Abstract
Due to increased obesity, non-alcoholic fatty liver disease (NAFLD) is now the most prevalent liver disease in the United States. NAFLD is considered a component of metabolic syndrome, a cluster of disorders that also includes diabetes mellitus, dyslipidemia, arteriosclerosis, and hypertension. Exposure to ambient air particulate matter with aerodynamic diameters < 2.5 µm (PM2.5) is a risk factor for arteriosclerosis as well as lung disease, but its effect on NAFLD is unknown. PM2.5 induces pulmonary dysfunction via toll-like receptor activation on alveolar macrophages. Toll-like receptor activation of Kupffer cells, resident hepatic macrophages, and subsequent pro-inflammatory cytokine production have been shown to play a key role in NAFLD progression. We hypothesized that PM2.5 exposure is a significant risk factor for progression of NAFLD. Thus, following exposure of male C57BL/6 mice fed high fat chow to concentrated air particulate matter (CAPs) or filtered air for 6 wk, progression of NAFLD was evaluated by standardized histological assessment of hepatic inflammation and fibrosis. In mice fed high fat chow, the hepatic inflammatory grade (3.00 ± 0.00 vs. 1.50 ± 0.71, p < 0.001) and fibrosis stage (1.00 ± 0.00 vs. 0.60 ± 0.52, p = 0.023) were both significantly higher in mice exposed to CAPs versus filtered air, respectively. Increased numbers of Kupffer cells contained PM in CAPs-exposed mice (2.00 ± 0.94 vs. 0.20 ± 0.42, respectively, p < 0.001). PM exposure increased IL-6 secretion up to seven fold in a dose-dependent manner by isolated wild-type but not TLR4−/− Kupffer cells (p < 0.050). Conclusion: Ambient PM2.5 exposure may be a significant risk factor for NAFLD progression.
Keywords: Toll-like receptor, Kupffer cell, steatohepatitis, fibrosis, IL-6
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States (McCullough, 2004), but its pathogenesis is poorly understood. Indeed, the condition was not recognized as a cause of cirrhosis (end-stage liver disease) until fairly recently. NAFLD ranges from simple fatty liver (hepatic steatosis) to cirrhosis with intervening steatohepatitis characterized by progressive amounts of inflammation and fibrosis. It is unclear why steatohepatitis develops in only 10% of those with hepatic steatosis, but increased release of pro-inflammatory cytokines is likely essential (McCullough, 2006). Increased hepatic cytokine release appears to be related to Toll-like receptor (TLR) activation on Kupffer cells (Isogawa et al., 2005; Yohe et al., 2006). Identification of factors that induce hepatic pro-inflammatory cytokine production is an important area of research, since prevention of progression from simple steatosis to steatohepatitis may be sufficient to prevent development of cirrhosis in NAFLD.
Epidemiological studies have demonstrated that exposure to airborne fine particulate matter (PM2.5) is positively associated with increases in the morbidity and mortality caused by cardiovascular disease (Mar et al., 2000; Schwartz et al., 2001; Sun et al., 2005) and pulmonary disease (Pope and Kanner, 1993; Sunyer et al., 2000). The main constituents of PM2.5 are organic carbon, elemental carbon, sulfates and nitrates (Fuentes et al., 2006). Little data exists on the relationship between air particulate matter exposure and liver disease. Air pollution exposure related to toxic waste sites has been associated with an increased prevalence of autoimmune liver disease (Ala et al., 2006; Stanca et al., 2008). Two recent animal studies suggest airborne pollutants may also play a role in the pathogenesis of NAFLD (Folkmann et al., 2007; Tomaru et al., 2007).
The mechanisms by which PM2.5 might affect liver disease are unclear. PM2.5 induces pulmonary damage at least partly through induction of cytokine release (Kumagai et al., 1997). that is dependent on Toll-like receptor (TLR) activation (Becker et al., 2005). A similar effect of PM2.5 in the liver is possible since activation of TLR4 on Kupffer cells results in the release of pro-inflammatory cytokines (Su et al., 2000; Uesugi et al., 2001). TLR4 signaling also enhances TGF-β responsiveness in hepatic stellate cells leading to their increased synthesis of collagen and fibrosis (Seki et al., 2007). Studies were done in vivo and in vitro to determine whether air particulate matter exposure enhances hepatic inflammation in NAFLD and induces Kupffer cell activation.
Materials and methods
Mice
Six-wk-old C57BL/6 male mice (Taconic Europe, Denmark) were enrolled and housed two to a cage in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal housing facility. They were fed either high fat chow (HFC) (n = 10; Adjusted Calories Diet, TD 88137, Harlan, Indianapolis, IND) or normal chow (NC) (n = 10; Ralston Purina Co., Chicago, IL) during exposure to PM2.5 or filtered air. Assignments to high-fat chow vs. normal chow and PM2.5 vs. filtered air were random. The Committees on Use and Care of Animals from New York University and Mount Sinai School of Medicine approved all experimental procedures.
For studies in vitro, cells were isolated from male wild-type (WT) and TLR4−/− C57BL/6 mice fed NC obtained from Dr. Maria T. Abreau (Adachi et al., 1998; Hoshino et al., 1999).
PM2.5 Inhalation
Mice were exposed from October 2006 through December 2006 to concentrated PM2.5 (CAPs) composed of the northeastern regional background at the AJ Lanza Laboratory of the Department of Environmental Medicine of New York University (NYU), which is located within Sterling Forest State Park in Tuxedo, NY, 40 miles northwest of Manhattan, where the PM2.5 is largely attributable to long-range transport. Sterling Forest is a largely undeveloped woodland park. The NYU laboratory is located near the center of the park, on a relatively lightly traveled two-lane road that bisects the park. There are no large power generators or industrial operations within 20 miles of the site. The ambient fine PM in Sterling Forest is, therefore, representative of regional background PM2.5 aerosol of the megalopolis that extends from Virginia to Maine. The PM in Sterling Forest has been characterized previously (Maciejczyk et al., 2005).
The CAPs were collected and concentrated by a versatile aerosol concentration enrichment system (Sioutas et al., 2005), which was modified for longer-term exposures (Chen and Nadziejko, 2005). The mice were exposed to PM2.5 (n = 10) at an average concentration of 85 µg/m3 for 6 hr/d, 5 d/wk, for 6 wk. The filtered air (FA, n = 10) control mice in the experiment were exposed to an identical protocol with the exception that a high efficiency particulate air filter (HEPA, Pall Life Sciences, East Hills, NY) was positioned in the inlet valve to the exposure system to remove all of the PM2.5 from the air stream. For the measurement of concentrated PM2.5 in the exposure chambers, PM2.5 samples were collected on Teflon filters (Gelman “Teflon” 37 mm, 0.2 µm pore) and the filters were weighed before and after sampling in a temperature and humidity controlled weighing room. The long-term average exposure that the mice received over this period (15 µg/m3) corresponds to the current EPA ambient air quality standard and the level of exposure for many U.S. residents. At the completion of the exposure period, mice were euthanized following anesthesia with ketamine/xylacine (100 mg/kg, intraperitoneally [ip]). Livers were then removed and fixed in 10% formalin.
Injection with PM
SRM 1469a, atmospheric particulate matter collected in the Washington, DC area in 1976–1977, was obtained from the National Institute of Standards and Technology (NIST; Gaithersburg, MD). SRM 1469a (500 µg) in PBS (n = 3) or PBS alone (n = 3) was injected into the retinal vein of WT C57BL/6 male mice. After 24 hr, the mice were euthanized following anesthetization with ketamine/xylacine (100 mg/kg, ip). Livers were removed and fixed in 10% formalin. Blinded, semi-quantitative scoring of the number of cells containing PM in ten high power fields was conducted as follows: no cells = 0; one to two cells = 1; three to five cells = 2; over five cells = 3.
Histological Staining
Formalin-fixed liver specimens were paraffin-embedded. Five mm sections were prepared and stained either with hematoxylin and eosin (H&E) or with 0.1% Sirius red F3B (Sigma, St Louis, MO) in saturated picric acid. The H&E sections were scored for inflammation and fibrosis using the modified Brunt classification system for fatty liver disease (Brunt et al., 1999) and for the presence of particulate matter by a liver histopathologist blinded as to sample source. Relative fibrosis area (expressed as % of total liver area) was assessed by analyzing 40 Sirius red-stained liver sections per animal. Each field was acquired at 10× magnification and then analyzed with a computerized Bioquant morphometry system (Bioquant, Nashville, TN). Subtraction of the vascular luminal area from the total field area yielded the net field area (Horani et al., 2007). To evaluate the relative fibrosis area, the measured collagen area (red) was divided by the net field area and then multiplied by 100.
Immunohistochemistry and Electron Microscopy
Formalin-fixed liver sections underwent sequential dehydration in ethanol, peroxide blocking with 0.03% hydrogen peroxide, and antigen retrieval with citrate buffer (pH 7.6) in a microwave for 20 min. Sections were stained with horseradish peroxidase-conjugated primary antibody or isotype control antibody for 1 hr. DAB substrate (3,3’-diaminobenzidine; Vector, Burlingame, CA) was used for detection. Anti-mouse F4/80 (Abcam, Cambridge, MA) and isotype control antibody (Jackson Immunoresearch, West Grove, PA) were purchased for macrophage detection. Blinded, semi-quantitative scoring of the number of cells in 20 high-power fields with positive staining was conducted as follows: < 10 positive cells = 0; 10–49 cells = 1; 50–99 = 2; 100 or more cells = 3. Glutaraldehyde-fixed sections from PM-exposed (n = 3) and FA-exposed (n = 3) mice for electron microscopy were prepared, imaged, and scanned as described previously (Phelps et al., 1989; Sicari et al., 1999).
Cells
The RAW 264.7 murine macrophage cell line was a kind gift of Dr. J. Unkeless (Mount Sinai School of Medicine, New York, NY). Cell viability was measured using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] cell viability kit and following the manufacturer’s instructions (Biotum Inc., Hayward, CA). Kupffer cells were isolated from 2-moold WT (n = 5) and TLR4−/− (n = 5) C57BL/6 mice. Following anesthetization with keta-mine/xylacine, a medial laparotomy was performed and the portal vein was catheterized. Livers were perfused through the portal vein in situ with 10 ml of Hanks’ Balanced Salt Solution (SAFC Biosciences, Lenexa, KA) followed by 10 ml of 0.02% collagenase IV (Sigma). The liver was removed and digested for another 30 min in 0.02% collagenase IV. The digested liver was minced and strained through a steel mesh (70 µm). The dispersed liver cells were collected by centrifugation at 50 × g for 10 min. at 4 °C. Cells were washed twice in PBS. Kupffer cells were then isolated by Percoll (GE Healthcare, Pittsburg, PA) gradient centrifugation at RT. The cells were washed again in PBS twice. Cells were counted and viability was checked by Trypan blue exclusion (Smedsrod et al., 1985). Only isolations with >95% cell viability were used. Cells were seeded in 24-well plates at 2 × 105 in RPMI with 10% heat-inactivated FBS for 72 hr before exposure to particulate matter. Stellate cells from WT and TLR4−/− C57BL/6 mice were immortalized as previously described (Guo et al., 2008).
Incubation of Cells In Vitro with PM
DMEM media supplemented with 10% heat-inactivated FBS, 1% l-glutamine, and 1% penicillin-streptomycin was used for all in vitro studies. All cells were cultured at 37°C and in a 5% CO2 atmosphere. Macrophages (RAW 264.7) were seeded at 8 × 105 cells/well; mouse stellate cells (WT and TLR−/−) were seeded at 4 × 105 cells/well; and primary Kupffer cells isolated from mice were seeded at 1 × 106 cells/well. All wells were at least 70% confluent after 24 hr. Media was completely replaced at 24 hr with media containing PM (SRM 1649a at 0–200 µg/ml). The doses of PM are based on prior studies of alveolar cells (Hetland et al., 2005; Imrich et al., 2007). TLR-blocking antibody (Ebioscience, San Diego, CA) or isotype control antibody (Jackson Immunoresearch, West Grove , PA) were added to specified wells at 20 µg/mL. The amount of bacterial lipopolysaccharide (LPS) contamination in SRM 1469a preparations was measured using the Chromogenic Limulus Amebocyte Lysate Test Kit (Cambrex, Walkersville, MD), according to manufacturer’s instructions. LPS concentrations in SRM 1469a were less than 125 pg/ml, well below the level known to stimulate macrophage cytokine secretion. In some experiments, conditioned media from macrophages exposed to particulate matter (0, 100, or 200 µg/ml) for 24 hr was employed. Stellate cells were then incubated in a 1:4 mixture of conditioned macrophage media and fresh media (final concentration of PM was 0, 20 or 40 µg/ml). Control stellate cells were likewise exposed to 0, 20, or 40 µg/ml of PM in fresh media. Cell culture supernatants and cells were retrieved at various timepoints, and mRNA and protein expression levels were assayed by qRT-PCR and enzyme-linked immunosorbent assay according to the manufacturer’s directions (R&D Systems, Minneapolis, MN), respectively. Measurements were performed in triplicate.
Statistical analysis
An unpaired Mann-Whitney U-test was used for non-parametric comparisons of histological staining. Fisher exact two-tailed test was used to compare the presence of hepatic PM in CAPs- vs. FA-exposed mice. A one-way ANOVA test was used for parametric comparisons. A p-value δ 0.050 was considered statistically significant for each test.
Results
Inhalation of PM2.5 increased hepatic inflammation and fibrosis
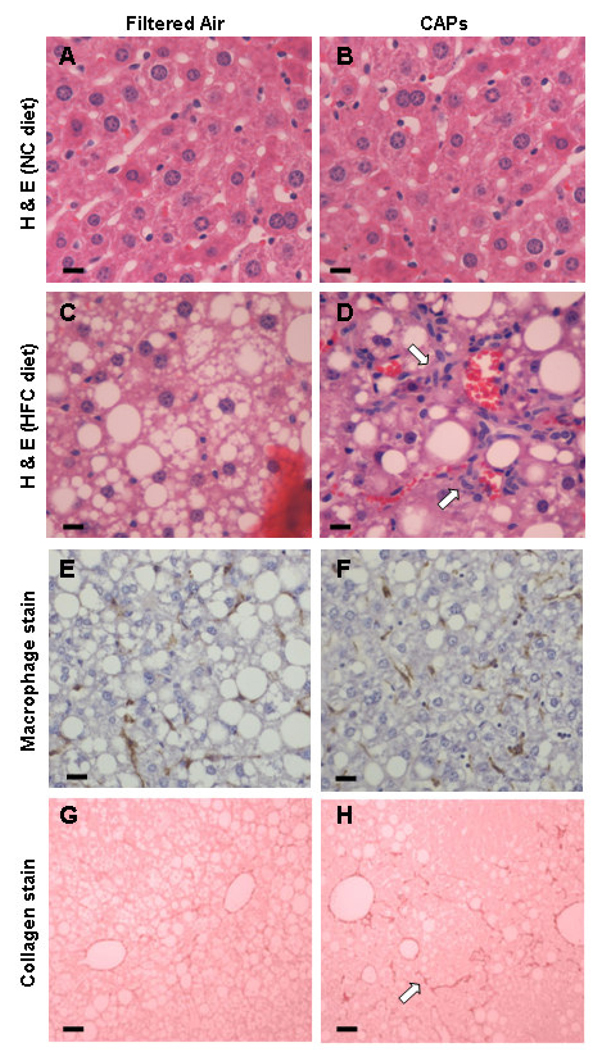
The livers of wild-type (WT) C57BL/6 mice exposed to either filtered air (FA) or concentrated air particles (CAPs) were examined histologically at the end of 6 wk. The average CAPs exposure that experimental mice received over this period (15 µg/m3) corresponds to the current EPA ambient air quality standard and the level of exposure for many U.S. residents. During exposure periods the average CAPs concentration was 85 µg/m3 for the CAPs-exposed mice and 0 µg/m3 for the FA-exposed mice. In mice fed normal chow (NC), no histological abnormalities were noted regardless of exposure to FA or CAPs (Figures 1A and 1B). In contrast, increased hepatic steatosis was evident in both groups of mice fed high fat chow (HFC) (Figures 1C and 1D). Lobular inflammation characterized by leukocyte infiltration appeared significantly increased along with hepatocyte ballooning and Mallory bodies in mice exposed to CAPs and fed HFC. Macrophage staining (Figures 1E and 1F) was significantly increased in the CAPs-exposed mice compared to in the FA-exposed mice fed HFC (2.4 ± 1.1 vs. 1.1 ± 1.2 cells/hpf, p = 0.045). Bioquant analysis of Sirius red staining suggested a trend toward increased collagen staining (Figures 1G and 1H) in CAPs-exposed versus FA-exposed mice (5.9 ± 2.7% vs. 4.5 ± 2.1%, respectively, p = 0.334). The collagen staining had the typical “chicken wire” pattern seen in human NAFLD.
Figure 1. Histological analysis of formalin-fixed sections from C57BL/6 mice exposed to FA or CAPs.
H&E staining demonstrated that mice fed NC did not develop fatty livers or hepatic inflammation regardless of exposure to FA (A) or PM (B). Mice receiving HFC and exposed to FA had fatty livers with minimal hepatic inflammation (C); whereas CAPs-exposed mice on HFC developed fatty livers with increased hepatic inflammation (as noted by arrows) (D). F4/80 macrophage staining was less significant in HFC mice exposed to FA (E) versus CAPs (F). Sirius red staining of collagen deposition in mice fed HFC was slightly less in mice exposed to FA (G) versus CAPs (H). For each panel a representative image is shown. Bars represent 10 µm.
Liver sections were quantitatively scored in a blinded fashion using the modified Brunt scoring system to assess steatohepatitis (Brunt et al., 1999). CAPs exposure did not significantly alter the amount of hepatic fat compared to FA exposure for mice receiving HFC. However, the overall mean steatohepatitis grade (inflammation) was significantly (p < 0 001) higher in the CAPs- vs. FA-exposed mice (Table 1). Increases in lobular inflammation, hepatocyte ballooning, and Mallory bodies were primarily responsible for the higher inflammatory grade in the CAPs-exposed mice. The mean fibrosis stage was also statistically significantly higher in the CAPs-versus FA-exposed mice (p = 0.023) after just 6 wk of exposure. Increased fibrosis typically follows increased inflammation in NAFLD (Jou et al., 2008). Similarly the NAFLD activity score (Kleiner et al., 2005) based on steatosis, lobular inflammation, and hepatocyte ballooning was also significantly higher in the CAPs versus the FA group (7.10 ± 0.88 vs. 4.50 ± 1.90, respectively, p = 0.002).
Table 1.
NAFLD grading and staging in C57BL/6 mice fed high fat chow.
| Steatosis | Ballooning | Lobular inflammation |
Portal inflammation |
Mallory bodies |
Grade (0–3) | Stage (0–3) | |
|---|---|---|---|---|---|---|---|
| FA (10) | 2.50±0.65 | 1.70±0.95 | 1.30±0.46 | 0.80±0.42 | 1.00±0.47 | 1.50±0.71 | 0.60±0.52 |
| PM (10) | 3.00±0.00 | 2.70±0.67 | 2.40±0.52 | 1.10±0.57 | 1.80±0.41 | 3.00±0.00 | 1.00.±000 |
| p value | 0.257 | 0.038 | 0.002 | 0.326 | 0.007 | <0.001 | 0.023 |
Mean±S.E.M. values are shown. Number of mice is given in parenthesis. P values were calculated using unpaired Manny Whitney U test.
PM localizes predominantly to Kupffer cells in the liver
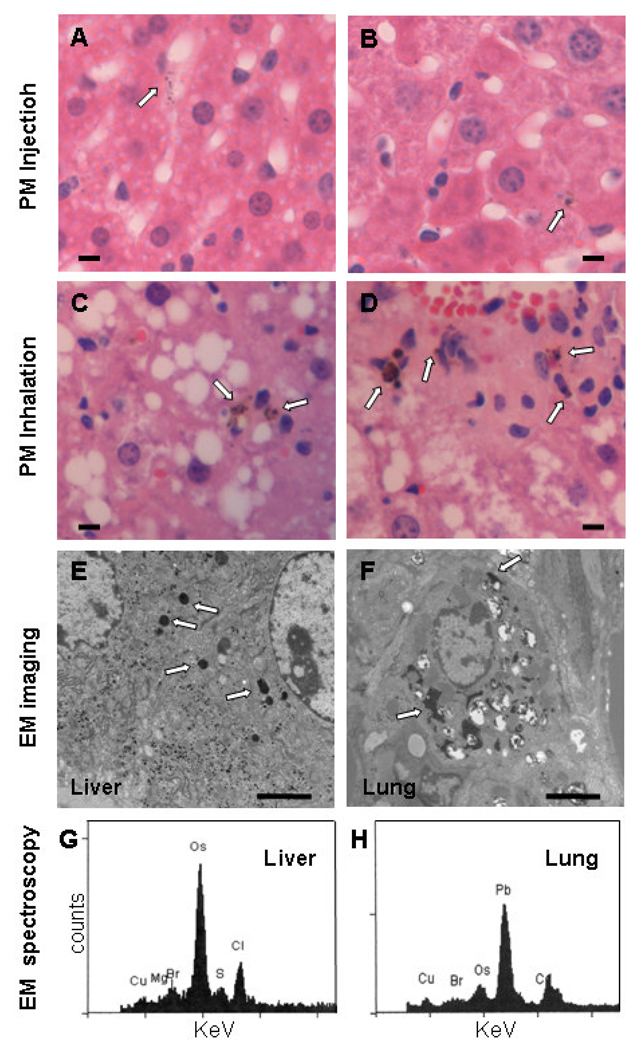
It is controversial whether PM reaches the liver following exposure to ambient CAPs. Prior to analyzing the liver PM content in the ambient CAPs- and FA-exposed mice, standard PM (SRM 1649a) suspended in PBS or PBS alone was injected intravenously into mice to determine if circulating PM is taken up by the liver. Twenty-four hours post-injection, H&E stained liver sections were prepared for review. Dark intra-cytoplasmic PM of less than 2 µm was detected only in the livers of PM injected mice (n=3), mainly within Kupffer cells (Fig. 2A&B), and not in PBS-injected mice (n = 3) (data not shown). Larger particles were not observed in the livers of PM- or PBS-injected mice. Similar dark, intra-cytoplasmic PM (< 2 µm) was present in the livers of 10 of 10 mice following ambient CAPs exposure (Figures 2C and 2D) and only 2 of 10 mice exposed to FA (data not shown) after a blinded analysis of H&E-stained liver sections (p < 0.001). PM was again observed predominantly in Kupffer cells. Semi-quantitative scoring (0–3) of the percentage of liver cells containing PM indicated significantly more cells contained PM in CAPs- vs. FA-exposed mice (2.00 ± 0.94 vs. 0.2 ± 0.42, respectively, p < 0.001). Prussian blue did not stain these particles, indicating little iron was in the PM (data not shown).
Figure 2. Examination of PM in liver sections.
Following injection of PM, fine particles less than 2 µm are observed in the cytosol of hepatic macrophages, primarily Kupffer cells as seen under high power (40× objective) in H&E-stained formalin–fixed sections (A and B; arrows mark PM; bar equals 10 µm). Similar PM was observed in hepatic macrophages following CAPs exposure (C and D; arrows mark PM; bar equals 10 µm). Imaging of glutaraldehyde-fixed sections by electron microscopy also identified electron dense fine PM in both livers (E) and lungs (F) of mice following exposure to ambient CAPs (arrows mark PM; bar = 2 µm). Electron dispersive spectroscopy of the electron dense intracellular particles revealed sulfur (S), chlorine (Cl), and bromine (Br) in the liver (G) and lead (Pb) and bromine (Br) in the lung (H). The osmium (Os) peaks indicate deposition of osmium tetroxide, which is used to fix membranes. Copper (Cu) peaks are from the copper grid used to mount the tissue section. (Counts = electron counts, KeV = kilovolts.). For each panel a representative image is shown.
Electron microscope imaging of liver sections from the CAPs-exposed mice revealed dense, intra-cytoplasmic PM in both the liver and lungs (Figures 2E and 2F). The PM in the lung cells appeared more heterogeneous in size and morphology than in the liver cells. Spectroscopic analysis of PM in liver cells identified sulfur, a major constituent of PM2.5 known to affect cytokine production (Figure 2G) (Fuentes et al., 2006; Lipfert et al., 2006; Duvall et al., 2008). Bromine and chlorine, common PM2.5 constituents, were present in the liver particles as well. The spectroscopic analysis of PM in aorta plaques identified similar elements as found in liver cell PM (data not shown). Lead, which is known to partition poorly from the lungs into the circulation (Wallenborn et al., 2007), was present only in the lung particles (Figure 2H).
PM exposure increased macrophage and Kupffer cell pro-inflammatory cytokine production in a TLR4-dependent manner
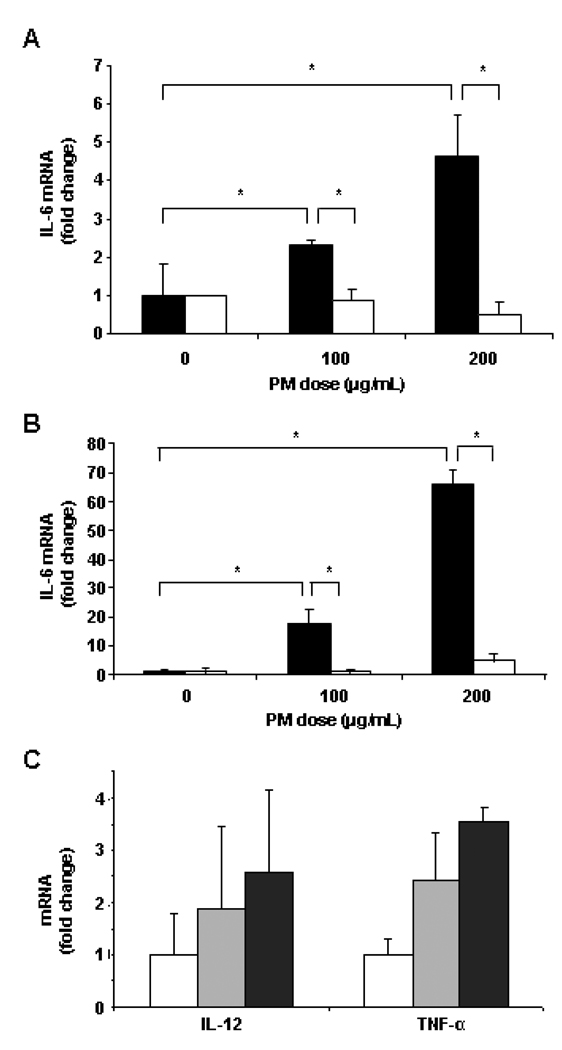
Studies were performed using cultured cells to determine the potential mechanism of any direct effect of PM exposure on inflammatory cytokine and collagen production by liver cells. Standard ambient PM (SRM 1469a) was diluted in media for these studies. The LPS contamination in the PM preparation was minimal, less than 125 pg/ml. Since PM accumulated mostly in Kupffer cells, resident hepatic macrophages, following exposure to ambient CAPs, a macrophage cell line was exposed to PM (SRM 1469a) in vitro to assess changes in pro-inflammatory cytokine production (interleukin [IL]-6, IL-12, and tumor necrosis factor [TNF]-α). IL-6 mRNA levels were significantly increased in macrophages in a dose and time dependent manner. As early as 8 hr after addition of PM, macrophage IL-6 mRNA levels were increased 2–5-fold relative to unexposed control cells (both p < 0.050) for the 100 µg/ml dose and the 200 µg/ml dose, respectively (Figure 3A, black columns). After 24 hr of exposure to PM (Figure 3 B, black columns), the fold increases in IL-6 mRNA levels were 17.65 ± 5.05 and 65.87 ± 4.93, respectively (both p < 0.010). Pre-incubation with inhibitory anti-TLR4 antibodies (white columns), as opposed to isotype control antibodies (black columns), completely blocked the increase in macrophage IL-6 mRNA levels due to PM exposure at both timepoints (Figures 3A and 3B). To a lesser extent, IL-12 and TNFα mRNA levels were increased 24 hr following PM exposure relative to control macrophages, but the increases were not statistically significant (p > 0.050) except the increase in TNFα mRNA concentration was significant after exposure to 200 µg/ml PM (Figure 3C).
Figure 3. PM effect on cytokine mRNA levels in cultured macrophages.
IL-6 mRNA levels were increased in murine RAW macrophages (black bars) both 8 hr (A) and 24 hr (B) after PM addition. Antibody blockade of TLR4 (white bars) inhibited the effect of PM on IL-6 mRNA levels. Isotype control antibody was present in other cultures (black bars). Macrophage IL-12 and TNFα mRNA levels were more modestly increased by PM exposure after 24 hr (C). The results are based on at least three independent experiments. *Statistically significant differences (p < 0.050).
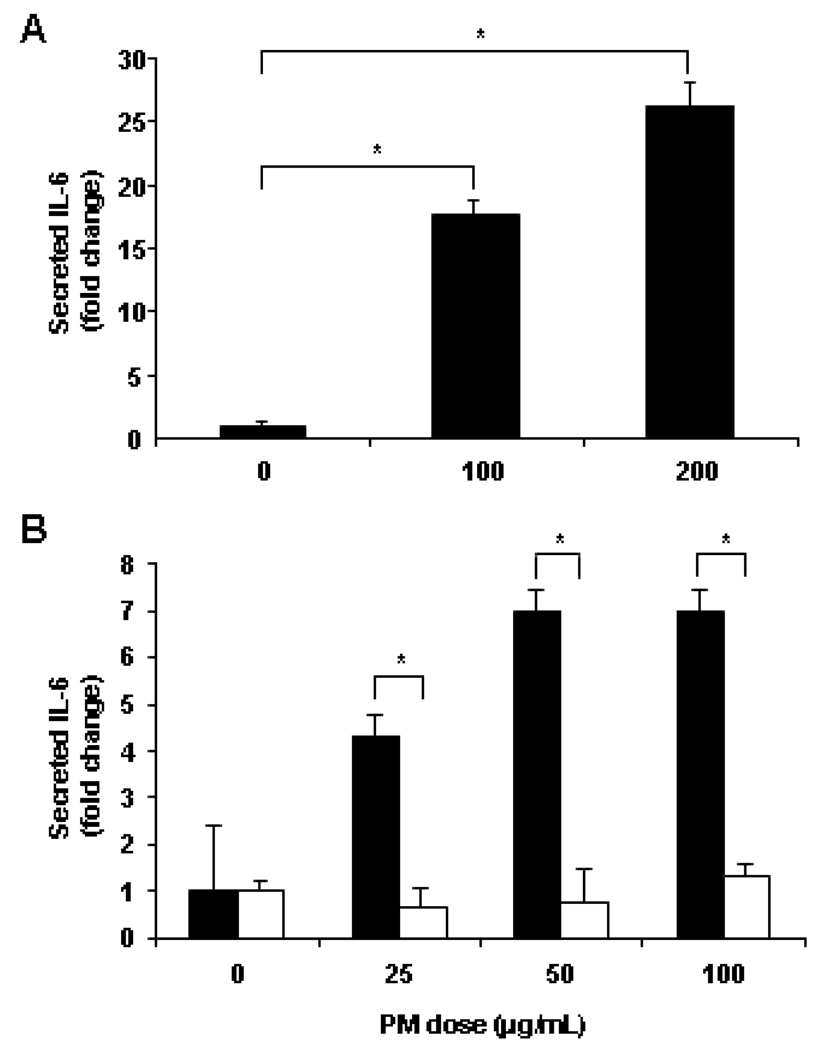
Changes due to PM exposure were also detectable at the protein level. Twenty-four hours post-PM addition to the macrophage cell line, IL-6 secretion increased 17-fold for the 100 µg/ml dose and 27-fold for the 200 µg/ml dose (both p < 0.010) compared to control macrophages (Figure 4A). The effect of PM on IL-6 cytokine secretion was also examined in Kupffer cells isolated from both WT and TLR4−/− mouse livers. There was a significant increase in IL-6 secretion in PM-exposed, WT Kupffer cells (25–100 µg/ml PM) compared to unexposed WT Kupffer cells (all < 0.050) (Figure 4B, black columns). The increase in IL-6 secretion plateaued at a lower dose of PM in Kupffer cells than in the RAW macrophage cell line. At each dose of PM, IL-6 secretion was significantly less in TLR4−/− Kupffer cells (Figure 4B, white columns) compared to WT Kupffer cells (black columns) (all p < 0.050).
Figure 4. PM effect on cultured macrophage and Kupffer cell IL-6 protein secretion.
PM addition significantly increased mouse macrophage cell line IL-6 secretion after 24 hr (A). PM stimulated IL-6 secretion by isolated WT Kupffer cells (black bars) but not TLR4 −/− Kupffer cells (white bars) after 24 hr (B). The results are based on at least three independent experiments. *Statistically significant differences (p < 0.050).
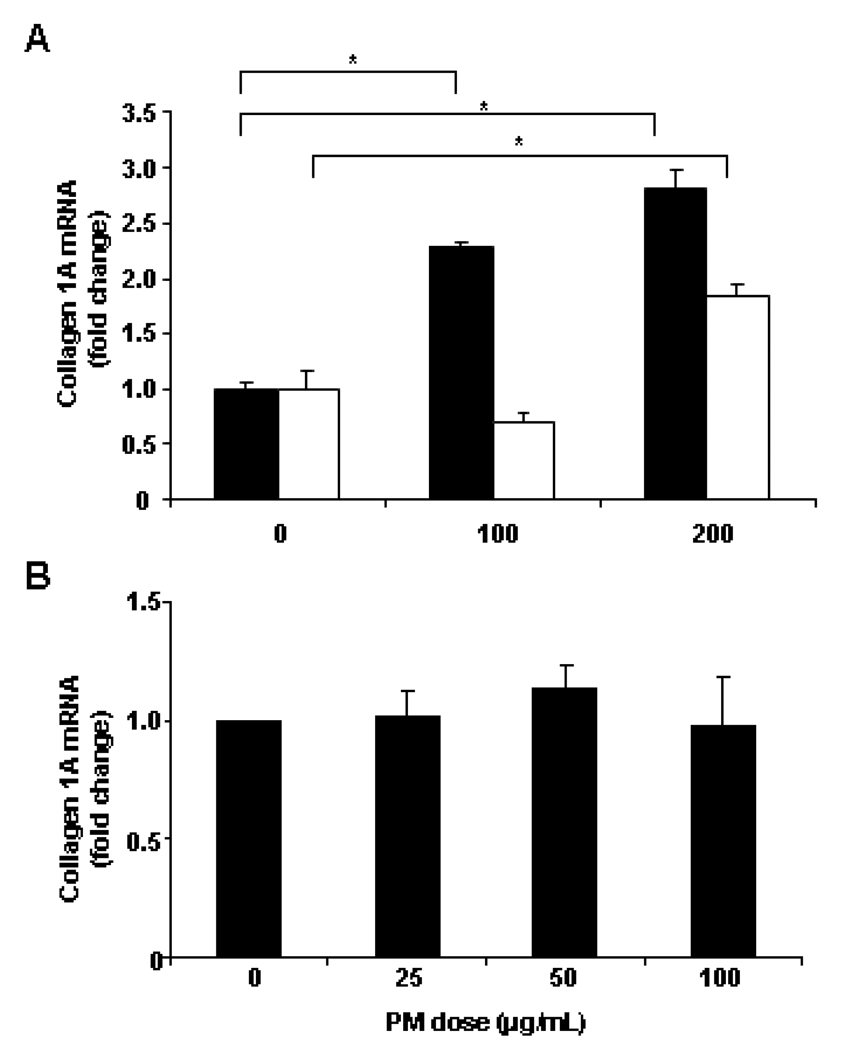
Hepatic stellate cell collagen expression was increased by conditioned media from PM-exposed macrophages, but not by direct PM exposure
Pro-inflammatory cytokines released by Kupffer cells may enhance fibrosis by increasing collagen 1A production by hepatic stellate cells, which are in close proximity to Kupffer cells in the hepatic sinusoids. Therefore, mouse hepatic stellate cells were incubated with 4-fold diluted, conditioned media from macrophages exposed to 0, 100, and 200 µg/ml PM for 24 hr. In the presence of conditioned media from PM exposed macrophages, collagen 1A mRNA expression increased significantly in WT (black columns) stellate cells (both p < 0.050) compared to WT stellate cells cultured in conditioned media from unexposed macrophages (Figure 5A). In TLR4−/− stellate cells (white columns), a significant increase in collagen 1A mRNA expression was observed in the presence of conditioned media from macrophages exposed to 200 µg/ml PM (p = 0.005). Exposure of stellate cells to PM in the absence of macrophage-conditioned media did not increase collagen 1A mRNA expression (Figure 5B).
Figure 5. PM effect on cultured stellate cells.
Collagen 1A mRNA levels in both WT (black bars) and TLR4−/− (white bars) hepatic stellate cells were increased by exposure to conditioned media from macrophages exposed to PM (A). Direct PM exposure had no significant effect on collagen 1A mRNA expression (B). The results are based on at least three independent experiments. *Statistically significant differences (p < 0.050).
Discussion
Since NAFLD is the most common chronic liver disease, identification of risk factors responsible for NAFLD progression will significantly help reduce morbidity and mortality from chronic liver disease. The need for liver transplantation would be reduced as well. Limited data is available concerning environmental risk factors associated with NAFLD progression. Our results suggest that chronic inhalation of low levels of ambient air PM is an important risk factor for progression of NAFLD. The minimal hepatic inflammation and fibrosis in mice exposed only to filtered air indicates that reductions in ambient air PM levels or specific PM components would reduce morbidity and mortality due to NAFLD.
To what extent reducing PM exposure will decrease progression of benign fatty liver to steatohepatitis is uncertain. Even if only a small percentage of those with NAFLD are affected by PM exposure, millions might benefit from reduced PM exposure since a large epidemiologic study suggested NAFLD affects up to 30% of the U.S. population as assessed by hepatic triglyceride content (Browning et al., 2004). Hispanics (45%) were most likely to have NAFLD and the prevalence of insulin resistance (58%) and metabolic syndrome (30%) was increased three fold in those with NAFLD. Of all factors analyzed, body mass index (i.e. obesity) had the closest positive correlation with hepatic triglyceride content. For now the prevalence of NAFLD is less in the pediatric population; however, a 4 fold increase in pediatric cases of NAFLD has been noted in the past two decades (Koebnick et al., 2009). This data suggests NAFLD will be even more prevalent amongst adults in the near future. Epidemiologic studies utilizing U.S. Environmental Protection Agency data on local PM levels may be helpful in assessing the importance of PM exposure in progression of NAFLD.
Our animal exposure study was physiologic in that it utilized ambient air PM at doses mimicking naturally occurring levels, daily exposure, natural inhalation, and diet-induced hepatic steatosis. Previous studies using less physiologic models have examined the effect of air pollutant exposure on the liver (Folkmann et al., 2007; Gong et al., 2007; Tomaru et al., 2007). Tomaru et al. demonstrated that obese diabetic mice injected intratracheally bi-weekly for four months with diesel exhaust particles developed increased hepatic steatosis and lipid peroxidation compared to mice exposed to vehicle only (Tomaru et al., 2007). Diesel exhaust particles are a major component of ambient PM in urban areas. Araujo et al. (2008) also observed increased hepatic lipid peroxidation in ApoE knockout mice fed NC and exposed three days a week for six weeks to ambient air CAPs collected 300 m from a Los Angeles freeway. Hepatic lipid peroxidation is indicative of oxidative stress, commonly associated with the transition from simple fatty liver to a pro-fibrotic inflammatory state (steatohepatitis) (Wortham et al., 2008). Unlike our study, the effect of air pollutant exposure on hepatic inflammation and fibrosis was not analyzed in these studies.
Along with oxidative stress, Kupffer cell/macrophage activation and cytokine/chemokine production play a central role in the progression of NAFLD (McCullough, 2006). PM was primarily observed in the cytosol of Kupffer cells and to a lesser extent in portal macrophages. Our studies in vitro indicate that ambient PM that reaches the liver has the potential to induce Kupffer cell cytokine secretion in vivo. Kupffer cell IL-6 secretion was greatly increased following PM2.5 exposure. IL-6 levels are significantly elevated in human NAFLD (Wieckowska et al., 2008) and are significantly higher in those with steatohepatitis as opposed to simple fatty liver (Wieckowska et al., 2008). Kupffer cell activation by PM was TLR4-dependent, consistent with recent studies indicating that macrophage TLR4 expression is pivotal for progression of NAFLD (Rivera et al., 2007). Similar to PM, bacterial LPS activation of hepatic macrophage via TLR4 may also play a role in NAFLD progression (Becker et al., 2002). Though LPS may be bound to ambient PM, the ambient PM used in our studies in vitro contained only trace amounts of LPS (< 125 pg/ml) insufficient for activation of macrophages.
It is uncertain whether the particles noted in the liver represent intact, ambient PM that translocated from the lungs into the circulation. The particles we observed in liver may be soluble components of PM that were released from their insoluble carbon backbone. Water or acid soluble metals have been shown to translocate to the systemic circulation and deposit in the rat liver following acute or chronic exposure to PM derived from incomplete oil or coal combustion, respectively (Mani et al., 2007; Wallenborn et al., 2007). In the latter study, increased hepatic inflammation was noted, but the exposure concentration was a thousand fold higher than in the current study. Seasonal and geographic variability in ambient PM concentrations and components alter its effect on pulmonary (Nadadur and Kodavanti, 2002; Prophete et al., 2006) and cardiovascular disease (Steerenberg et al., 2006). Further studies are needed to determine the route and extent to which inhaled particulate matter reaches the liver.
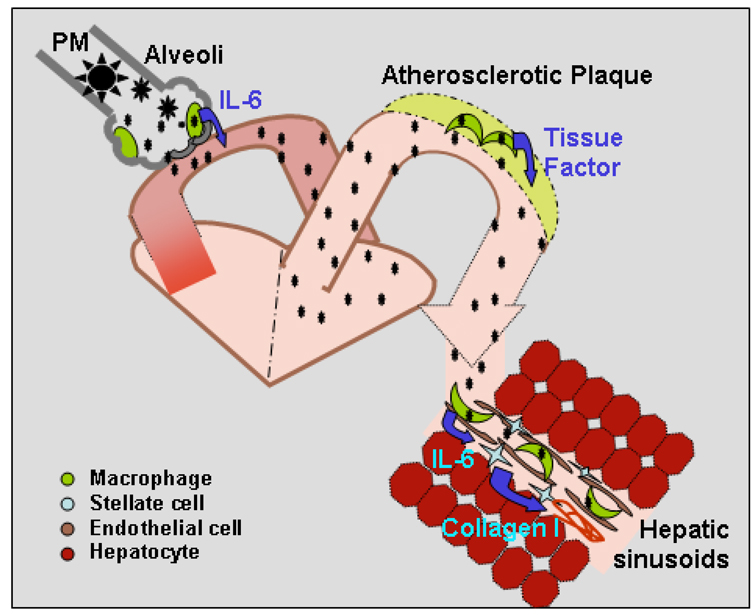
Our working model (Figure 6) illustrates how inhaled fine PM may reach the liver by crossing the alveolar membranes to reach the circulation. Circulating fine PM may then accumulate in both atherosclerotic plaques (Sun et al., 2005) and hepatic Kupffer cells. TLR4-dependent activation of cytokine release by Kupffer cells may then trigger inflammation and hepatic stellate cell collagen synthesis. A better understanding of the impact of ambient PM exposure on NAFLD progression may require studies utilizing a variety of ambient PM sources. Lastly, the increased inflammation and fibrosis induced by ambient PM exposure in this animal model may facilitate evaluation of treatments to slow fatty liver disease progression.
Figure 6. Dissemination of ambient PM2.5 and activation of tissue macrophages.
Ambient PM2.5 may stimulate alveolar macrophage activation and IL-6 production. Soluble PM2.5 may then enter the circulation and enhance tissue macrophage production of pro-inflammatory cytokines and chemokines. In arteries, this process may enhance progression of atherosclerosis while in the liver progression of NAFLD may be accelerated.
Acknowledgements
C. E. Alvarez' technical support for mRNA analysis and Kupffer cell isolation were invaluable. C. E. Jackson’s helpful review and suggestions during manuscript preparation are much appreciated.
Footnotes
Declaration of interest
The Authors report no conflicts of interest. The Authors alone are responsible for the content and writing of the paper.
References
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. _targeted disruption of the MyD88 gene results in loss of IL-1- and IL- 18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Ala A, Stanca CM, Bu-Ghanim M, Ahmado I, Branch AD, Schiano TD, Odin JA, Bach N. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology. 2006;43:525–531. doi: 10.1002/hep.21076. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ. Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Dailey LA, Soukup JM, Grambow SC, Devlin RB, Huang YC. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ. Health Perspect. 2005;113:1032–1038. doi: 10.1289/ehp.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Fenton MJ, Soukup JM. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am. J. Respir. Cell. Mol. Biol. 2002;27:611–618. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbset HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Non-alcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- Chen LC, Nadziejko C. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. V. CAPs exacerbate aortic plaque development in hyperlipidemic mice. Inhal. Toxicol. 2005;17:217–224. doi: 10.1080/08958370590912815. [DOI] [PubMed] [Google Scholar]
- Duvall RM, Norris GA, Dailey LA, Burke JM, McGee JK, Gilmour MI, Gordon T, Devlin RB. Source apportionment of particulate matter in the U.S. and associations with lung inflammatory markers. Inhal. Toxicol. 2008;20:671–683. doi: 10.1080/08958370801935117. [DOI] [PubMed] [Google Scholar]
- Folkmann JK, Risom L, Hansen CS, Loft S, Moller P. Oxidatively damaged DNA and inflammation in the liver of dyslipidemic ApoE−/ − mice exposed to diesel exhaust particles. Toxicology. 2007;237:134–144. doi: 10.1016/j.tox.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Fuentes M, Song HR, Ghosh SK, Holland DM, Davis JM. Spatial association between speciated fine particles and mortality. Biometrics. 2006;62:855–863. doi: 10.1111/j.1541-0420.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- Gong KW, Zhao W, Li N, Barajas B, Kleinman M, Sioutas C, Horvath S, Lusis AJ, Nel A, Araujo JA. Air-pollutant chemicals and oxidized lipids exhibit genomewide synergistic effects on endothelial cells. Genome Biol. 2007;8:R149. doi: 10.1186/gb-2007-8-7-r149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, Tarocchi M, Abar OT, Huang H, Sninsky JJ, Friedman SL. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetland RB, Cassee FR, Lag M, Refsnes M, Dybing E, Schwarze PE. Cytokine release from alveolar macrophages exposed to ambient particulate matter: Heterogeneity in relation to size, city and season. Part. Fibre Toxicol. 2005;2:4–15. doi: 10.1186/1743-8977-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horani A, Muhanna N, Pappo O, Melhem A, Alvarez CE, Doron S, Wehbi W, Dimitrios K, Friedman SL, Safadi R. Beneficial effect of glatiramer acetate (Copaxone) on immune modulation of experimental hepatic fibrosis. Am. J. Physiol. 2007;292:G628–G638. doi: 10.1152/ajpgi.00137.2006. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Imrich A, Ning Y, Lawrence J, Coull B, Gitin E, Knutson M, Kobzik L. Alveolar macrophage cytokine response to air pollution particles: Oxidant mechanisms. Toxicol. Appl. Pharmacol. 2007;218:256–264. doi: 10.1016/j.taap.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in non-alcoholic fatty liver disease. Semin. Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Koebnick C, Getahun D, Reynolds K, Coleman KJ, Porter AH, Lawrence JM, Punyanitya M, Quinn VP, Jacobsen SJ. Trends in nonalcoholic fatty liver disease-related hospitalizations in US children, adolescents, and young adults. J Pediatr Gastroenterol Nutr. 2009;48(5):597–603. doi: 10.1097/MPG.0b013e318192d224. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Arimoto T, Shinyashiki M, Shimojo N, Nakai Y, Yoshikawa T, Sagai M. Generation of reactive oxygen species during interaction of diesel exhaust particle components with NADPH-cytochrome P450 reductase and involvement of the bioactivation in the DNA damage. Free Rad. Biol. Med. 1997;22:479–487. doi: 10.1016/s0891-5849(96)00341-3. [DOI] [PubMed] [Google Scholar]
- Lipfert FW, Baty JD, Miller JP, Wyzga RE. PM2.5 constituents and related air quality variables as predictors of survival in a cohort of U.S. military veterans. Inhal. Toxicol. 2006;18:645–657. doi: 10.1080/08958370600742946. [DOI] [PubMed] [Google Scholar]
- Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal. Toxicol. 2005;17:189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- Mani U, Prasad AK, Suresh Kumar V, Lal K, Kanojia RK, Chaudhari BP, Murthy RC. Effect of fly ash inhalation on biochemical and histomorphological changes in rat liver. Ecotoxicol. Environ. Safety. 2007;68:126–133. doi: 10.1016/j.ecoenv.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ. Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AJ. The clinical features, diagnosis and natural history of non-alcoholic fatty liver disease. Clin. Liver. Dis. 2004;8:521–533. doi: 10.1016/j.cld.2004.04.004. viii. [DOI] [PubMed] [Google Scholar]
- McCullough AJ. Pathophysiology of non-alcoholic steatohepatitis. J. Clin. Gastroenterol. 2006;40:S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- Nadadur SS, Kodavanti UP. Altered gene expression profiles of rat lung in response to an emission particulate and its metal constituents. J. Toxicol. Environ. Health A. 2002;65:1333–1350. doi: 10.1080/00984100290071559. [DOI] [PubMed] [Google Scholar]
- Phelps RG, Bernstein LE, Harpaz N, Gordon RE, Cruickshank FA, Schwartz E. Characterization of a dermal derived malignant mesenchymal tumor arising in ultraviolet irradiated mice. Am. J. Pathol. 1989;135:149–159. [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Kanner RE. Acute effects of PM10 pollution on pulmonary function of smokers with mild to moderate chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1993;147:1336–1340. doi: 10.1164/ajrccm/147.6_Pt_1.1336. [DOI] [PubMed] [Google Scholar]
- Prophete C, Maciejczyk P, Salnikow K, Gould T, Larson T, Koenig J, Jaques P, Sioutas C, Lippmann M, Cohen M. Effects of select PM-associated metals on alveolar macrophage phosphorylated ERK1 and -2 and iNOS expression during ongoing alteration in iron homeostasis. J. Toxicol. Environ. Health A. 2006;69:935–951. doi: 10.1080/15287390500362030. [DOI] [PubMed] [Google Scholar]
- Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J. Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Ballester F, Saez M, Perez-Hoyos S, Bellido J, Cambra K, Arribas F, Canada A, Perez-Boillos MJ, Sunyer J. The concentration-response relation between air pollution and daily deaths. Environ. Health Perspect. 2001;109:1001–1006. doi: 10.1289/ehp.011091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGFβ signaling and hepatic fibrosis. Nat. Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- Sicari MC, Lebwohl M, Baral J, Wexler P, Gordon RE, Phelps RG. Photo-induced dermal pigmentation in patients taking tricyclic antidepressants: Histology, electron microscopy, and energy dispersive spectroscopy. J. Am. Acad. Dermatol. 1999;40:290–293. doi: 10.1016/s0190-9622(99)70467-6. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ. Health Perspect. 2005;113:947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrod B, Pertoft H, Eggertsen G, Sundstrom C. Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res. 1985;241:639–649. doi: 10.1007/BF00214586. [DOI] [PubMed] [Google Scholar]
- Stanca CM, Babar J, Singal V, Ozdenerol E, Odin JA. Pathogenic role of environmental toxins in immune-mediated liver diseases. J. Immunotoxicol. 2008;5:59–68. doi: 10.1080/15476910802019086. [DOI] [PubMed] [Google Scholar]
- Steerenberg PA, van Amelsvoort L, Lovik M, Hetland RB, Alberg T, Halatek T, Bloemen HJ, Rydzynski K, Swaen G, Schwarze P, Dybing E, Cassee FR. Relation between sources of particulate air pollution and biological effect parameters in samples from four European cities: An exploratory study. Inhal Toxicol. 2006;18:333–346. doi: 10.1080/08958370500515913. [DOI] [PubMed] [Google Scholar]
- Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. Kupffer cell activation by lipopolysaccharide in rats: Role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932–936. doi: 10.1053/he.2000.5634. [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Schwartz J, Tobias A, Macfarlane D, Garcia J, Anto JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: A case-crossover analysis. Am. J. Epidemiol. 2000;151:50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- Tomaru M, Takano H, Inoue K, Yanagisawa R, Osakabe N, Yasuda A, Shimada A, Kato Y, Uematsu H. Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int. J. Mol. Med. 2007;19:17–22. [PubMed] [Google Scholar]
- Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- Wallenborn JG, McGee JK, Schladweiler MC, Ledbetter AD, Kodavanti UP. Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicol. Sci. 2007;98:231–239. doi: 10.1093/toxsci/kfm088. [DOI] [PubMed] [Google Scholar]
- Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human non-alcoholic steatohepatitis. Am. J. Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Wortham M, He L, Gyamfi M, Copple BL, Wan YJ. The transition from fatty liver to NASH associates with SAMe depletion in db/db mice fed a methionine choline-deficient diet. Dig. Dis. Sci. 2008;53:2761–2774. doi: 10.1007/s10620-007-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohe HC, O'Hara KA, Hunt JA, Kitzmiller TJ, Wood SG, Bement JL, Bement WJ, Szakacs JG, Wrighton SA, Jacobs JM, Kostrubsky V, Sinclair PR, Sinclair JF. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am. J. Physiol. 2006;290:G1269–G1279. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]