Abstract
Blood gene expression profiles of very brief (5 and 10 mins) focal ischemia that simulates transient ischemic attacks in humans were compared with ischemic stroke (120 mins focal ischemia), sham, and naïve controls. The number of significantly regulated genes after 5 and 10 mins of cerebral ischemia was 39 and 160, respectively (fold change ⩾∣1.5∣ and P<0.05). There were 103 genes common to brief focal ischemia and ischemic stroke. Ingenuity pathway analysis showed that genes regulated in the 5 mins group were mainly involved in small molecule biochemistry. Genes regulated in the 10 mins group were involved in cell death, development, growth, and proliferation. Such genes were also regulated in the ischemic stroke group. Genes common to ischemia were involved in the inflammatory response, immune response, and cell death—indicating that these pathways are a feature of focal ischemia, regardless of the duration. These results provide evidence that brief focal ischemia differentially regulates gene expression in the peripheral blood in a manner that could distinguish brief focal ischemia from ischemic stroke and controls in rats. We postulate that this will also occur in humans.
Keywords: blood, focal cerebral ischemia, gene expression, rat, stroke, transient ischemic attack
Introduction
Stroke is the leading cause of adult disability, and the third leading cause of death in the United States. Among patients with ischemic stroke, 15% report a preceding history of transient ischemic attack (TIA) (Nguyen-Huynh and Johnston, 2007). Indeed, one-third of classical TIA patients go on to have ischemic strokes, the majority occurring within the first week (Rothwell et al, 2006). Transient ischemic attacks often show evidence of cerebral infarction, with 25% to 40% of patients having restricted diffusion on magnetic resonance imaging (Rothwell et al, 2006). Related to this finding, we have shown that very brief focal ischemia in rats simulating human TIAs, results in brain microinfarctions and an inflammatory response in one-third of the animals (Zhan et al, 2008).
Transient ischemic attacks are underrecognized, underreported, undertreated, and often do not have an identified etiology. Analysis of gene expression after TIAs is one method to gain insight into this heterogeneous disease. Ischemic brain tissue induces a response in the peripheral circulation, including an inflammatory response, which can be detected by genomic analysis of the blood (Tang et al, 2001; Schwab et al, 2001; Gelderblom et al, 2009). Several gene expression studies have been performed in animals and humans to identify genes in brain and blood associated with ischemic stroke (Tang et al, 2001, 2006; Lu et al, 2003; Moore et al, 2005; Grond-Ginsbach et al, 2008; Xu et al, 2008). Gene expression has also been studied in the brain after brief periods of focal ischemia that results in ischemia preconditioning (Stenzel-Poore et al, 2003, 2004; Dhodda et al, 2004).
Though the effects of brief focal ischemia on brain have been described, this has not been done for blood. We have shown that 10 mins of global cerebral ischemia does change gene expression (RNA expression levels) in peripheral blood of rodents (Tang et al, 2003). Moreover, the RNA expression profile in peripheral blood correlates with neuronal cell death in rat brain after the global ischemia (Tang et al, 2003). In this study we aimed to determine whether brief focal ischemia, which simulates TIAs in humans, changes RNA expression levels in blood. Adult rats were subjected to 120 mins of focal cerebral ischemia (stroke), 10 mins of focal cerebral ischemia, 5 mins of focal cerebral ischemia, sham surgery, and compared with naïve, untouched controls. Whole blood was obtained 24 h later and RNA expression assessed on whole genome Affymetrix microarrays.
We report several novel observations. (1) Very brief focal cerebral ischemia is associated with specific gene expression profiles in rat blood, which differ from sham controls and animals with ischemic stroke. (2) Different durations of focal cerebral ischemia can regulate duration-specific genes in blood. (3) Many of the genes regulated by very brief focal ischemia are common to ischemic stroke genes and are involved in pathways relating to the inflammatory response, immune response, and cell death. These data support the possibility that human patients with TIAs may have changes in blood gene expression, and that some of these changes may depend on the duration of cerebral ischemia.
Matherials and methods
Animals
Fifteen male Sprague–Dawley rats weighing 285 to 338 g (Charles River Labs, Hollister, CA, USA) were used in this study. The Institutional Animal Care and Use Committee at the University of California at Davis approved the animal protocol in accordance with NIH guidelines.
Focal Ischemia
Brief focal cerebral ischemia was produced by occluding the middle cerebral artery using the intraluminal suture technique. Briefly, rats were anesthetized with 3% isoflurane in a 2 L induction chamber and maintained with 1.5% isoflurane in 100% oxygen. The right common carotid artery was exposed through a ventral midline incision. To occlude the middle cerebral artery, a 3–0 monofilament nylon suture with the tip rounded by heat was inserted into the external carotid artery and advanced into the internal carotid artery approximately 20 to 23 mm beyond the carotid bifurcation until mild resistance was felt. Rectal temperature was maintained between 36.6°C and 37.3°C with a heating blanket throughout the procedure.
Rats were subjected to 5, 10, or 120 mins of focal ischemia. The suture was then removed, animals allowed to recover from anesthesia and to survive 24 h. Sham-operated rats were subjected to the identical surgical protocol except that no suture was inserted into external carotid artery. Naïve rats were left in their home cages up until the time of anesthesia and killing.
RNA Isolation
After 24 h of reperfusion, animals were anesthetized with 3% isoflurane in a 2 L induction chamber and maintained with 1.5%. isoflurane in 100% oxygen. Whole blood (5 mL) was then collected from the right ventricles into 2 PAXgene vacutainers (PreAnalytiX, Hilden, Germany) from each animal. PAXgene tubes contain a proprietary reagent that immediately stabilizes RNA, thus reducing RNA degradation and inhibiting gene induction after phlebotomy. Total RNA was isolated according to the manufacturer's protocol (PAXgene blood RNA kit; PreAnalytiX). The RNA isolated with this protocol comes from all blood cells, including white blood cells (neutrophils, basophils, eosinophils, lymphocytes, and macrophages/monocytes), platelets, and red blood cells. RNA quality and quantity was assessed as described earlier (Xu et al, 2008).
Microarray Hybridization
Biotin-labeled cDNA was synthesized from 50 ng of total RNA using the Ovation Whole Blood Solution (Nugen, San Carlos, CA, USA) kit according to the manufacturer's protocol. Each RNA sample was hybridized on Affymetrix Rat Genome 230 2.0 microarrays. Microarray processing followed the Affymetrix protocol.
Microarray Data Normalization and Analysis
Partek Genomic Suite 6.04. software was used for gene expression analysis. After the arrays were scanned, the raw expression values for each gene were saved. CEL file data were then normalized using GC-robust multiarray average. A P<0.05 was considered significant following a one-way analysis of variance and the Fisher's least significant difference test.
Results
Blood Genomic Response
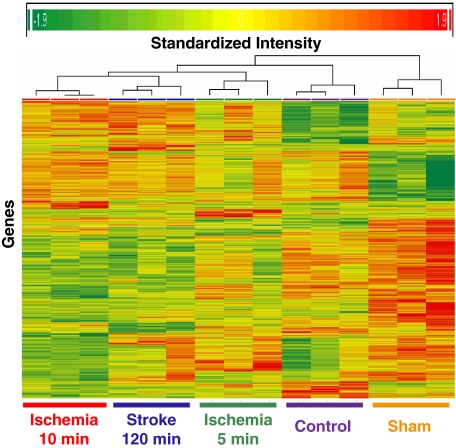
A total of 3,318 (out of 31,099) genes were significantly regulated in whole blood of adult rats 24 h after 120 mins of focal cerebral ischemia (stroke), 10 mins of focal ischemia, 5 mins of focal ischemia, and sham surgery as compared with naïve control (one-way analysis of variance, P<0.05). A cluster analysis of these 3,318 genes showed that the changes in gene expression from the same condition clustered together, suggesting that the changes in each group were reliable (Figure 1). Among these significant genes, 973 were associated with 5 mins ischemia, 1,336 with 10 mins ischemia, 1,369 with 120 mins ischemia (stroke), and 1,637 with sham operation. Many of these genes were nonspecific, meaning that they were regulated by all experimental conditions (120, 10, 5 mins, and sham) compared with naïve controls. To show that brief focal ischemia can selectively modify peripheral blood gene expression, we excluded genes that were common to brief focal ischemia, stroke, and sham operation. Thus, we created 5, 10, 120 mins and sham-specific gene lists. The numbers of regulated genes were derived using several different criteria: (1) ⩾∣2∣ fold change, ⩾∣1.5∣ fold change, or ⩾∣1.2∣ fold change; and (2) these genes had to be significantly different from the naïve controls, P 0.05 (Table 1).
Figure 1.
Heat map of genes (n=3,318) significantly regulated (P<0.05, one-way analysis of variance) in whole blood of adult rats 24 h after sham surgery (Sham), 5 mins of focal ischemia (ischemia 5 mins), 10 mins of focal ischemia (ischemia 10 mins), 120 mins of focal ischemia (stroke 120 mins) as compared with naïve control subjects (control). These genes were identified using RNA isolated from whole blood, whole genome Affymetrix rat microarrays, GC-robust multiarray average normalization and a one-way analysis of variance. The x axis shows treatment for the three animals in each group and the y axis shows individual genes. Note that genes from the three different animals in each group all clustered together. Red=upregulation; yellow=no change; green=downregulation.
Table 1. Numbers of specifically regulated genes for each treatment compared with naïve controls (P<0.05).
| Treatment | Regulation | Numbers of regulated gene probes | ||
|---|---|---|---|---|
| ⩾2-fold change | ⩾1.5-fold change | ⩾1.2-fold change | ||
| 5 mins ischemia | Upregulated | 10 | 21 | 38 |
| Downregulated | 4 | 18 | 40 | |
| 10 mins ischemia | Upregulated | 34 | 62 | 102 |
| Downregulated | 35 | 98 | 212 | |
| 120 mins ischemia | Upregulated | 40 | 62 | 64 |
| Downregulated | 15 | 80 | 170 | |
| Sham | Upregulated | 14 | 68 | 308 |
| Downregulated | 163 | 322 | 410 | |
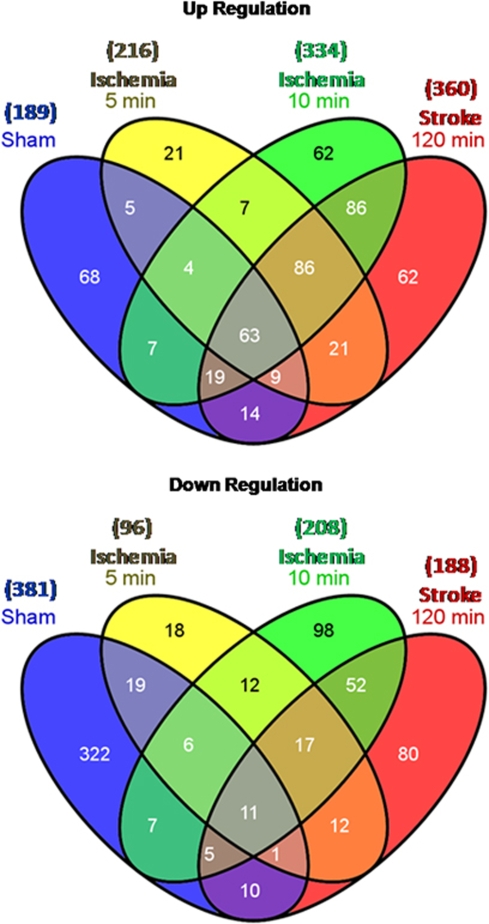
We focused on the changes of gene expression using the moderately stringent criteria of ⩾∣1.5∣ fold change and P<0.05 to display and interpret findings. Figure 2 shows the number of upregulated (top panel) and downregulated genes (bottom panel) in separate Venn diagrams using these criteria. The probe ID, gene symbols, fold change, and gene names for 5 mins ischemia, 10 mins ischemia, 120 mins ischemia (stroke), and sham-regulated genes are listed in Supplementary Tables 1–4, respectively.
Figure 2.
Venn diagrams showing the numbers of genes upregulated (top panel) or downregulated (lower panel) at least 1.5-fold in blood of adult rats 24 h after 5 mins of focal brain ischemia (ischemia 5 mins), 10 mins of focal brain ischemia (ischemia 10 mins), 120 mins of focal brain ischemia (ischemia 120 mins=stroke) and sham surgery (sham) as compared with naïve, untouched controls.
Ischemia-Specific Common Genes
Using the Venn diagrams (Figure 2), we determined the genes that were regulated in common by 5 mins ischemia, 10 mins ischemia, and 120 mins ischemia but not by sham operations. These ‘ischemia'-regulated genes are listed in Supplementary Table 5. There were 86 upregulated ‘ischemia' genes, and 17 downregulated ‘ischemia' genes. A few genes were regulated by both 5 and 10 mins of ischemia but not 120 mins of ischemia. These included C2 (complement component 2), GZMM (granzyme M-lymphocyte met-ase 1), IIGP1 (interferon inducible GTPase 1), SIRPA (signal-regulatory protein α, also known as CD172A, OX41), and PTEN (phosphatase and tensin homolog). These genes seem to indicate leukocyte signaling that occurs in response to durations of ischemia on the threshold (10 mins) of infarction.
Ingenuity pathway analysis (http://www.ingenuity.com) showed that the genes common to all three ischemia groups were involved in many functions and pathways (P<0.05). The top disease or disorder was inflammatory response and the top physiological system development and function was cell-mediated immune response. The top molecular and cellular functions and disease-related conditions are listed in Supplementary Table 9.
Brief Focal Ischemia or Stroke-Specific Genes
To show that very brief focal cerebral ischemia and 120 mins ischemia (which produces stroke in these studies) can produce specific gene expression profiles in peripheral blood, we identified the genes specific for 5 mins ischemia, genes specific for 10 mins ischemia, and genes specific for 120 mins ischemia as compared with the controls using the Venn diagrams in Figure 2. The probe ID, gene symbols, fold changes, and gene names that are specific for 5 mins ischemia, 10 mins ischemia, and 120 mins ischemia (stroke) are shown in Supplementary Tables 6–8, respectively. The top three molecular and cellular functions and neurological diseases in which the ischemia genes are involved are listed in Supplementary Table 9. The inflammatory response and cell-mediated immune response were the top functions shared by ischemia.
Genes Specific for 5 mins of Focal Ischemia
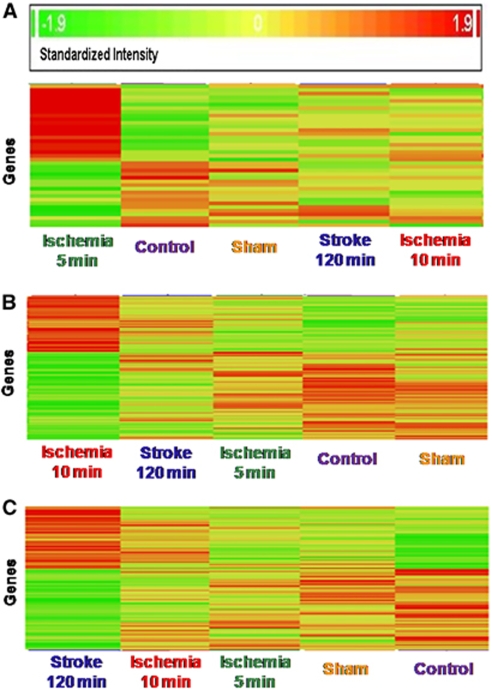
The specific upregulated and downregulated gene numbers after 5 mins of ischemia were 21 and 18, respectively. Brief focal ischemia of 5 mins duration specifically regulated angio-associated migratory cell protein (AAMP) and CLDN10 (claudin 10) gene transcripts. Angio-associated migratory cell protein encodes an immunoglobulin-type protein. The protein contains a heparin-binding domain and mediates heparin-sensitive cell adhesion. CLDN10 encodes claudin 10 protein, a cellular adhesion molecule. Claudins are integral membrane proteins and components of tight junctions. One transcript, a serine (or cysteine) peptidase inhibitor, clade B 11 (SERPINB11), was downregulated about 41-fold by 5 mins ischemia. The heat map of the 39 genes specific for 5 mins ischemia is shown in Figure 3A.
Figure 3.
Heat maps of genes regulated in whole blood of adult rats 24 h after brief focal ischemia (ischemia 5 mins; ischemia 10 mins); 24 h after 120 mins of focal ischemia (ischemia 120 mins=stroke); 24 h after sham surgery (sham); and in naïve control rats (control). (A) Genes specifically regulated in blood 24 h after 5 mins of focal cerebral ischemia (ischemia 5 mins, left column) compared with the other conditions.(B) Genes specifically regulated in blood 24 h after 10 mins of focal cerebral ischemia (ischemia 10 mins, left column) compared with the other conditions.(C) Genes specifically regulated in blood 24 h after 120 mins of focal ischemia (ischemia 120 mins=stroke, left column) compared with the other conditions. The columns on the x axis show the five treatment groups (three animals in each group) and the y axis shows individual genes. The genes in the left column in panels (A, B, and C) changed by at least 1.5-fold as compared with naïve controls with P<0.05. Red=upregulation; yellow=no change; green=downregulation.
Genes Specific for 10 mins of Focal Ischemia
The number of specific upregulated and downregulated genes after 10 mins ischemia was 62 and 98, respectively. Ten minutes of focal ischemia specifically regulated CBFB (core-binding factor β), CCL6 (chemokine (C-C motif) ligand 6), CD63 (CD63 molecule, also known as LAMP-3), CD8B (CD8b molecule), COL10A1 (Collagen-type X), CREBZF (CREB/ATF bZIP transcription factor), FCGR2B (Fc fragment of IgG, low affinity II b, receptor (CD32), ID1 (inhibitor of DNA-binding 1), NOTCH1 (Notch homolog 1, translocation-associated (Drosophila)), PIM1(pim-1 oncogene), PMPCB (peptidase β-mitochondrial processing), LILRA6 (leukocyte IgG-like receptor, subfamily A (with TM domain) 6, also known as (CD85b), MX1(myxovirus (influenza virus) resistance 1), MX2 (myxovirus (influenza virus) resistance 2), and THY-1 (Thy-1 cell surface antigen, also known as CD7, CD90). Two transcripts were regulated more than eight-fold after 10 mins ischemia: Glutathiolne S-transferase theta1///theta3 (Gstt1///Gstt3; 18.9-fold upregulation); and leukocyte IgG-like receptor B (LILRB3; 8.9-fold upregulation). The heat map of these 160 specific genes is shown in Figure 3B. These findings suggest that additional cell types and signaling pathways are regulated after the 10 mins compared with 5 mins of brief local ischemia.
Genes Specific for 120 mins of Focal Ischemia
The number of specific upregulated and downregulated genes after 120 mins ischemia was 62 and 80, respectively. These genes are fairly specific for brain infarction (stroke). They included BCL6 (B-cell CLL/lymphoma 6), CTRB2 (chymotrypsinogen B2), HLA-G (MHC class I, G), IRAK2 (interleukin-1 receptor-associated kinase 2), ITGA9 (α9 integrin), ITM2C (integral membrane protein 2C), MAP3K3 (mitogen-activated protein kinase kinase 3), SRPRB (signal recognition particle receptor B), and TSPO (translocator protein). The pathways and functions of these and other ‘stroke-specific' genes are discussed below. The heat map of these 142 stroke (120 mins ischemia)-specific genes is shown in Figure 3C. Overall, different durations of focal ischemia lead to ischemia duration-specific changes of gene expression involved in the inflammatory/immune response.
Genes Specific for Sham Operation
Sham operation regulated 390 genes (68 upregulated and 322 downregulated) compared with naïve control animals. See Supplementary Table 4 for a list of these genes. A total of 63 upregulated genes and 11 separate downregulated genes were common to all treatments (120 mins ischemia, 10 mins ischemia, 5 mins ischemia, and sham) compared with naïve controls.
Discussion
The major finding of this study is that specific patterns of gene expression occur in the peripheral blood of rats after episodes of brief focal cerebral ischemia compared with ischemic stroke and sham controls. These findings point to signaling differences based on duration of cerebral ischemia. This may help in understanding the role of the immune system in the transition from reversible ischemia (TIAs) to irreversible ischemia and perhaps to distinguish these events at the molecular and cellular levels in animals and in humans.
Gene Expression Profiles After Brief Focal Ischemia, Stroke, and Sham Operation
Though brief focal ischemia and stroke specifically regulated many genes, there were many genes shared by sham surgery and ischemia. These are nonspecific genes and likely reflect variables common to all of these animals such as stress, anesthesia, and the surgery itself.
In contrast, there were also genes common to 120, 10, and 5 mins ischemia—genes that presumably indicate brain ischemia independent of ischemia duration. The fact that gene expression changes in a single sample of blood after ischemia suggests many of the cells in blood responded. How this occurs is unclear, but could be related to local release or changes of various molecules by ischemic endothelium and/or brain (hormones, cytokines, chemokines, trophic factors, expression of cell adhesion molecules, and many others) that affect the circulating cells in blood.
Inflammatory Response and Cell-Mediated Immune Response
The top disorders for the genes regulated by 5 to 120 mins of ischemia were inflammatory response and cell-mediated immune response. Ischemia-specific genes include many inflammatory mediators: cytokines (IL1B—interleukin-1β); transmembrane receptors (CLEC7A—C-type lectin domain family 7A, CSF3R [CD114]—colony stimulating factor 3 receptor; IFNGR1 [CD119]—interferon γ receptor 1; IL13RA1—interleukin-13 receptor α1; IL1R2—interleukin-1 receptor, type II; KLRK1 [CD314]—killer cell lectin-like receptor subfamily K, member 1); G-protein-coupled receptors (IL8RB—interleukin-8 receptor β; PTAFR—platelet-activating factor receptor); integrin (ITGA5—integrin α5); immunoglobulin superfamily 6; junctional adhesion molecule 2(JAM2); and metallopeptidase-related molecules (TIMP2—TIMP metalloproteinase inhibitor 2; CTSF—cathepsin F, cysteine-type peptidase; ECE1—endothelin converting enzyme 1). These molecules are involved in leukocyte extravasation, a central process to inflammatory immune responses. Leukocyte extravasation from the circulation into sites of tissue inflammation is a tightly controlled process involving the multistep action of traffic signals and adhesion molecules that mediate tethering, rolling, adhesion, and diapedesis (transendothelial migration) (Weber et al, 2007). Selectins initiate leukocyte tethering and rolling along inflamed endothelium. Rolling slows down circulating leukocytes and allows the binding of chemokines such as IL-8 on endothelium to their specific G-protein-coupled chemokine receptors on leukocytes (Weber et al, 2007). Thus, the upregulation of both IL8RB (IL-8 receptor β) and PTAFR likely contribute to rolling of leukocytes even in the absence of tissue infarction (brief focal ischemia) in addition to focal stroke.
Activation of chemokine receptors triggers intracellular signaling pathways that activate leukocyte integrins. Interactions between leukocyte integrins and their immunoglobulin ligands on endothelial cells mediate the adhesion of leukocytes (Weber et al, 2007). In this study, ITGA5 and two immunoglobulin superfamilies, JAM2 (also known as JAM-B), and immunoglobulin superfamily 6 (IGSF6) are specifically upregulated by brief focal ischemia and stroke in peripheral blood. The function of α5 integrin remains unclear. JAM2 is a ligand for the integrin very late antigen 4 (VLA4, also known as α4β1) (Cunningham et al, 2002). VLA4 is an important molecule that mediates adhesion as well as rolling (Steeber et al, 2005). JAM2 is expressed by brain endothelial cells and is specifically enriched in certain vessels, such as high endothelial venules and lymphatics (Palmeri et a, 2000). The origins of JAM2 in the blood remain to be clarified.
The final step of leukocyte extravasation is diapedesis, which requires an elaborate series of adhesive interactions and involving specific molecules (such as CD31, JAM, and CD99) expressed at junctions between adjacent endothelial cells (Nourshargh and Marelli-Berg, 2005). Besides the JAM2 detected in brief focal ischemia and stroke peripheral blood, a tissue inhibitor of metallopeptidase, TIMP2, was upregulated specifically by brief focal ischemia and stroke. It likely regulates diapedesis as well.
Diapedesis is crucial to the cellular functions of leukocytes in both innate and adaptive immunity. In the innate immune response, migration of neutrophils, monocytes, and natural killer cells is initiated through local generation of inflammatory mediators (Nourshargh and Marelli-Berg, 2005), including the cytokine IL1β, which is upregulated by brief focal ischemia and stroke in peripheral blood. In adaptive immunity, expression of specific adhesion and chemokine receptors is regulated in T lymphocytes in response to antigen-initiated differentiation (Nourshargh and Marelli-Berg, 2005). In this study, the IL1β receptor IL1R2 and other transmembrane receptors (CLEC7A, CSF3R (CD114), IFNGR1 (CD119), and IL13RA1) were upregulated by brief ischemia and stroke, indicating that both the innate and adaptive immune responses are modulated during brief focal ischemia and ischemic stroke.
Small Molecule Biochemistry
The top molecular and cellular function after 5 mins of focal ischemia was small molecule biochemistry. Such genes were mainly involved in the synthesis of eicosanoids, nitric oxide, proteoglycans, leukotrienes (LT) D4, LTE4, and release of eicosanoids and arachidonic acid.
Eicosanoids are signaling molecules that function in many systems, including inflammation, immunity (O'Donnell et al, 2009), and the central nervous system (Tassoni et al, 2008). There are four families of eicosanoids—prostaglandins (PG), prostacyclins, thromboxanes (TX) and LT. IL1B and PTAFR can increase the synthesis of PGE2 (Pei et al, 1998; Zhu et al, 2003). IL1B can increase the release of TXA2 (Woodrow, 2003).
Nitric oxide is an important signaling molecule involved in a variety of biological processes, including vessel homeostasis through inhibition of vascular smooth muscle contraction, platelet aggregation, and leukocyte adhesion (Walter et al, 1995). IFNGR1, IL1B, and KLRK1 are reported to increase the synthesis of nitric oxide (Rieger et al, 1998; Trauner et al, 1999; Diefenbach et al, 2003), all of which were regulated by brief focal ischemia and stroke.
Proteoglycans are a major component of the extracellular matrix, breakdown of which facilitates leukocyte transmigration in inflammation and immunity. IL1B reportedly decreases the synthesis of proteoglycan (Attur et al, 2000).
Leukotrienes are potent proinflammatory molecules involved in leukocyte recruitment, adhesion, and transmigration and are synthesized from arachidonic acid by 5-lipoxygenase. ALOX5AP (arachidonate 5-lipoxygenase-activating protein), GGT5 (γ-glutamyltransferase 5), and SOAT1 (sterol O-acyltransferase 1) affect the synthesis of LTD4 or LTE4 and are regulated by focal ischemia in this study.
Five minutes of focal ischemia also regulated metabolism of N-acetylmannosamine and ceramide. NAGK encodes N-acetylglucosamine kinase, which catalyzes ATP and N-acetyl--glucosamine (NAG) into ADP and N-acetyl--glucosamine 6-phsophate. In addition to being catalyzed by NAGK, ATP can also be converted to ADP by ATPases. ATP6V1B2 encodes an ATPase (ATPase, H+ transporting, lysosomal V1 subunit B2) and this ATPase can also convert ATP into ADP. ADP is also converted back to ATP by ATP synthases. The conversion of these two molecules is critical for supplying energy for many important processes. ADP is usually stored in platelets and is released on platelet activation. When endothelium is injured, platelets become activated by the interaction of ADP and other molecules (Mustard and Packham, 1975; Colman, 1990). Accordingly, upregulation of NAGK and ATP6V1B2 expression may contribute to energy loss and platelet activation after brief focal ischemia.
Five minutes of focal ischemia downregulated CREM, which encodes a transcriptional inhibitor, cAMP responsive element modulator. Decreasing CREM expression in pinealocytes leads to enhanced synthesis of melatonin (Maronde et al, 1999). Melatonin inhibits the aggregation of the amyloid β protein that may affect vascular function and be involved in Alzheimer's disease. SERPINB11 is downregulated about 41-fold after 5 mins of focal ischemia. SERPINB11 encodes a serine protease inhibitor B11, Serpin B11. Tissue-type plasminogen activator (tPA), which is an endogenous thrombolytic enzyme inhibitor of clot formation, is a serine proteinase. As most serpins have a very important function in the inhibition of tPA the decreased Serpin B11 could activate tPA thrombolysis after brief durations of focal ischemia.
Cell Death
Stroke and 10 mins of focal ischemia regulated many genes related to cell death, whereas 5 mins focal ischemia regulated very few. Thus, 10 mins of focal ischemia, which is on the threshold of producing infarction, regulated many genes in peripheral blood related to cell death. These included CD40LG (CD40 ligand), RARA (retinoic acid receptor α), SLK (STE20-like kinase), DHCR24 (24-dehydrocholesterol reductase), DUSP1 (dual specificity phosphatase 1), FN1 (fibronectin 1), and NOTCH1 (Notch gene homolog 1)—which are involved in the cell death of fibroblasts. PTEN (phosphatase and tensin homolog), CD40LG, and FN1 are regulated by stroke and can increase the cell death of T lymphocytes and B lymphocytes. Other stroke-regulated genes FCGR2B, RARA, PEA15 (phosphoprotein enriched in astrocytes 15A), SOD2 (superoxide dismutase 2, mitochondrial), and ZYX (zyxin) can decrease the cell death of dendritic cells, pre-B lymphocytes, activated T lymphocytes, hematopoietic progenitor cells, epidermal cells, neurons, and cardiomyocytes. Notably, CLU (clusterin), CREM (cAMP responsive element modulator), and GSK3B (glycogen synthase kinase 3β) can increase the cell death of cortical neurons. ID1 (inhibitor of DNA-binding 1), PTEN, and SIRPA (single regulatory protein α, increase cell death of sympathetic neurons. IL1β can modulate cell death of thymocytes, macrophages, dendritic cells, and neutrophils, and LGALS3 (lectin, galactose binding, soluble 3) can increase cell death of CD4+ T lymphocytes.
The above ‘cell death' genes are regulated by 10 mins of focal ischemia, stroke, or both. The effect of brain ischemia on cell death-related genes in leukocytes is unclear, but likely relates to turnover of cells and production of specific cell types that respond to brain ischemia and stroke. Indeed, the changes of gene expression themselves may well relate in part to changes in total numbers of specific cell types—which is a balance of birth and death of each cell type. For example, 5 mins of focal ischemia specifically upregulated CFLAR (CASP8 and FADD-like apoptosis regulator, also known as c-FLIP and FLIP). c-FLIP downregulates apoptotic molecules in part by promoting NFκB survival signaling in leukocytes (Matsumori et al, 2006; Ran et al, 2004). A number of studies have showed increased circulating neutrophil counts and decreased lymphocyte counts in mice (Rosenzweig et al, 2004), rats (Relton et al, 2001), and humans (Emsley et al, 2003; Ross et al, 2007; Klehmet et al, 2009) after TIAs and stroke. The degree to which these changes in cell counts affect gene expression will have to be addressed in future studies.
This study shows specific changes in gene expression in the peripheral blood of rats in response to very brief focal cerebral ischemia. Though the functions of some are known, the functions of the large majority of the regulated genes in response to focal ischemia remain unclear. For example, stroke downregulates CTRB1 (chymotrypsinogen B1) 373-fold and upregulates EHD2 (EPS15 homology domain protein, endocytic recycling gene) 24-fold. However, their role and that of many other genes in blood after cerebral ischemia is unknown. The present findings suggest that examination of activated and suppressed pathways through gene expression studies in blood after stroke may provide novel insights in addition to those obtainable by standard immunological approaches.
Acknowledgments
This study was supported by NIH/NINDS grants NS028167, NS043252 and NS054652 to FRS.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow and Metabolism website (http://www.nature.com/jcbfm)
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
References
- Attur MG, Dave M, Cipolletta C, Kang P, Goldring MB, Patel IR, Abramson SB, Amin AR. Reversal of autocrine paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J Biol Chem. 2000;275:40307–40315. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- Colman RW. Aggregin: a platelet ADP receptor thatmediates activation. FASEB J. 1990;4:1425–1435. doi: 10.1096/fasebj.4.5.2407587. [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–27592. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochemistry. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Hsia JK, Hsiung MY, Raulet DH. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. Eur J Immunol. 2003;33:381–391. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, Hallenbeck JM, del Zoppo GJ, Rothwell NJ, Tyrrell PJ, Hopkins SJ. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93–101. doi: 10.1016/s0165-5728(03)00134-6. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Grond-Ginsbach C, Hummel M, Wiest T, Horstmann S, Pfleger K, Hergenhahn M, Hollstein M, Mansmann U, Grau AJ, Wagner S. Gene expression in human peripheral blood mononuclear cells uponacute ischemic stroke. J Neurol. 2008;255:723–731. doi: 10.1007/s00415-008-0784-z. [DOI] [PubMed] [Google Scholar]
- Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, Meisel A, Meisel C. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR. Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:786–810. doi: 10.1097/01.WCB.0000062340.80057.06. [DOI] [PubMed] [Google Scholar]
- Maronde E, Pfeffer M, Olcese J, Molina CA, Schlotter F, Dehghani F, Korf HW, Stehle JH. Transcription factors in neuroendocrine regulation: rhythmic changes in pCREB and ICER levels frame melatonin synthesis. J Neurosci. 1999;19:3326–3336. doi: 10.1523/JNEUROSCI.19-09-03326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumori Y, Northington FJ, Hong SM, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke. 2006;37:507–512. doi: 10.1161/01.STR.0000199057.00365.20. [DOI] [PubMed] [Google Scholar]
- Moore DF, Li H, Jeffries N, Wright V, Cooper RA, Jr, Elkahloun A, Gelderman MP, Zudaire E, Blevins G, Yu H, Goldin E, Baird AE. Using peripheral blood mononuclear cells to determine a gene expression profile of acute ischemic stroke: a pilot investigation. Circulation. 2005;111:212–221. doi: 10.1161/01.CIR.0000152105.79665.C6. [DOI] [PubMed] [Google Scholar]
- Mustard JF, Packham MA. Platelets, thrombosis and drugs. Drugs. 1975;9:19–76. doi: 10.2165/00003495-197509010-00003. [DOI] [PubMed] [Google Scholar]
- Nguyen-Huynh MN, Johnston SC. Evaluation and management of transient ischemic attack: an important component of stroke prevention. Nat Rev Cardiol. 2007;4:310–318. doi: 10.1038/ncpcardio0889. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Marelli-Berg FM. Transmigration through venular walls: a key regulator of leukocyte phenotype and function. Trends Immunol. 2005;26:157–165. doi: 10.1016/j.it.2005.01.006. [DOI] [PubMed] [Google Scholar]
- O'Donnell VB, Maskrey B, Taylor GW. Eicosanoids: generation and detection in mammalian cells. Methods Mol Biol. 2009;462:5–23. [PubMed] [Google Scholar]
- Palmeri D, van Zante A, Huang CC, Hemmerich S, Rosen SD. Vascular endothelial junction-associated molecule, a novel member of the immunoglobulin superfamily, is localized to intercellular boundaries of endothelial cells. J Biol Chem. 2000;275:19139–19145. doi: 10.1074/jbc.M003189200. [DOI] [PubMed] [Google Scholar]
- Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley SW, Dy LC, Fertel RH, Nguyen TM, Williams DA, Travers JB. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, Sharp FR. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18:1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32:199–205. doi: 10.1161/01.str.32.1.199. [DOI] [PubMed] [Google Scholar]
- Rieger J, Ständer M, Löschmann PA, Heneka M, Dichgans J, Klockgether T, Weller M. Synthesis and biological effects of NO in malignant glioma cells: modulation by cytokines including CD95 L and TGF-beta, dexamethasone, and p53 gene transfer. Oncogene. 1998;17:2323–2332. doi: 10.1038/sj.onc.1202154. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Ross AM, Hurn P, Perrin N, Wood L, Carlini W, Potempa K. Evidence of the peripheral inflammatory response in patients with transient ischemic attack. J Stroke Cerebrovasc Dis. 2007;16:203–207. doi: 10.1016/j.jstrokecerebrovasdis.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Buchan A, Johnston SC. Recent advances in management of transient ischaemic attacks and minor ischaemic strokes. Lancet Neurol. 2006;5:323–331. doi: 10.1016/S1474-4422(06)70408-2. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Nguyen TD, Meyermann R, Schluesener HJ. Human focal cerebral infarctions induce differential lesional interleukin-16 (IL-16) expression confined to infiltrating granulocytes, CD8+ T-lymphocytes and activated microglia/macrophages. J Neuroimmunol. 2001;114:232–241. doi: 10.1016/s0165-5728(00)00433-1. [DOI] [PubMed] [Google Scholar]
- Steeber DA, Venturi GM, Tedder TF. A new twist to the leukocyte adhesion cascade: intimate cooperation is key. Trends Immunol. 2005;26:9–12. doi: 10.1016/j.it.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Simon RP. Genomics of preconditioning. Stroke. 2004;35:2683–2686. doi: 10.1161/01.STR.0000143735.89281.bb. [DOI] [PubMed] [Google Scholar]
- Tang Y, Lu A, Aronow BJ, Sharp FR. Blood genomic responses differ after stroke, seizures, hypoglycemia, and hypoxia: blood genomic fingerprints of disease. Ann Neurol. 2001;50:699–707. doi: 10.1002/ana.10042. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nee AC, Lu A, Ran R, Sharp FR. Blood genomic expression profile for neuronal injury. J Cereb Blood Flow Metab. 2003;23:310–319. doi: 10.1097/01.WCB.0000048518.34839.DE. [DOI] [PubMed] [Google Scholar]
- Tang Y, Xu H, Du X, Lit L, Walker W, Lu A, Ran R, Gregg JP, Reilly M, Pancioli A, Khoury JC, Sauerbeck LR, Carrozzella JA, Spilker J, Clark J, Wagner KR, Jauch EC, Chang DJ, Verro P, Broderick JP, Sharp FR. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: a microarray study. J Cereb Blood Flow Metab. 2006;26:1089–1102. doi: 10.1038/sj.jcbfm.9600264. [DOI] [PubMed] [Google Scholar]
- Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia Pac J Clin Nutr. 2008;17:220–228. [PubMed] [Google Scholar]
- Trauner M, Fickert P, Stauber RE. Inflammation-induced cholestasis. J Gastroenterol Hepatol. 1999;14:946–959. doi: 10.1046/j.1440-1746.1999.01982.x. [DOI] [PubMed] [Google Scholar]
- Walter U, Geiger J, Haffner C, Markert T, Nehls C, Silber RE, Schanzenbächer P. Platelet-vessel wall interactions, focal adhesions, and the mechanism of action of endothelial factors. Agents Actions Suppl. 1995;45:255–268. doi: 10.1007/978-3-0348-7346-8_35. [DOI] [PubMed] [Google Scholar]
- Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- Woodrow P. Anthrax: forms, symptoms and treatment. Nurs Stand. 2003;17:33–37. doi: 10.7748/ns.17.48.33.s50. [DOI] [PubMed] [Google Scholar]
- Xu H, Tang Y, Liu DZ, Ran R, Ander BP, Apperson M, Liu XS, Khoury JC, Gregg JP, Pancioli A, Jauch EC, Wagner KR, Verro P, Broderick JP, Sharp FR. Gene expression in peripheral blood differs after cardioembolic compared with large-vessel atherosclerotic stroke: biomarkers for the etiologyof ischemic stroke. J Cereb Blood Flow Metab. 2008;28:1320–1328. doi: 10.1038/jcbfm.2008.22. [DOI] [PubMed] [Google Scholar]
- Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce Hsp70 protein. Brain Res. 2008;1234:183–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hua P, Lance P. Cyclooxygenase-2 expression and prostanoid biogenesis reflect clinical phenotype in human colorectal fibroblast strains. Cancer Res. 2003;63:522–526. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.