Abstract
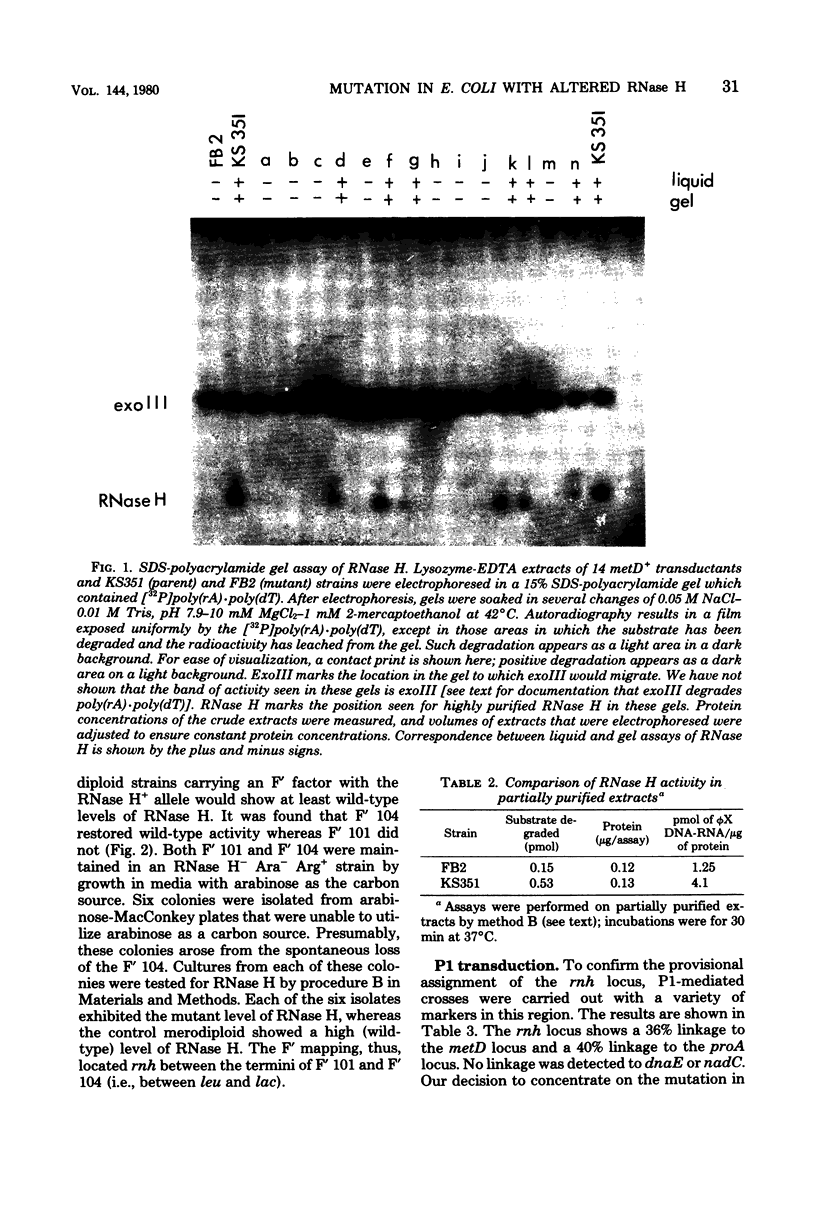
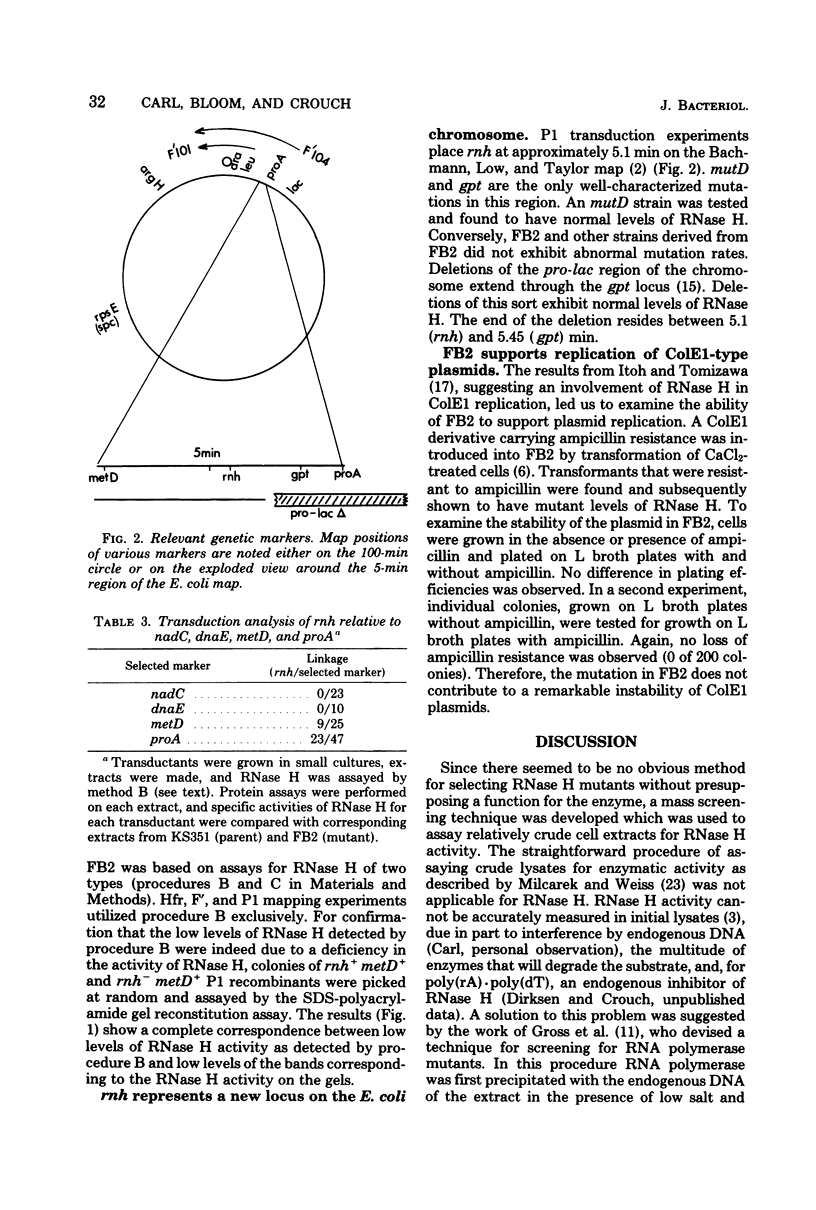
A mutant of Escherichia coli with altered levels of ribonuclease (RNase) H was isolated after mutagenesis with ethyl methane sulfonate. A procedure for assaying RNase H in partially purified extracts was used to screen approximately 1,500 colonies for variations in RNase H activity. Confirmation of a lower level of RNase H in the mutant was accomplished by analysis of RNase H in sodium dodecyl sulfate-polyacrylamide gels. By Hfr, F', and P1 transduction mapping, the genetic locus responsible for the lower levels of RNase H was located at 5.1 min on the E. coli chromosome. This mutation (rnh) represents a new locus on the E. coli chromosome. The only phenotypic characteristic of this mutation which has been observed to date is the lower level of RNase H (30% of parental values).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D., Watson N. Mapping and characterization of a mutation in Escherichia coli that reduces the level of ribonuclease III specific for double-stranded ribonucleic acid. J Bacteriol. 1975 Oct;124(1):317–324. doi: 10.1128/jb.124.1.317-324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkower I., Leis J., Hurwitz J. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid-deoxyribonucleic acid hybrid structures. J Biol Chem. 1973 Sep 10;248(17):5914–5921. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Büsen W., Hausen P. Distinct ribonuclease H activities in calf thymus. Eur J Biochem. 1975 Mar 3;52(1):179–190. doi: 10.1111/j.1432-1033.1975.tb03985.x. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Panasenko S. M., Lehman I. R., Davis R. W. In vitro construction of bacteriophage lambda carrying segments of the Escherichia coli chromosome: selection of hybrids containing the gene for DNA ligase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3416–3420. doi: 10.1073/pnas.72.9.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Rech J., Huet J., Jeanteur P. Isolation and characterization of two types of ribonucleases H in Krebs II ascites cells. J Biol Chem. 1979 Aug 10;254(15):7353–7361. [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Darlix J. L. Stimultaneous purification of Escherichia coli termination factor rho, RNAase III and RNAase H. Eur J Biochem. 1975 Feb 21;51(2):369–376. doi: 10.1111/j.1432-1033.1975.tb03937.x. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Gross C., Engbaek F., Flammang T., Burgess R. Rapid micromethod for the purification of Escherichia coli ribonucleic acid polymerase and the preparation of bacterial extracts active in ribonucleic acid synthesis. J Bacteriol. 1976 Oct;128(1):382–389. doi: 10.1128/jb.128.1.382-389.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkern R. C., Cantoni G. L. Studies on a calf thymus ribonuclease specific for ribonucleic acid-deoxyribonucleic acid hybrids. Biochemistry. 1973 Jun 19;12(13):2389–2395. doi: 10.1021/bi00737a004. [DOI] [PubMed] [Google Scholar]
- Hall S. H., Crouch R. J. Isolation and characterization of two enzymatic activities from chick embryos which degrade double-stranded RNA. J Biol Chem. 1977 Jun 25;252(12):4092–4097. [PubMed] [Google Scholar]
- Henry C. M., Ferdinand F. J., Knippers R. A hydridase from Escherichia coli. Biochem Biophys Res Commun. 1973 Feb 5;50(3):603–611. doi: 10.1016/0006-291x(73)91287-4. [DOI] [PubMed] [Google Scholar]
- Holden J. A., Harriman P. D., Wall J. D. Escherichia coli mutants deficient in guanine-xanthine phosphoribosyltransferase. J Bacteriol. 1976 Jun;126(3):1141–1148. doi: 10.1128/jb.126.3.1141-1148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi T., Maki H., Sekiguchi M. A new conditional lethal mutator (dnaQ49) in Escherichia coli K12. Mol Gen Genet. 1978 Jul 25;163(3):277–283. doi: 10.1007/BF00271956. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Initiation of replication of plasmid ColE1 DNA by RNA polymerase, ribonuclease H, and DNA polymerase I. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):409–417. doi: 10.1101/sqb.1979.043.01.047. [DOI] [PubMed] [Google Scholar]
- Keller W., Crouch R. Degradation of DNA RNA hybrids by ribonuclease H and DNA polymerases of cellular and viral origin. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3360–3364. doi: 10.1073/pnas.69.11.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. RNA-primed DNA synthesis in vitro. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1560–1564. doi: 10.1073/pnas.69.6.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. A conditional lethal mutant of Escherichia coli K12 defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A. 1974 May;71(5):2048–2051. doi: 10.1073/pnas.71.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman I. R., Chien J. R. Persistence of deoxyribonucleic acid polymerase I and its 5'--3' exonuclease activity in PolA mutants of Escherichia coli K12. J Biol Chem. 1973 Nov 25;248(22):7717–7723. [PubMed] [Google Scholar]
- Leis J. P., Berkower I., Hurwitz J. Mechanism of action of ribonuclease H isolated from avian myeloblastosis virus and Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):466–470. doi: 10.1073/pnas.70.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease 3. J Mol Biol. 1972 Jul 21;68(2):303–318. doi: 10.1016/0022-2836(72)90215-x. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Riggs A. D., Gill G. N. Ribonuclease H (hybrid) in Escherichia coli. Identification and characterization. J Biol Chem. 1973 Apr 10;248(7):2621–2624. [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Olivera R. M., Bonhoeffer E. Replication of Escherichia coli requires DNA polymerase I. Nature. 1974 Aug 9;250(5466):513–514. doi: 10.1038/250513a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Stein H., Hausen P. Enzyme from calf thymus degrading the RNA moiety of DNA-RNA Hybrids: effect on DNA-dependent RNA polymerase. Science. 1969 Oct 17;166(3903):393–395. doi: 10.1126/science.166.3903.393. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J Bacteriol. 1975 Oct;124(1):307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Ikeda J. E., Benz E., Marians K. J., Vicuna R., Sugrue S., Zipursky S. L., Hurwitz J. Replication of phiX174 DNA: in vitro synthesis of phiX RFI DNA and circular, single-stranded DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):311–329. doi: 10.1101/sqb.1979.043.01.038. [DOI] [PubMed] [Google Scholar]
- Vicuna R., Hurwitz J., Wallace S., Girard M. Selective inhibition of in vitro DNA synthesis dependent on phiX174 compared with fd DNA. I. Protein requirements for selective inhibition. J Biol Chem. 1977 Apr 25;252(8):2524–2533. [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wyers F., Sentenac A., Fromageot P. Role of DNA-RNA hybrids in eukaryotes. Ribonuclease H in yeast. Eur J Biochem. 1973 Jun;35(2):270–281. doi: 10.1111/j.1432-1033.1973.tb02835.x. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]