Abstract
Salmonella typhimurium causes a localized enteric infection in immunocompetent individuals, whereas HIV-infected individuals develop a life-threatening bacteremia. Here we show that simian immunodeficiency virus (SIV) infection results in depletion of T helper type 17 (TH17) cells in the ileal mucosa of rhesus macaques, thereby impairing mucosal barrier functions to S. typhimurium dissemination. In SIV-negative macaques, the gene expression profile induced by S. typhimurium in ligated ileal loops was dominated by TH17 responses, including the expression of interleukin-17 (IL-17) and IL-22. TH17 cells were markedly depleted in SIV-infected rhesus macaques, resulting in blunted TH17 responses to S. typhimurium infection and increased bacterial dissemination. IL-17 receptor–deficient mice showed increased systemic dissemination of S. typhimurium from the gut, suggesting that IL-17 deficiency causes defects in mucosal barrier function. We conclude that SIV infection impairs the IL-17 axis, an arm of the mucosal immune response preventing systemic microbial dissemination from the gastrointestinal tract.
Although nontyphoidal Salmonella serotypes (NTS) are common agents causing acute food-borne disease worldwide, it is unusual to isolate them from the blood of adults, because in immunocompetent individuals these pathogens remain localized to the intestine and cause a self-limiting gastroenteritis1. However, in people with underlying immunosuppression, NTS may spread beyond the intestine and reach the bloodstream, a serious complication known as NTS bacteremia2. The rise in the number of people with AIDS in sub-Saharan Africa has led to a dramatic increase in the frequency of NTS bacteremia3. In marked contrast to the pre-AIDS era4, NTS is currently a leading cause of hospital admission of adults and among the most common bacterial blood isolates from hospitalized adults in sub-Saharan Africa5, the vast majority of whom are HIV positive3. NTS infection in HIV-positive African adults is associated with high acute mortality rates (47%)6. Although the occurrence of NTS bacteremia in HIV-positive people is well documented, there are no reports investigating the mechanisms by which HIV infection increases susceptibility.
Human infections with NTS are characterized by a rapidly developing acute inflammatory reaction in the terminal ileum and colon1. Initial inflammatory responses elicited by S. typhimurium in the intestine are important for the disease outcome, as they enable immunocompetent people to contain bacteria at the site of infection, thereby causing a localized gastroenteritis. The induction of this rapid inflammatory response can be studied by using S. typhimurium infection of bovine ligated ileal loops, where inflammation is initiated by bacterial invasion of the intestinal epithelium7, resulting in translocation into the lamina propria within 1 to 2 h after infection8. Within the lamina propria, S. typhimurium is observed within mononuclear phagocytes or neutrophils9. Stimulation of mononuclear phagocytes with S. typhimurium in vitro triggers the induction of a proinflammatory gene expression profile10. However, host responses observed in vitro may not account for all changes in the gene expression profiles observed in vivo, because inflammatory responses may be amplified by paracrine mechanisms in tissue.
Like humans, rhesus macaques (Macaca mulatta) develop a localized gastroenteritis in response to infection with S. typhimurium11. SIV infection of rhesus macaques is an established model for studying human HIV disease12. By establishing the loop procedure in the rhesus macaque, we were able to determine how an underlying SIV infection changes host responses elicited by S. typhimurium.
RESULTS
TH17 responses dominate the Salmonella gene expression profile
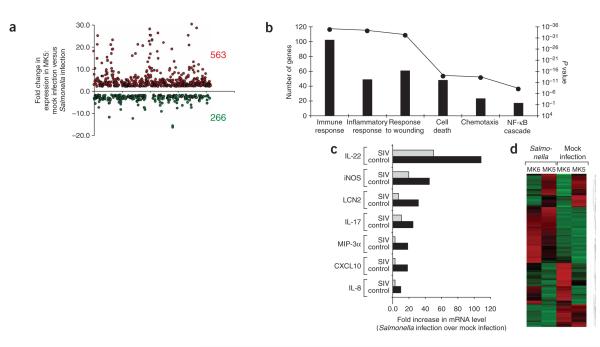
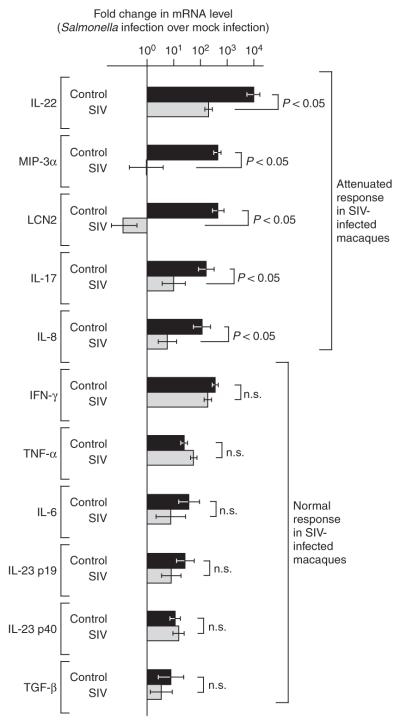
Four young adult healthy rhesus macaques (MK5, MK7, MK8 and MK9) underwent loop surgery. Loops of each macaque were inoculated by injecting either a S. typhimurium culture or sterile culture medium (mock infection) into the intestinal lumen, and individual loops of each treatment group were removed surgically at 2, 5 and 8 h after inoculation. We monitored the host response to S. typhimurium infection in vivo at defined early time points after infection and compared it to responses in mock-infected tissue collected from the same macaque at the same time point. S. typhimurium infection resulted in marked enteritis and fluid accumulation at 5 and 8 h after inoculation. To characterize mucosal responses to S. typhimurium infection, we performed gene expression profiling by comparing mRNA levels from S. typhimurium–infected or mock-infected loops (Fig. 1). The gene expression profile induced by S. typhimurium was dominated by responses linked to immunity and inflammation. The strongest up-regulation of gene expression was observed for IL-22, followed by IFN-γ and IL-26 (Fig. 1c and data not shown). IL-22 and IFN-γ synergize to induce inducible nitric oxide synthase (iNOS) expression13, whose mRNA levels were markedly increased after S. typhimurium infection (Fig. 1c). Furthermore, S. typhimurium induced a marked upregulation of IL-17 expression and the expression of genes induced by IL-17, including those encoding lipocalin-2 (LCN2)14, CCL20 (MIP-3α)15, CXCL8 (IL-8)16 and CXCL10 (ref. 17). To verify changes in cytokine expression detected by gene expression profiling, we determined the mRNA levels of IL-17 and IL-17–regulated genes (those coding for MIP-3α, lipocalin-2 and IL-8) in samples from all four macaques by real-time PCR. Cytokines whose expression is not affected by IL-17, including interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-6, IL-23 p19, IL-12 and IL-23 p40, and transforming growth factor-β (TGF-β) were analyzed for comparison (Fig. 2). These results show a marked increase in the mRNA levels of all proinflammatory cytokines after S. typhimurium infection.
Figure 1.
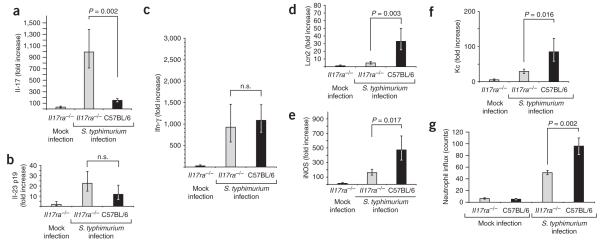
Gene expression profiling of the host response to S. typhimurium infection. (a,b) Host response elicited 5 h after S. typhimurium infection in a healthy young adult macaque (MK5). (a) The graph shows the fold changes in gene expression for genes that were significantly (P < 0.05) upregulated (563) or downregulated (266) during S. typhimurium infection compared to mock-infected tissue. (b) Genes with significantly (P < 0.05) altered expression were subjected to hierarchical clustering, followed by functional and statistical analysis of the genes in each subcluster. The number of genes in each category (bars) and the corresponding P values for each biological process (filled circles) are indicated. (c,d) Comparison of host responses elicited 5 h after S. typhimurium infection in an SIV-infected macaque (MK6) compared to a control macaque (MK5). (c) IL-17 responses elicited 5 h after S. typhimurium inoculation of loops in an SIV-infected macaque (MK6) compared to an SIV-negative control macaque (MK5). (d) A heat diagram of changes in gene expression detected in S. typhimurium–infected tissue compared to mock-infected control tissue. Relative increase (red) or decrease (green) of mRNA level is shown.
Figure 2.
Cytokine expression elicited by S. typhimurium in SIV-infected (n = 4) and SIV-negative control macaques (n = 4) 5 h after infection. Fold changes in gene expression observed in a S. typhimurium–infected loop compared to a mock-infected loop from the same macaque were determined. Data are shown as geometric means of fold-increases ± s.e.m. Statistically significant (P < 0.05) differences are indicated. n.s., not significant.
All macaques used in this study were raised in a Salmonella-free colony and tested negative for Salmonella serotypes. Although there was no evidence of previous exposure of the macaques to this pathogen, mRNAs whose expression was upregulated most strongly at a very early time point (that is, at 5 h) after S. typhimurium infection included IL-22, IL-26 and IL-17 (Fig. 1c). The main cellular sources of these cytokines are T cells18–20. The importance of T cells in generating early inflammatory changes in response to S. typhimurium infection has not been apparent from previous studies10,21. Thus, the increased expression of these cytokines attracted our attention because T cell depletion has been linked to an inability of HIV-affected individuals to contain S. typhimurium at the site of infection (that is, the intestinal mucosa), resulting in the development of a life-threatening bacteremia22. Therefore, we wanted to determine whether the IL-17 responses elicited by S. typhimurium were altered by an underlying SIV infection.
Salmonella-induced TH17 responses are blunted by SIV infection
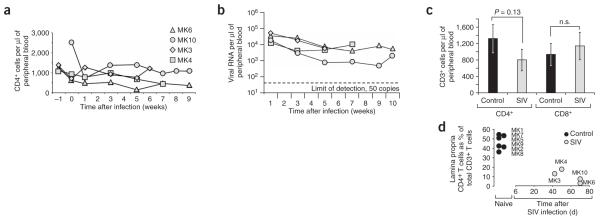
Four young adult healthy rhesus macaques (MK3, MK4, MK6 and MK10) were infected with SIV (SIV mac251), and blood samples were collected to monitor the number of CD4+ lymphocytes in the peripheral blood and the plasma viral load (Fig. 3). SIV-infected macaques underwent surgery between 6 and 10 weeks after inoculation. Compared to the SIV-negative macaques (see above), SIV-infected macaques had similar CD8+ counts in the peripheral blood at the time of surgery, whereas the numbers of CD4+ lymphocytes were lower. Loops of each macaque underwent S. typhimurium infection or mock infection as described above.
Figure 3.
Kinetics of lymphocyte depletion and viral replication in SIV-infected macaques compared to naive controls. (a) CD4+ T cell counts in peripheral blood of SIV-infected macaques (MK3, MK4, MK6 and MK10) over the time course of the experiment. (b) Viral loads in peripheral blood of SIV-infected macaques over the time course of the experiment. A dashed line indicates the limit of detection. (c) CD4+ and CD8+ T cell counts in peripheral blood of naive controls compared to SIV-infected macaques at the time of surgery. (d) Fraction of CD4+ T cells in the ileal lamina propria of naive controls compared to SIV-infected macaques at the time of surgery.
To identify mucosal responses elicited by S. typhimurium that were modified by an underlying SIV infection, gene expression profiling was performed with mRNA from S. typhimurium–infected and mock-infected loops collected from an SIV-infected macaque (MK6; Fig. 1d). The mRNA levels for genes involved in epithelial repair and maintenance were markedly upregulated in S. typhimurium–infected tissue from the SIV-negative macaque (MK5), and these responses were blunted by SIV infection (MK6) (Supplementary Fig. 1 online). Decreased expression levels of genes regulating epithelial barrier maintenance are also observed in people with HIV23. However, mRNA expression of genes linked to cell death and stress response were induced more strongly during S. typhimurium challenge of the SIV-infected macaque than the control macaque. Notably, mRNA expression of IL-22, IL-17 and genes regulated by IL-17 was blunted in the SIV-infected macaque compared to the SIV-negative macaque (Fig. 1c). To verify changes in mRNA levels detected by gene expression profiling, samples from all four SIV-infected macaques taken throughout the time course of S. typhimurium infection were analyzed by real-time PCR (Fig. 2). No significant differences between SIV-infected and SIV-negative macaques were observed for the S. typhimurium–mediated induction of IFN-γ, TNF-α, TGF-β, IL-6, IL-23 p19 or IL-12 and IL-23 p40 expression. In contrast, S. typhimurium challenge resulted in significantly (P < 0.05) higher mRNA levels of IL-17, IL-22, MIP-3α, LCN2 and IL-8 in SIV-negative compared to SIV-infected macaques. These data suggest that SIV-positive macaques develop a cytokine deficiency during S. typhimurium infection because IL-17 responses, which dominate the gene expression profile elicited by this bacterial pathogen, are blunted in these macaques.
SIV-induced CD4+ depletion reduces IL-17 production
IL-17 production detected by fluorescence microscopy of loop tissue suggested a cellular localization for IL-17 in mock-infected loops (Supplementary Fig. 2 online). Staining with antibody to Il-17 was markedly increased in S. typhimurium–infected loops of control macaques but produced a diffuse background signal, presumably because much of this cytokine is released during infection (Supplementary Fig. 2). To study the cellular sources of IL-17, we performed in vitro experiments with lymphocyte populations in which the release of IL-17 was prevented by treatment with brefeldin A.
Because T cells are a major source of IL-17, we investigated whether SIV-mediated T cell depletion affects IL-17 production in mucosal T cells. Memory CD4+ T cell depletion in the intestinal mucosa occurs within the first few weeks after HIV infection in humans or SIV infection in rhesus macaques24–28. Lymphocyte populations isolated from the ileal mucosa of four SIV-infected macaques (MK3, MK4, MK6 and MK10) and six control macaques (MK1, MK2, MK5, MK7, MK8 and MK9) were analyzed for expression of surface markers (CD3, CD4, CD8 and CD95) and for their capacity to produce IL-17 and IFN-γ. To this end, one 10-cm segment of the ileum that had not been exposed to S. typhimurium was collected from SIV-negative and SIV-infected macaques, and lamina propria lymphocytes were isolated and analyzed by flow cytometry. The CD4+ T cell population was markedly depleted in the intestinal mucosa of SIV-infected macaques (P = 0.0005), and this was accompanied by a relative increase in the fraction of CD8+ T cells (Fig. 4a). For the eight macaques that underwent loop surgery (MK3–10), the severity of CD4+ T cell depletion in the ileal mucosa (measured in tissue that had not been exposed to S. typhimurium) correlated directly with the magnitude of IL-17 mRNA expression induced in the same macaque 5 h after in vivo challenge of the loops with S. typhimurium (Fig. 4b).
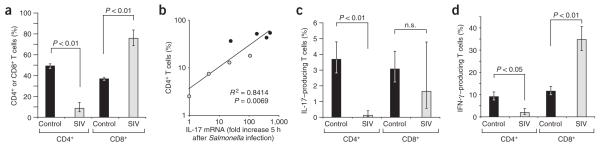
Figure 4.
Analysis of lamina propria T lymphocytes. (a) T cells in the ileal lamina propria of SIV-infected macaques (n = 4) compared to naive controls (n = 6). The y-axis indicates the numbers of CD4+ or CD8+ T cells as percentage of the total number of lamina propria CD3+ Tcells. (b) Correlation between the fraction of CD4+CD8− T cells and the fold increases in IL-17 expression elicited in the ileal mucosa 5 h after S. typhimurium infection of SIV-infected macaques (n = 4) or SIV-negative macaques (n = 4). The y-axis indicates the numbers of CD4+ T cells as percentage of the total number of lamina propria CD3+ Tcells. (c,d) Cytokine expression by CD3+ lamina propria T cells from SIV-infected macaques (n = 4) or naive controls (n = 6) in response to PMA-ionomycin stimulation. (c) The y-axis indicates the numbers of IL-17–producing CD4+ or CD8+ T cells as percentage of the total number of lamina propria CD3+ Tcells. (d) The y-axis indicates the numbers of IFN-γ–producing CD4+ or CD8+ T cells as percentage of the total number of lamina propria CD3+ T cells. Bars represent geometric means ± s.e.m. Statistically significant (P < 0.05) differences are indicated.
We next investigated whether ileal T cell populations in SIV-negative macaques (n = 6) differed from those in SIV-infected macaques (n = 4) in their capacity to produce IL-17. To this end, lamina propria lymphocytes from each macaque (isolated from tissue that had not been exposed to S. typhimurium) were stimulated ex vivo with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of brefeldin A (to prevent cytokine secretion) and then analyzed by multicolor flow cytometry to assess IL-17 and IFN-γ production by T cells (Fig. 4c,d). The majority of lamina propria CD3+ T cells showed high expression of CD95, indicative of a memory phenotype. The fraction of CD3+ T cells that showed a TH1 phenotype (that is, IFN-γ–producing CD4+ T cells) was significantly (P = 0.019) smaller in SIV-infected macaques (1.9% on average) compared to control macaques (9.2% on average; Fig. 4d). This depletion of TH1 cells did not, however, result in reduced IFN-γ expression during S. typhimurium infection (Fig. 2), presumably because these cells are not an important source of this cytokine at early times after infection. No significant differences between SIV-infected and SIV-negative macaques were observed in IL-17 production by CD8+ T cells in response to PMA-ionomycin stimulation. In contrast, the average percentage of lamina propria CD3+ T cells that was IL-17–producing and CD4+ (TH17 cells) was significantly (P = 0.005) lower in SIV-infected macaques (0.12% on average) compared to control macaques (3.7% on average; Fig. 4c). These data show that SIV infection significantly lowers the number of TH17 cells in the intestinal mucosa, which is predominantly the result of the overall CD4+ T cell depletion caused by the infection. The finding that SIV-mediated memory CD4+ T cell depletion significantly (P < 0.01) lowers the number of TH17 cells provides a plausible explanation for the blunted IL-17 response elicited by S. typhimurium in SIV-infected macaques (Fig. 2).
IL-17 deficiency accelerates Salmonella dissemination
At the end of the experimental procedure (8 h after inoculation of loops), samples of the draining mesenteric lymph nodes of rhesus macaques were processed for quantification of S. typhimurium. SIV-infected macaques had significantly higher numbers (330-fold increase, P = 0.019) of S. typhimurium in the mesenteric lymph node than SIV-negative macaques (Fig. 5a). These data provide a noteworthy illustration of the magnitude by which SIV-induced defects in mucosal barrier function increase the ability of S. typhimurium to disseminate within its host.
Figure 5.
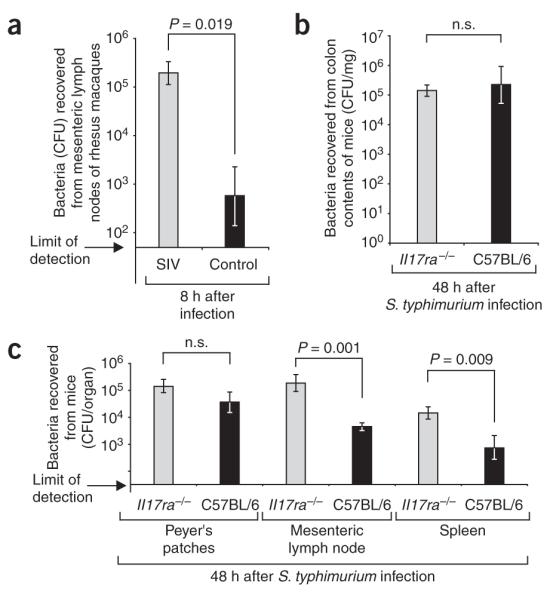
Bacterial translocation from the intestinal mucosa to internal organs in rhesus macaques and mice. (a) Recovery of S. typhimurium from the mesenteric lymph nodes of macaques 8 h after infection of ligated ileal loops. Data represent geometric means from control macaques or SIV-infected macaques ± s.e.m. (b,c) Recovery of S. typhimurium from the cecum (b) or internal organs (c) of C57BL/6 mice or Il17ra−/− mice 48 h after infection. Data represent geometric means ± s.e.m. Statistically significant (P < 0.05) differences are indicated.
To determine whether there is a causal link between the defect in IL-17 mRNA expression (Fig. 2) and increased bacterial dissemination (Fig. 5a), we used a well-established mouse model of S. typhimurium infection. Whereas mice normally develop few inflammatory changes in the intestinal mucosa during S. typhimurium infection, pretreatment with streptomycin drastically exacerbates neutrophil influx and inflammation in the cecal mucosa29. By using this model, we have previously shown that Il-17 mRNA expression is increased 150-fold in the cecal mucosa of streptomycin–pretreated mice by 48 h after S. typhimurium infection30. To assess the importance of IL-17 in controlling S. typhimurium infection, we pretreated two groups of six IL-17 receptor–deficient mice (Il17ra−/− mice) bred on a C57BL/6 background with streptomycin and inoculated them with S. typhimurium or sterile LB broth. As a control, two groups of C57BL/6 mice were treated identically. Organs were collected 48 h after infection (Fig. 5b,c). A significantly increased ability of S. typhimurium to disseminate to the mesenteric lymph nodes (40-fold, P = 0.001) and the spleens (42-fold, P = 0.009) was detected in Il17ra−/− mice compared to C57BL/6 mice (Fig. 5c). These data provide direct evidence that the IL-17–IL-17RA axis limits bacterial translocation from the intestinal mucosa to systemic sites of infection.
We further investigated the host response elicited by S. typhimurium in Il17ra−/− mice by measuring cytokine responses in the cecal mucosa by real-time PCR. S. typhimurium infection resulted in similar IFN-γ and IL-23 p19 mRNA levels in C57BL/6 mice as in Il17ra−/− mice (Fig. 6). A compensatory increase in Il-17 mRNA levels was observed during S. typhimurium infection of Il17ra−/− mice. Compared to infection of Il17ra−/− mice, S. typhimurium infection of C57BL/6 mice elicited significantly increased mRNA levels of lipocalin-2 (eightfold, P = 0.003). These data suggest a cause-and-effect relationship between blunted Il-17 responses in SIV-positive macaques and the inability of these macaques to increase lipocalin-2 expression in response to S. typhimurium infection (Fig. 2). S. typhimurium elicited significantly higher mRNA levels of the keratinocyte-derived chemokine (Kc; threefold, P = 0.016), a murine neutrophil chemoattractant related to CXCL1, in C57BL/6 mice than in Il17ra−/− mice (Fig. 6). Increased expression of Kc mRNA in the cecal mucosa was accompanied by an increased neutrophil influx detected in histological sections from the cecal mucosa of C57BL/6 mice compared to Il17ra−/− mice. In summary, our data show that IL-17 is required for the full induction of responses that lead to neutrophil influx and the production of antimicrobials in the cecal mucosa during S. typhimurium infection.
Figure 6.
Host responses elicited 48 h after S. typhimurium infection in the cecal mucosa of C57BL/6 mice orIl17ra−/− mice. (a–f) Data are expressed as fold changes of mRNA levels over mRNA levels detected in mock-infected C57BL/6 mice. Data represent geometric means ± s.e.m. Statistically significant (P < 0.05) differences are indicated. (g) Numbers of neutrophils per microscopic field (y-axis) were determined by a veterinary pathologist during a blinded examination of slides from the cecal mucosa. Data represent means ± s.e.m. Statistically significant (P < 0.05) differences are indicated.
DISCUSSION
To our knowledge, this study is the first to investigate the gene expression profile elicited by S. typhimurium in the ileal mucosa in vivo. We found that in contrast to gene expression profiling performed with macrophages or epithelial cells10,21, in vivo gene expression profiling revealed that T cell products, including IL-17 and IL-22, are among the genes whose expression is upregulated most strongly during S. typhimurium infection. Studies of a mouse model of Klebsiella pneumoniae lung infection show that bacterial stimulation of pattern recognition receptors on mononuclear cells results in IL-23 production, which in turn triggers the production of IL-17 by T cells31,32. This paracrine IL-23–mediated mechanism also triggers the release of IL-22 and IL-17 from T cells in other models of inflammation33,34. These studies suggest that TH17 cells amplify signals (for example, IL-23) generated by mononuclear cells after encounter of invasive bacteria in the intestinal mucosa. The existence of such a paracrine amplification loop would explain why IL-17 responses were among the most prominent changes in gene expression observed in the ileal mucosa during S. typhimurium infection in this study.
Here we show that SIV infection selectively blunts IL-17 responses elicited by S. typhimurium in rhesus macaques. This is probably caused by a general depletion of CD4+ T cells in the ileal mucosa, as indicated by the similar depletion of both TH1 and TH17 populations. Our data specify a concrete immune defect that impairs the ability of SIV-infected macaques to orchestrate a normal mucosal inflammatory response to S. typhimurium infection. In our study, an underlying SIV infection markedly increased the ability of S. typhimurium to disseminate to the mesenteric lymph node of rhesus macaques. Experiments with Il17ra−/− mice demonstrated that defective IL-17 responses accelerate bacterial translocation to mesenteric lymph node and spleen. These data suggested that an important function of IL-17 is to orchestrate immune responses that limit bacterial dissemination from the intestinal mucosa. SIV selectively impaired this arm of the immune response, which provides an attractive explanation as to why people with HIV are at an increased risk of developing NTS bacteremia35–37. This SIV-mediated defect in mucosal barrier function may also help explain the presence of lipopolysaccharide in the circulation of people with HIV38.
It has been proposed that one function of TH17 cells is to regulate innate immune responses by producing cytokines20. IL-22 and IL-17 _target epithelial cells and modulate their expression of antimicrobial peptides18,39, migration and cell turnover in the intestinal epithelium40,41; they also modulate intestinal proinflammatory responses40,42. Among the cytokines whose expression is induced by IL-17 in epithelial cells are neutrophil chemoattractants such as CXCL8 (IL-8)16. Compared to wild-type mice, Il17ra−/− mice showed reduced mRNA levels of the neutrophil chemoattractant Kc and reduced neutrophil recruitment into the cecal mucosa during S. typhimurium infection. Finally, IL-17 induces the production of granulocyte colony–stimulating factor43, which, in turn, is required for neutrophil functionality44. Neutrophils from people with HIV show reduced antimicrobial activity45, and this defect can be restored by treatment with granulocyte colony–stimulating factor46,47. It is currently not clear which of the responses controlled by IL-17 is most important for limiting S. typhimurium dissemination from the intestinal mucosa. The fact that neutropenia is associated with NTS bacteremia in other clinical settings48 points to a reduced neutrophil recruitment, reduced neutrophil functionality, or both as possible causes for a defect in mucosal barrier function in people with HIV. NTS bacteremia is commonly found in HIV-infected individuals when CD4+ T cell counts in the blood fall below 200/μl5, which suggests a contribution of systemic defects, possibly because depletion of TH17 cells at extraintestinal sites may also impair neutrophil functionality in regional lymph nodes or in the circulation. Further work is necessary to precisely pinpoint the defect in mucosal barrier function caused by IL-17 deficiency.
METHODS
Animal experiments
All experiments were approved by the Institutional Animal Care and Use Committee at the University of California, Davis. We used eight healthy, Salmonella-free, male rhesus macaques ranging from 2 to 4 years of age (MK3–10) for ligated ileal loop surgery. We isolated lamina propria T cells from all macaques undergoing loop surgery and two additional macaques (MK1 and MK2). We inoculated four macaques (MK3, MK4, MK6 and MK10) intravenously with previously titrated frozen stocks of SIV mac251. We determined plasma viral loads as previously described49. Macaques were pre-anesthetized with ketamine (10 mg/kg; Parke-Davis), followed by placement of an endotracheal tube and maintenance of the anesthesia with isofluorane. When needed, we kept the macaques under a positive-pressure respirator. We performed a laparotomy, exposing the ileum and ligating 13 loops with an average of 4 cm in length, leaving 1-cm spacer loops in between. We inoculated the loops by intralumenal injections of 1 ml of either sterile LB broth or a logarithmically grown culture containing 1 × 109 colony-forming units (CFU) of wild-type S. typhimurium (IR715)30. We collected loops at 2, 5, or 8 h after inoculation.
We streptomycin-pretreated and orally infected groups of eight female C57BL/6 mice or six female Il17ra−/− mice (B6.IL-17R mice, Taconic), aged 8–10 weeks, with S. typhimurium as described previously30. At 48 h after infection, we killed the mice and collected the organs for mRNA isolation and bacteriology. A veterinary pathologist performed blinded histopathological evaluation of H&E-stained sections from the cecum.
Bacteriology
We collected two 6-mm biopsy punches from each loop. At the end of the surgery, we collected two biopsy punches (6-mm) from the mesenteric lymph node. We incubated biopsy punches for 1 h in PBS containing 50 mg/l of gentamicin, homogenized them, serially diluted the homogenate and plated on LB agar plates containing the appropriate antibiotics.
RNA analysis
We collected two 6-mm biopsy punches from each loop for RNA isolation, which provided comparable tissue samples across different areas of the intestinal mucosa (gut-associated lymphoid tissue in rhesus macaques was composed of randomly distributed individual lymphoid follicles). We performed RNA extraction from intestinal biopsy punches from macaques or organs collected from mice as previously described30.
We monitored gene expression profiles in ileal tissues with newly developed rhesus macaque genome–specific high-density oligonucleotide microarrays (Affymetrix). The arrays contained probe sets representing over 20,000 rhesus macaque genes. To minimize the occurrence of false positives in the array data, we used a minimum twofold difference in mRNA levels (P value <0.05; 95% confidence) between control and experimental samples as a criterion for identifying a change in gene expression. We determined statistical confidence through analysis of at least 11 independent 25-nucleotide oligonucleotide probes for each gene in each sample. We performed real-time PCR as described previously30 with primers listed in Supplementary Table 1 online.
Fluorescence microscopy
We stained rhesus ileal sections with rabbit polyclonal antibody to human IL-17 (Santa Cruz Biotechnology) and FITC-conjugated antibody to rabbit IgG. We mounted sections with Slow Fade with DAPI (Invitrogen) and captured images by confocal laser microscopy using LSM 5 and PASCAL software (Zeiss)23.
Cell isolation, stimulation and flow cytometry
For each macaque, we collected one 10-cm segment of the terminal ileum at surgery. We incubated the tissue in 2.5 mM EDTA, washed it twice with PBS and digested with collagenase type IV (Sigma). After filtering the cell suspension over glass wool, we washed the cells and incubated them with or without 50 ng/ml PMA and 1,000 ng/ml ionomycin in the presence of 0.01 mg/ml brefeldin A for 6 h. We blocked the cells with 2% human γ-globulin (Sigma) and then stained with Amcyan live amine dye (Invitrogen). We used antibodies to CD3 (allophycocyanin (APC)-Cy7–labeled; SP34.2), CD4 (Pacific Blue–labeled; OKT4), CD8 (APC-Cy5.5-labeled; 3B5), γδ T cell receptor (phycoerythrin-labeled; 5A6.E9) and CD95 (phycoerythrin-Cy5–labeled; DX2), followed by intracellular staining with FITC-labeled antibody to IL-17 (eBio64CAP17) and APC-labeled antibody to IFN-γ (4SB3) using 2 × Perm/Fix solution (BD). We performed multicolor immunophenotyping on a modified LSRII with a minimum of 500,000 events collected.
Statistical analyses
We analyzed microarray data using model-based algorithms (dChip, http://biosun1.harvard.edu/complab/dchip) and t-tests. We performed biological analysis of microarray data with the Affymetrix NetAFFX web interface and the DAVID (http://david.abcc.ncifcrf.gov/) annotation tool. Statistically over-represented (P < 0.05) biological processes within sub-clusters were identified with EASE (http://david.abcc.ncifcrf.gov/). Fold changes in mRNA levels measured by real-time PCR and CFU numbers underwent logarithmic transformation, and percentage values underwent angular transformation before ANOVA followed by either Student’s t-test or the Student-Newman-Keuls test.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank C. Bevins and R. Tsolis for helpful suggestions to improve the manuscript. We would like to thank E. Reay and L. Hirst for their invaluable help in coordinating the macaque studies and Taconic Corporation for providing Il17ra−/− mice for this study.
Work in A.J.B.’s laboratory was supported by US Public Health Service grants AI040124, AI044170 and AI065534. Work in S.D.’s laboratory was supported by US Public Health Service grants DK43183, DK61297 and AI43274. T.A.P. and R.L.S. were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil. R.L.S. was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil. I.G. was supported by Public Health Service grant AI06055.
Footnotes
Accession codes. Gene Expression Omnibus microarray accession code, GSE10567.
Note: Supplementary information is available on the Nature Medicine website.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Zhang S, et al. Molecular pathogenesis of Salmonella enterica serotype typhimurium–induced diarrhea. Infect. Immun. 2003;71:1–12. doi: 10.1128/IAI.71.1.1-12.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohmann EL. Nontyphoidal salmonellosis. Clin. Infect. Dis. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 3.Gordon MA, et al. Bacteraemia and mortality among adult medical admissions in Malawi-predominance of non-typhi salmonellae and Streptococcus pneumoniae. J. Infect. 2001;42:44–49. doi: 10.1053/jinf.2000.0779. [DOI] [PubMed] [Google Scholar]
- 4.Alausa KO, et al. Septicaemia in the tropics. A prospective epidemiological study of 146 patients with a high case fatality rate. Scand. J. Infect. Dis. 1977;9:181–185. doi: 10.3109/inf.1977.9.issue-3.05. [DOI] [PubMed] [Google Scholar]
- 5.Kankwatira AM, Mwafulirwa GA, Gordon MA. Non-typhoidal salmonella bacteraemia—an under-recognized feature of AIDS in African adults. Trop. Doct. 2004;34:198–200. doi: 10.1177/004947550403400404. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MA, et al. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS. 2002;16:1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, et al. The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 2002;70:3843–3855. doi: 10.1128/IAI.70.7.3843-3855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reis BP, et al. The attenuated sopB mutant of Salmonella enterica serovar typhimurium has the same tissue distribution and host chemokine response as the wild type in bovine Peyer’s patches. Vet. Microbiol. 2003;97:269–277. doi: 10.1016/j.vetmic.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Santos RL, Zhang S, Tsolis RM, Bäumler AJ, Adams LG. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet. Pathol. 2002;39:200–215. doi: 10.1354/vp.39-2-200. [DOI] [PubMed] [Google Scholar]
- 10.Nau GJ, Schlesinger A, Richmond JF, Young RA. Cumulative Toll-like receptor activation in human macrophages treated with whole bacteria. J. Immunol. 2003;170:5203–5209. doi: 10.4049/jimmunol.170.10.5203. [DOI] [PubMed] [Google Scholar]
- 11.Kent TH, Formal SB, Labrec EH. Salmonella gastroenteritis in rhesus monkeys. Arch. Pathol. 1966;82:272–279. [PubMed] [Google Scholar]
- 12.Daniel MD, et al. Simian models for AIDS. Cancer Detect. Prev. Suppl. 1987;1:501–507. [PubMed] [Google Scholar]
- 13.Ziesche E, Bachmann M, Kleinert H, Pfeilschifter J, Muhl H. The inter-leukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J. Biol. Chem. 2007;282:16006–16015. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]
- 14.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17– and TNF-α–induced genes in bone cells. J. Leukoc. Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 15.Kao CY, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-κB-dependent signaling pathway. J. Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 16.Laan M, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 17.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium–induced colitis in mice. Clin. Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by TH17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington LE, et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 20.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng H, et al. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 2003;171:3668–3674. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 22.Thamlikitkul V, Dhiraputra C, Paisarnsinsup T, Chareandee C. Non-typhoidal Salmonella bacteraemia: clinical features and risk factors. Trop. Med. Int. Health. 1996;1:443–448. doi: 10.1046/j.1365-3156.1996.d01-92.x. [DOI] [PubMed] [Google Scholar]
- 23.Sankaran S, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heise C, Miller CJ, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 25.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veazey RS, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 28.Mattapallil JJ, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 29.Barthel M, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffatellu M, et al. The capsule-encoding viaB locus reduces IL-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype typhi. Infect. Immun. 2007;75:4342–4350. doi: 10.1128/IAI.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Happel KI, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Happel KI, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, et al. Interleukin-22, a TH17 cytokine, mediates IL-23–induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 34.Umemura M, et al. IL-17–mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein LJ, Krieger BZ, Novick B, Sicklick MJ, Rubinstein A. Bacterial infection in the acquired immunodeficiency syndrome of children. Pediatr. Infect. Dis. 1985;4:472–475. doi: 10.1097/00006454-198509000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Eng RH, Bishburg E, Smith SM, Geller H, Kapila R. Bacteremia and fungemia in patients with acquired immune deficiency syndrome. Am. J. Clin. Pathol. 1986;86:105–107. doi: 10.1093/ajcp/86.1.105. [DOI] [PubMed] [Google Scholar]
- 37.Gilks CF, et al. Life-threatening bacteraemia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet. 1990;336:545–549. doi: 10.1016/0140-6736(90)92096-z. [DOI] [PubMed] [Google Scholar]
- 38.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 39.Kao CY, et al. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J. Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 40.Brand S, et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz S, Beaulieu JF, Ruemmele FM. Interleukin-17 is a potent immuno-modulator and regulator of normal human intestinal epithelial cell growth. Biochem. Biophys. Res. Commun. 2005;337:505–509. doi: 10.1016/j.bbrc.2005.09.075. [DOI] [PubMed] [Google Scholar]
- 42.Andoh A, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 43.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony–stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. Granulocyte colony–stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J. Clin. Invest. 1998;102:663–670. doi: 10.1172/JCI2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitrak DL. Neutrophil deficiency and dysfunction in HIV-infected patients. Am. J. Health Syst. Pharm. 1999;56(Suppl 5):S9–S16. doi: 10.1093/ajhp/56.suppl_5.S9. [DOI] [PubMed] [Google Scholar]
- 46.George S, et al. Neutrophils from AIDS patients treated with granulocyte colony–stimulating factor demonstrate enhanced killing of Mycobacterium avium. J. Infect. Dis. 1998;178:1530–1533. doi: 10.1086/314460. [DOI] [PubMed] [Google Scholar]
- 47.Pitrak DL. Filgrastim treatment of HIV-infected patients improves neutrophil function. AIDS. 1999;13(Suppl 2):S25–S30. [PubMed] [Google Scholar]
- 48.Karim M, Khan W, Farooqi B, Malik I. Bacterial isolates in neutropenic febrile patients. J. Pak. Med. Assoc. 1991;41:35–37. [PubMed] [Google Scholar]
- 49.George MD, Sankaran S, Reay E, Gelli AC, Dandekar S. High-throughput gene expression profiling indicates dysregulation of intestinal cell cycle mediators and growth factors during primary simian immunodeficiency virus infection. Virology. 2003;312:84–94. doi: 10.1016/s0042-6822(03)00207-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.