [PubMed] [Google Scholar]
- 156. Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006 [DOI] [PubMed] [Google Scholar]
- 157. Young D, O'Neill K, Jessell T, Wigler M. Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proc Natl Acad Sci U S A 85: 5339–5342, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Yousif MH, Kehinde EO, Benter IF. Different responses to angiotensin-(1–7) in young, aged and diabetic rabbit corpus cavernosum. Pharmacol Res 56: 209–216, 2007 [DOI] [PubMed] [Google Scholar]
- 159. Zhang J, Noble NA, Border WA, Huang Y. Infusion of angiotensin-(1–7) reduces glomerulosclerosis through counteracting angiotensin II in experimental glomerulonephritis. Am J Physiol Renal Physiol 298: F579–F588, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


